A Beneficial Plant-Associated Fungus Shifts the Balance toward Plant Growth over Resistance, Increasing Cucumber Tolerance to Root Herbivory
Abstract
:1. Introduction
2. Results
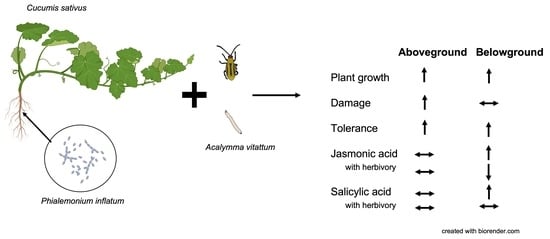
2.1. Phialemonium Inflatum Increases Cucumber Plant Germination, Biomass, and Reproduction Potential
2.2. Seed Treatment with Phialemonium Inflatum Reduces Cucumber Plant Resistance to Adult Cucumber Beetles
2.3. Cucumber Plants Treated with Phialemonium Inflatum Were more Tolerant to Root Herbivory
2.4. Roots but Not Leaves of Cucumber Plants Treated with Phialemonium Inflatum had Compromised Defense Responses to Herbivory
2.5. Phialemonium Inflatum Grows on the Surface of Cucumber Roots, but Does Not Colonize Cucumber Tissues
3. Discussion
4. Materials and Methods
4.1. Plants, Fungus, and Insects
4.2. Plant Growth and Reproductive Potential
4.3. Adult Cucumber Beetle Feeding and Preference
4.4. Larval Cucumber Beetle Feeding and Performance
4.5. Plant Tolerance to Herbivory by Cucumber Beetle Larvae
4.6. Plant Defense Signaling (Phytohormones)
4.7. Phialemonium Inflatum Growth and Colonization of Cucumber Tissues
4.8. Microscopic Observations of P. inflatum on Cucumber Roots
4.9. Statistical Analyses
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ruan, Y.-L.; Patrick, J.W.; Shabala, S.; Slewinski, T. Uptake and regulation of resource allocation for optimal plant performance and adaptation to stress. Front. Plant Sci. 2013, 4, 455. [Google Scholar] [CrossRef] [Green Version]
- Lokesha, R.; Vasudeva, R. Influence of a biotic stress (leaf curl viral infection) on the sex ratio and resource allocation in Momordica tuberosa (Roxb.) Cogn.—A monoecious perennial herb. Curr. Sci. 1993, 65, 238–242. [Google Scholar]
- Keller, I.; Rodrigues, C.M.; Neuhaus, H.E.; Pommerrenig, B. Improved resource allocation and stabilization of yield under abiotic stress. J. Plant Physiol. 2021, 257, 153336. [Google Scholar] [CrossRef] [PubMed]
- Mcconnaughay, K.D.M.; Coleman, J.S. Biomass allocation in plants: Ontogeny or optimality? A test along three resource gradients. Ecology 1999, 80, 2581–2593. [Google Scholar] [CrossRef]
- Boege, K.; Marquis, R.J. Facing herbivory as you grow up: The ontogeny of resistance in plants. Trends Ecol. Evol. 2005, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Boege, K. Influence of plant ontogeny on compensation to leaf damage. Am. J. Bot. 2005, 92, 1632–1640. [Google Scholar] [CrossRef]
- Osier, T.L.; Lindroth, R.L. Genotype and environment determine allocation to and costs of resistance in quaking aspen. Oecologia 2006, 148, 293–303. [Google Scholar] [CrossRef]
- Cailleau, A.; Grimanelli, D.; Blanchet, E.; Cheptou, P.-O.; Lenormand, T. Dividing a maternal pie among half-sibs: Genetic conflicts and the control of resource allocation to seeds in maize. Am. Nat. 2018, 192, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.R.; Poveda, K. Resource allocation trade-offs and the loss of chemical defences during apple domestication. Ann. Bot. 2019, 123, 1029–1041. [Google Scholar] [CrossRef]
- Olsson, P.A.; Jakobsen, I.; Wallander, H. Foraging and resource allocation strategies of mycorrhizal fungi in a patchy environment. In Mycorrhizal Ecology; Springer: Berlin/Heidelberg, Germany, 2002; pp. 93–115. [Google Scholar]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef] [Green Version]
- Loomis, W.E. Growth-differentiation balance vs. carbohydrate-nitrogen ratio. Proc. Am. Soc. Hortic. Sci. 1932, 29, 240–245. [Google Scholar]
- Stamp, N. Can the growth–differentiation balance hypothesis be tested rigorously? Oikos 2004, 107, 439–448. [Google Scholar] [CrossRef]
- Neuser, J.; Metzen, C.C.; Dreyer, B.H.; Feulner, C.; van Dongen, J.T.; Schmidt, R.R.; Schippers, J.H.M. HBI1 mediates the trade-off between growth and immunity through Its impact on apoplastic ROS homeostasis. Cell Rep. 2019, 28, 1670–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zust, T.; Agrawal, A.A. Trade-Offs between plant growth and defense against insect herbivory: An emerging mechanistic synthesis. Annu. Rev. Plant Biol. 2017, 68, 513–534. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Zebelo, S.; McNear, D.; Kloepper, J.; Fadamiro, H. Plant growth-promoting rhizobacteria induce changes in Arabidopsis thaliana gene expression of nitrate and ammonium uptake genes. J. Plant Interact. 2019, 14, 224–231. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and applications. Scientifica (Cairo) 2012, 2012, 963401. [Google Scholar] [CrossRef] [Green Version]
- Bashan, Y.; Levanony, H. Current status of Azospirillum inoculation technology: Azospirillum as a challenge for agriculture. Can. J. Microbiol. 2011, 36, 591–608. [Google Scholar] [CrossRef] [Green Version]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schardl, C.L.; Florea, S.; Pan, J.; Nagabhyru, P.; Bec, S.; Calie, P.J. The epichloae: Alkaloid diversity and roles in symbiosis with grasses. Curr. Opin. Plant Biol. 2013, 16, 480–488. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M.; Nl, C.M.J.P.; Nl, C.Z.; Nl, R.L.B.; Nl, S.V.; et al. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Gómez, S.; Stuefer, J.F. Members only: Induced systemic resistance to herbivory in a clonal plant network. Oecologia 2005, 147, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Heil, M. The ecological concept of costs of Induced Systemic Resistance (ISR). Eur. J. Plant Pathol. 2001, 107, 137–146. [Google Scholar] [CrossRef]
- Shoresh, M.; Yedidia, I.; Chet, I. Involvement of Jasmonic Acid/Ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 2007, 95, 76–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.J.; Wees, S.C.M. Van Shifting from priming of salicylic acid- to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Medina, A.; Van Wees, S.C.M.; Pieterse, C.M.J. Airborne signals from Trichoderma fungi stimulate iron uptake responses in roots resulting in priming of jasmonic acid-dependent defences in shoots of Arabidopsis thaliana and Solanum lycopersicum. Plant. Cell Environ. 2017, 40, 2691–2705. [Google Scholar] [CrossRef] [Green Version]
- Ek-Ramos, M.J.; Zhou, W.; Valencia, C.U.; Antwi, J.B.; Kalns, L.L.; Morgan, G.D.; Kerns, D.L.; Sword, G.A. Spatial and temporal variation in fungal endophyte communities isolated from cultivated cotton (Gossypium hirsutum). PLoS ONE 2013, 8, e66049. [Google Scholar] [CrossRef]
- Zhou, W.; Wheeler, T.A.; Starr, J.L.; Valencia, C.U.; Sword, G.A. A fungal endophyte defensive symbiosis affects plant-nematode interactions in cotton. Plant Soil 2018, 422, 251–266. [Google Scholar] [CrossRef]
- Lopez, D.C.; Sword, G.A. The endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea). Biol. Control 2015, 89, 53–60. [Google Scholar] [CrossRef]
- Castillo Lopez, D.; Zhu-Salzman, K.; Ek-Ramos, M.J.; Sword, G.A. The entomopathogenic fungal endophytes Purpureocillium lilacinum (formerly Paecilomyces lilacinus) and Beauveria bassiana negatively affect cotton aphid reproduction under both greenhouse and field conditions. PLoS ONE 2014, 9, e103891. [Google Scholar] [CrossRef] [Green Version]
- Sword, G.A.; Tessnow, A.; Ek-Ramos, M.J. Endophytic fungi alter sucking bug responses to cotton reproductive structures. Insect Sci. 2017, 24, 1003–1014. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; Martinez-Montiel, N.; Cruz-Lopez, M.d.C.; Martinez-Contreras, R.D. Fungal diversity and community composition of culturable fungi in Stanhopea trigrina cast gibberellin producers. Front. Microbiol. 2018, 9, 612. [Google Scholar] [CrossRef]
- Rathnayake, G.R.N.; Kumar, N.S.; Jayasinghe, L.; Araya, H.; Fujimoto, Y. Chemical investigation of metabolites produced by an endophytic fungi Phialemonium curvatum from the leaves of Passiflora edulis. Nat. Prod. Res. 2018, 32, 2483–2486. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Verhage, A.; Fernández, I.; García, J.M.; Azcón-Aguilar, C.; Flors, V.; Pozo, M.J. Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J. Exp. Bot. 2010, 61, 2589. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Huang, Y.; Ge, W.; Jia, Z.; Song, S.; Zhang, L.; Huang, Y. Involvement of jasmonic acid, ethylene and salicylic acid signaling pathways behind the systemic resistance induced by Trichoderma longibrachiatum H9 in cucumber. BMC Genom. 2019, 20, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Schmelz, E.A.; Engelberth, J.; Alborn, H.T.; O’Donnell, P.; Sammons, M.; Toshima, H.; Tumlinson, J.H. Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 10552–10557. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.L.; De Moraes, C.M.; Mescher, M.C. Jasmonate- and salicylate-mediated plant defense responses to insect herbivores, pathogens and parasitic plants. Pest Manag. Sci. 2009, 65, 497–503. [Google Scholar] [CrossRef]
- Bazzaz, F.; Grace, J. Plant Resource Allocation; Bazzaz, F.A., Grace, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-Defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef] [Green Version]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zehnder, G.; Kloepper, J.; Tuzun, S.; Yao, C.; Wei, G.; Chambliss, O.; Shelby, R. Insect feeding on cucumber mediated by rhizobacteria-induced plant resistance. Entomol. Exp. Appl. 1997, 83, 81–85. [Google Scholar] [CrossRef]
- Zehnder, G.; Kloepper, J.; Yao, C.; Wei, G. Induction of Systemic Resistance in cucumber against cucumber beetles (Coleoptera: Chrysomelidae) by Plant Growth-Promoting Rhizobacteria. J. Econ. Entomol. 1997, 90, 391–396. [Google Scholar] [CrossRef]
- Koricheva, J.; Gange, A.C.; Jones, T. Effects of mycorrhizal fungi on insect herbivores: A meta-analysis. Ecology 2009, 90, 2088–2097. [Google Scholar] [CrossRef]
- Grunseich, J.M.; Thompson, M.N.; Hay, A.A.; Gorman, Z.; Kolomiets, M.V.; Eubanks, M.D.; Helms, A.M. Risky roots and careful herbivores: Sustained herbivory by a root-feeding herbivore attenuates indirect plant defences. Funct. Ecol. 2020, 34, 1779–1789. [Google Scholar] [CrossRef]
- Marmolejo, L.O.; Thompson, M.N.; Helms, A.M. Defense suppression through Interplant communication depends on the attacking herbivore species. J. Chem. Ecol. 2021, 1, 1–13. [Google Scholar] [CrossRef]
- Grunseich, J.M.; Thompson, M.N.; Aguirre, N.M.; Helms, A.M. The role of plant-associated microbes in mediating host-plant selection by insect herbivores. Plants 2019, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Dey, A.; Kumar, V.; Batiha, G.E.S.; El-Esawi, M.A.; Tomczyk, M.; Ray, P. Fungal endophyte: An interactive endosymbiont with the capability of modulating host physiology in myriad ways. Front. Plant Sci. 2021, 12, 1780. [Google Scholar] [CrossRef] [PubMed]
- Lee Díaz, A.S.; Macheda, D.; Saha, H.; Ploll, U.; Orine, D.; Biere, A. Tackling the context-dependency of microbial-induced resistance. Agronomy 2021, 11, 1293. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Agrawal, A.A. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 1999, 14, 179–185. [Google Scholar] [CrossRef]
- Garcia, L.C.; Eubanks, M.D. Overcompensation for insect herbivory: A review and meta-analysis of the evidence. Ecology 2019, 100, e02585. [Google Scholar] [CrossRef]
- Kaplan, I.; Halitschke, R.; Kessler, A.; Rehill, B.J.; Sardanelli, S.; Denno, R.F. Physiological integration of roots and shoots in plant defense strategies links above- and belowground herbivory. Ecol. Lett. 2008, 11, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Jogesh, T.; Stanley, M.C.; Berenbaum, M.R. Evolution of tolerance in an invasive weed after reassociation with its specialist herbivore. J. Evol. Biol. 2014, 27, 2334–2346. [Google Scholar] [CrossRef]
- Ali, J.G.; Agrawal, A.A. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci. 2012, 17, 293–302. [Google Scholar] [CrossRef]
- Erb, M.; Glauser, G.; Robert, C.A.M. Induced immunity against belowground insect herbivores- activation of defenses in the absence of a jasmonate burst. J. Chem. Ecol. 2012, 38, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Cosme, M.; Lu, J.; Erb, M.; Stout, M.J.; Franken, P.; Wurst, S. A fungal endophyte helps plants to tolerate root herbivory through changes in gibberellin and jasmonate signaling. New Phytol. 2016, 211, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.A.M.; Ferrieri, R.A.; Schirmer, S.; Babst, B.A.; Schueller, M.J.; Machado, R.A.R.; Arce, C.C.M.; Hibbard, B.E.; Gershenzon, J.; Turlings, T.C.J.; et al. Induced carbon reallocation and compensatory growth as root herbivore tolerance mechanism. Plant Cell Environ. 2014, 11, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Harth, J.E.; Ferrari, M.J.; Tooker, J.F.; Stephenson, A.G. Zucchini yellow mosaic virus infection limits establishment and severity of powdery mildew in wild populations of Cucurbita pepo. Front. Plant Sci. 2018, 9, 792. [Google Scholar] [CrossRef]
- Heinrich, M.; Hettenhausen, C.; Lange, T.; Wünsche, H.; Fang, J.; Baldwin, I.T.; Wu, J. High levels of jasmonic acid antagonize the biosynthesis of gibberellins and inhibit the growth of Nicotiana attenuata stems. Plant J. 2013, 73, 591–606. [Google Scholar] [CrossRef]
- Hummel, G.M.; Schurr, U.; Baldwin, I.T.; Walter, A. Herbivore-induced jasmonic acid bursts in leaves of Nicotiana attenuata mediate short-term reductions in root growth. Plant. Cell Environ. 2009, 32, 134–143. [Google Scholar] [CrossRef]
- Mozon, G.; Pinedo, M.; Lamattina, L.; de la Canal, L. Sunflower root growth regulation: The role of jasmonic acid and its relation to auxins. Plant Growth Regul. 2011, 66, 129–136. [Google Scholar]
- Kluczek-Turpeinen, B.; Steffen, K.T.; Tuomela, M.; Hatakka, A.; Hofrichter, M. Modification of humic acids by the compost-dwelling deuteromycete Paecilomyces inflatus. Appl. Microbiol. Biotechnol. 2004, 66, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Zavarzina, A.G.; Lisov, A.A.; Zavarzin, A.A.; Leontievsky, A.A. Fungal oxidoreductases and humification in forest soils. In Soil Enzymology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 207–228. [Google Scholar]
- Frey, S.D. Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 237–259. [Google Scholar] [CrossRef]
- Mishra, S.; Kour, D.; Yadav, N.; Kumar, A. Agriculturally Important Fungi for Sustainable Agriculture; Yadav, A.N., Ed.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-45970-3. [Google Scholar]
- Rasool, S.; Vidkjær, N.H.; Hooshmand, K.; Jensen, B.; Fomsgaard, I.S.; Meyling, N.V. Seed inoculations with entomopathogenic fungi affect aphid populations coinciding with modulation of plant secondary metabolite profiles across plant families. New Phytol. 2021, 229, 1715–1727. [Google Scholar] [CrossRef]
- Löser, T.B.; Mescher, M.C.; De Moraes, C.M.; Maurhofer, M. Effects of root-colonizing fluorescent Pseudomonas strains on Arabidopsis resistance to a pathogen and an herbivore. Appl. Environ. Microbiol. 2021, 87, e0283120. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Del-Val, E.; Larsen, J. The root endophytic fungus Trichoderma atroviride induces foliar herbivory resistance in maize plants. Appl. Soil Ecol. 2018, 124, 45–53. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Engelberth, J.; Tumlinson, J.H.; Block, A.; Alborn, H.T. The use of vapor phase extraction in metabolic profiling of phytohormones and other metabolites. Plant J. 2004, 39, 790–808. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Starr, J.L.; Krumm, J.L.; Sword, G.A. The fungal endophyte Chaetomium globosum negatively affects both above- and belowground herbivores in cotton. FEMS Microbiol. Ecol. 2016, 92, fiw158. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Vega, L.J.; Grunseich, J.M.; Aguirre, N.M.; Valencia, C.U.; Sword, G.A.; Helms, A.M. A Beneficial Plant-Associated Fungus Shifts the Balance toward Plant Growth over Resistance, Increasing Cucumber Tolerance to Root Herbivory. Plants 2022, 11, 282. https://doi.org/10.3390/plants11030282
Rivera-Vega LJ, Grunseich JM, Aguirre NM, Valencia CU, Sword GA, Helms AM. A Beneficial Plant-Associated Fungus Shifts the Balance toward Plant Growth over Resistance, Increasing Cucumber Tolerance to Root Herbivory. Plants. 2022; 11(3):282. https://doi.org/10.3390/plants11030282
Chicago/Turabian StyleRivera-Vega, Loren J., John M. Grunseich, Natalie M. Aguirre, Cesar U. Valencia, Gregory A. Sword, and Anjel M. Helms. 2022. "A Beneficial Plant-Associated Fungus Shifts the Balance toward Plant Growth over Resistance, Increasing Cucumber Tolerance to Root Herbivory" Plants 11, no. 3: 282. https://doi.org/10.3390/plants11030282
APA StyleRivera-Vega, L. J., Grunseich, J. M., Aguirre, N. M., Valencia, C. U., Sword, G. A., & Helms, A. M. (2022). A Beneficial Plant-Associated Fungus Shifts the Balance toward Plant Growth over Resistance, Increasing Cucumber Tolerance to Root Herbivory. Plants, 11(3), 282. https://doi.org/10.3390/plants11030282