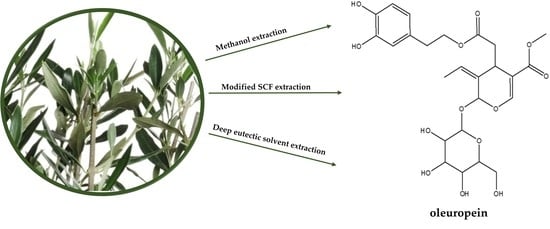
The Effect of Drying Methods and Extraction Techniques on Oleuropein Content in Olive Leaves
Abstract
:1. Introduction
2. Results
2.1. Results of Drying Olive Leaves (Air Drying at Room T, at T = 105 °C, and Freeze Drying)
2.2. Results of Ethanol-Modified Supercritical Extraction of Olive Leaves
2.2.1. DPPH Activity of SCE Extracts
2.2.2. HPLC–AD Analysis of SC Extracts
2.3. Results of Methanol Extraction of Olive Leaves
2.3.1. DPPH Activity of Methanol Extracts
2.3.2. HPLC–DAD Analysis of Methanol Extracts
2.4. Results of Ultrasound Extraction with Deep Eutectic Solvent
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Drying
4.2.2. Ethanol-Modified Supercritical Extraction
4.2.3. Methanol Extraction
4.2.4. Ultrasound Extraction with Deep Eutectic Solvent (DES)
4.2.5. DPPH Radical Scavenging Assay
4.2.6. HPLC—DAD-MS/MS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Şahin, S.; Bilgin, M. Study on Oleuropein Extraction from Olive Tree (Olea Europaea) Leaves by Means of SFE: Comparison of Water and Ethanol as Co-Solvent. Sep. Sci. Technol. 2012, 47, 2391–2398. [Google Scholar] [CrossRef]
- Tabera, J.; Guinda, Á.; Ruiz-Rodríguez, A.; Señoráns, F.J.; Ibáñez, E.; Albi, T.; Reglero, G. Countercurrent Supercritical Fluid Extraction and Fractionation of High-Added-Value Compounds from a Hexane Extract of Olive Leaves. J. Agric. Food Chem. 2004, 52, 4774–4779. [Google Scholar] [PubMed]
- Guinda, Á. Use of Solid Residue from the Olive Industry. Grasas Aceites 2006, 57, 107–115. [Google Scholar]
- Ranalli, A.; Contento, S.; Lucera, L.; Di Febo, M.; Marchegiani, D.; Di Fonzo, V. Factors Affecting the Contents of Iridoid Oleuropein in Olive Leaves (Olea Europaea L.). J. Agric. Food Chem. 2006, 54, 434–440. [Google Scholar] [CrossRef]
- Cordell, G.A. Biodiversity and Drug Discovery—A Symbiotic Relationship. Phytochemistry 2000, 55, 463–480. [Google Scholar]
- Lee, O.-H.; Lee, B.-Y. Antioxidant and Antimicrobial Activities of Individual and Combined Phenolics in Olea Europaea Leaf Extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar]
- De Leonardis, A.; Aretini, A.; Alfano, G.; Macciola, V.; Ranalli, G. Isolation of a Hydroxytyrosol-Rich Extract from Olive Leaves (Olea Europaea L.) and Evaluation of Its Antioxidant Properties and Bioactivity. Eur. Food Res. Technol. 2008, 226, 653–659. [Google Scholar]
- Eltringham, W.; Catchpole, O.J. Processing of Fish Oils by Supercritical Fluids. In Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds; CRC Press: Boca Raton, FL, USA, 2007; pp. 141–188. [Google Scholar]
- Şanal, İ.; Bayraktar, E.; Mehmetoğlu, Ü.; Çalımlı, A. Determination of Optimum Conditions for SC-(CO2+ Ethanol) Extraction of β-Carotene from Apricot Pomace Using Response Surface Methodology. J. Supercrit. Fluids 2005, 34, 331–338. [Google Scholar]
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic Compounds and Antimicrobial Activity of Olive (Olea Europaea L. Cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar]
- Sun, W.; Wang, X.; Hou, C.; Yang, L.; Li, H.; Guo, J.; Huo, C.; Wang, M.; Miao, Y.; Liu, J. Oleuropein Improves Mitochondrial Function to Attenuate Oxidative Stress by Activating the Nrf2 Pathway in the Hypothalamic Paraventricular Nucleus of Spontaneously Hypertensive Rats. Neuropharmacology 2017, 113, 556–566. [Google Scholar] [PubMed]
- Bučar-Miklavčič, M.; Butinar, B.; Valenčič, V.; Bešter, E.; Korošec, M.; Golob, T.; Možina, S.S. Extra Virgin Olive Oil and Table Olives from Slovenian Istria. In Traditional Foods; Springer: Berlin, Germany, 2016; pp. 377–385. [Google Scholar]
- Malik, N.S.; Bradford, J.M. Changes in Oleuropein Levels during Differentiation and Development of Floral Buds in ‘Arbequina’ Olives. Sci. Hortic. 2006, 110, 274–278. [Google Scholar]
- Silva, S.; Gomes, L.; Leitao, F.; Coelho, A.V.; Boas, L.V. Phenolic Compounds and Antioxidant Activity of Olea Europaea L. Fruits and Leaves. Food Sci. Technol. Int. 2006, 12, 385–395. [Google Scholar]
- Ben-Amor, I.; Gargouri, B.; Attia, H.; Tlili, K.; Kallel, I.; Musarra-Pizzo, M.; Sciortino, M.T.; Pennisi, R. In Vitro Anti-Epstein Barr Virus Activity of Olea Europaea L. Leaf Extracts. Plants 2021, 10, 2445. [Google Scholar] [CrossRef] [PubMed]
- Sarbishegi, M.; Mehraein, F.; Soleimani, M. Antioxidant Role of Oleuropein on Midbrain and Dopaminergic Neurons of Substantia Nigra in Aged Rats. Iran. Biomed. J. 2014, 18, 16. [Google Scholar] [PubMed]
- Luccarini, I.; Dami, T.E.; Grossi, C.; Rigacci, S.; Stefani, M.; Casamenti, F. Oleuropein Aglycone Counteracts Aβ42 Toxicity in the Rat Brain. Neurosci. Lett. 2014, 558, 67–72. [Google Scholar] [PubMed]
- Daccache, A.; Lion, C.; Sibille, N.; Gerard, M.; Slomianny, C.; Lippens, G.; Cotelle, P. Oleuropein and Derivatives from Olives as Tau Aggregation Inhibitors. Neurochem. Int. 2011, 58, 700–707. [Google Scholar] [PubMed]
- Menendez, J.A.; Vazquez-Martin, A.; Colomer, R.; Brunet, J.; Carrasco-Pancorbo, A.; Garcia-Villalba, R.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Olive Oil’s Bitter Principle Reverses Acquired Autoresistance to Trastuzumab (HerceptinTM) in HER2-Overexpressing Breast Cancer Cells. BMC Cancer 2007, 7, 1–19. [Google Scholar]
- Athanasiadis, V.; Grigorakis, S.; Lalas, S.; Makris, D.P. Highly Efficient Extraction of Antioxidant Polyphenols from Olea Europaea Leaves Using an Eco-Friendly Glycerol/Glycine Deep Eutectic Solvent. Waste Biomass Valorization 2018, 9, 1985–1992. [Google Scholar]
- Chen, Y.; Mu, T. Application of Deep Eutectic Solvents in Biomass Pretreatment and Conversion. Green Energy Environ. 2019, 4, 95–115. [Google Scholar]
- Alañón, M.E.; Ivanović, M.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Segura-Carretero, A. Choline Chloride Derivative-Based Deep Eutectic Liquids as Novel Green Alternative Solvents for Extraction of Phenolic Compounds from Olive Leaf. Arab. J. Chem. 2020, 13, 1685–1701. [Google Scholar] [CrossRef]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel Glycerol-Based Natural Eutectic Mixtures and Their Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Agri-Food Waste Biomass. Waste Biomass Valorization 2016, 7, 1377–1387. [Google Scholar]
- Bučar-Miklavčič, M.; Butinar, B.; Jančar, M.; Soltar, M.; Vesel, V.; Kleva, L.; Primožič, Z.; Sedmak, D. Oljka in Oljčno Olje; Kmečki glas: Ljubljana, Slovenia, 1997; ISBN 9612031290. [Google Scholar]
- Bučar-Miklavčič, M.; Bešter, E.; Butinar, B.; Čalija, D.; Podgornik, M.; Valenčič, V. Oljčno Olje, Oljke in Senzorično Ocenjevanje: Olive Oil, Olives and Organoleptic Assessment; Univerzitetna založba Annales: Koper, Slovenia, 2016; ISBN 961-6964-66-6. [Google Scholar]
- Babu, A.K.; Kumaresan, G.; Raj, V.A.A.; Velraj, R. Review of Leaf Drying: Mechanism and Influencing Parameters, Drying Methods, Nutrient Preservation, and Mathematical Models. Renew. Sustain. Energy Rev. 2018, 90, 536–556. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 10th ed.; European Directorate for the Quality of Medicines and Healthcare: Strasbourg, France, 2021; Volume 2021. [Google Scholar]
- Tanahashi, T.; Sakai, T.; Takenaka, Y.; Nagakura, N.; Chen, C.-C. Structure Elucidation of Two Secoiridoid Glucosides from Jasminum Officinale L. Var. Grandiflorum (L.) Kobuski. Chem. Pharm. Bull. 1999, 47, 1582–1586. [Google Scholar]
- Taamalli, A.; Arráez-Román, D.; Ibañez, E.; Zarrouk, M.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Optimization of Microwave-Assisted Extraction for the Characterization of Olive Leaf Phenolic Compounds by Using HPLC-ESI-TOF-MS/IT-MS2. J. Agric. Food Chem. 2012, 60, 791–798. [Google Scholar]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of Phenolic Compounds of ˝Sikitita˝ Olive Leaves by HPLC-DAD-TOF-MS. Comparison with Its Parents ‘Arbequina’ and ‘Picual’ Olive Leaves. LWT-Food Sci. Technol. 2014, 58, 28–34. [Google Scholar]
- Afaneh, I.; Yateem, H.; Al-Rimawi, F. Effect of Olive Leaves Drying on the Content of Oleuropein. Am. J. Anal. Chem. 2015, 6, 246–252. [Google Scholar]
- Hata, S. Effect of Drying Temperature on the Oleuropein Content of Olive (Olea Europea L.) Leaves. Food Preserv. Sci. 2004, 30, 191–193. [Google Scholar]
- Şahin, S.; Elhussein, E.; Bilgin, M.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S. Effect of Drying Method on Oleuropein, Total Phenolic Content, Flavonoid Content, and Antioxidant Activity of Olive (Olea Europaea) Leaf. J. Food Process. Preserv. 2018, 42, e13604. [Google Scholar]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green Extracts from Coratina Olive Cultivar Leaves: Antioxidant Characterization and Biological Activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Kontominas, M.G.; Kontogiorgis, C.; Hadjipavlou-Litina, D.; Moustakas, A.; Kiritsakis, A. Composition and Antioxidant Activity of Olive Leaf Extracts from Greek Olive Cultivars. J. Am. Oil Chem. Soc. 2010, 87, 369–376. [Google Scholar] [CrossRef]
- Bouaziz, M.; Sayadi, S. Isolation and Evaluation of Antioxidants from Leaves of a Tunisian Cultivar Olive Tree. Eur. J. Lipid Sci. Technol. 2005, 107, 497–504. [Google Scholar]
- Baldino, L.; Della Porta, G.; Osseo, L.S.; Reverchon, E.; Adami, R. Concentrated Oleuropein Powder from Olive Leaves Using Alcoholic Extraction and Supercritical CO2 Assisted Extraction. J. Supercrit. Fluids 2018, 133, 65–69. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Barrajón-Catalán, E.; Micol, V.; Mulet, A.; García-Pérez, J.V. Influence of Freezing and Dehydration of Olive Leaves (Var. Serrana) on Extract Composition and Antioxidant Potential. Food Res. Int. 2013, 50, 189–196. [Google Scholar]
- Baruca Arbeiter, A.; Jakše, J.; Bandelj, D. Paternity Analysis of the Olive Variety “Istrska Belica” and Identification of Pollen Donors by Microsatellite Markers. Sci. World J. 2014, 2014, 208590. [Google Scholar]
- Hadolin, M.; Škerget, M.; Knez, Z.; Bauman, D. High Pressure Extraction of Vitamin E-Rich Oil from Silybum Marianum. Food Chem. 2001, 74, 355–364. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
- Quirantes-Piné, R.; Lozano-Sánchez, J.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC–ESI–QTOF–MS as a Powerful Analytical Tool for Characterising Phenolic Compounds in Olive-leaf Extracts. Phytochem. Anal. 2013, 24, 213–223. [Google Scholar] [PubMed]






| Cultivar | Type of Drying | Time of Drying | Water Content [%] |
|---|---|---|---|
| Istrska belica | air–room temperature | 10 days | 4.98 ± 0.25 |
| air–dryer T = 105 °C | 90 min | 3.42 ± 0.17 | |
| freeze–dryer | until the constant weight | 6.24 ± 0.31 | |
| Leccino | air–room temperature | 10 days | 4.87 ± 0.22 |
| air–dryer T = 105 °C | 90 min | 3.39 ± 0.21 | |
| freeze dryer | until the constant weight | 6.37 ± 0.33 |
| Sample ID | Type | Extraction Time [Min] | Fraction Yield [%] | Extraction Yield [%] |
|---|---|---|---|---|
| SC *_1 | Istrska belica, air–room T | 30 | 4.597 | |
| SC_2 | 60 | 3.519 | 12.6 | |
| SC_3 | 90 | 3.380 | ||
| SC_4 | 120 | 1.065 | ||
| SC_5 | Leccino air–room T | 120 | / | 6.8 |
| SC_6 | Istrska belica air–dryer T = 105 °C | 30 | 2.964 | |
| SC_7 | 60 | 1.393 | 7.5 | |
| SC_8 | 90 | 1.572 | ||
| SC_9 | 120 | 1.004 | ||
| SC_10 | Leccino air–dryer T = 105 °C | 120 | / | 1.2 |
| SC_11 | Istrska belica freeze–dryer | 30 | / | |
| SC_12 | 60 | 6.047 | 9.7 | |
| SC_13 | 90 | 3.087 | ||
| SC_14 | 120 | 0.457 | ||
| SC_15 | Leccino freeze–dryer | 120 | / | 4.3 |
| Type | Istrska belica | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Air Drying, Room T | Air Drying, T = 105 °C | Freeze Drying | |||||||||
| Sample ID | SC_1 | SC_2 | SC_3 | SC_4 | SC_6 | SC_7 | SC_8 | SC_9 | SC_12 | SC_13 | SC_14 |
| Time SCE (min) | 30 | 60 | 90 | 120 | 30 | 60 | 90 | 120 | 60 | 90 | 120 |
| Compound | mgc/g d.w. | ||||||||||
| HO-Tyr-O-Glu 1 | n.d. 16 | 0.015 ± 0.001 | 0.092 ± 0.003 | 0.027 ± 0.001 | 0.036 ± 0.001 | 0.038 ± 0.0008 | 0.055 ± 0.0012 | 0.056 ± 0.001 | n.d. | 0.338 ± 0.007 | 0.237 ± 0.005 |
| 2-MeO-OLE a 2 | 0.396 ± 0.01 | 0.201 ± 0.006 | 0.108 ± 0.004 | 0.065 ± 0.002 | 0.096 ± 0.002 | 0.0271 ± 0.0006 | 0.0272 ± 0.0007 | 0.0181 ± 0.0004 | 0.0021 ± 0.0001 | n.d. | 0.0171 ± 0.0004 |
| 2-MeO-OLE b 3 | 0.259 ± 0.007 | 0.143 ± 0.004 | 0.054 ± 0.002 | 0.028 ± 0.001 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.162 ± 0.003 | 0.037 ± 0.001 |
| Api 4 | 0.015 ± 0.001 | 0.014 ± 0.004 | 0.016 ± 0.001 | 0.01 ± 0.0004 | 0.0192 ± 0.0004 | 0.00621 ± 0.00013 | 0.0051 ± 0.0001 | 0.0040 ± 0.0001 | 0.00201 ± 0.00004 | 0.035 ± 0.001 | 0.0152 ± 0.0003 |
| Api-7-O-Glu 5 | 0.335 ± 0.01 | 0.231 ± 0.007 | 0.208 ± 0.007 | 0.13 ± 0.005 | 0.0451 ± 0.001 | 0.0473 ± 0.0009 | 0.0581 ± 0.0012 | 0.054 ± 0.001 | n.d | 0.492 ± 0.010 | 0.300 ± 0.006 |
| Glu-O-OLE-O-Glu 6 | 0.176 ± 0.005 | 0.094 ± 0.003 | 0.060 ± 0.002 | 0.036 ± 0.001 | 0.0261 ± 0.0006 | 0.0181 ± 0.0004 | 0.0191 ± 0.0004 | 0.0163 ± 0.0003 | n.d. | 0.119 ± 0.002 | 0.058 ± 0.001 |
| HO-Ole 7 | n.d. | 0.014 ± 0.001 | 0.011 ± 0.001 | 0.032 ± 0.001 | n.d | 0.0141 ± 0.0003 | 0.0171 ± 0.0004 | 0.0161 ± 0.0003 | 0.012 ± 0.0003 | 0.148 ± 0.003 | 0.109 ± 0.002 |
| Lig 8 | 0.171 ± 0.005 | 0.142 ± 0.005 | 0.15 ± 0.005 | 0.091 ± 0.003 | 0.0421 ± 0.001 | 0.042 ± 0.001 | 0.044 ± 0.001 | 0.0331 ± 0.0007 | 0.0011 ± 0.0002 | 0.226 ± 0.005 | 0.129 ± 0.003 |
| Lu 9 | 0.021 ± 0.001 | 0.016 ± 0.003 | 0.012 ± 0.001 | 0.018 ± 0.001 | 0.0251 ± 0.001 | 0.0084 ± 0.0002 | 0.0081 ± 0.0002 | 0.0062 ± 0.0001 | 0.0010 ± 0.0002 | 0.041 ± 0.001 | 0.0190 ± 0.0004 |
| Lu-7-O-Glu 10 | 0.067 ± 0.002 | 0.087 ± 0.002 | 0.199 ± 0.239 | 0.127 ± 0.005 | 0.0261 ± 0.048 | 0.043 ± 0.001 | 0.064 ± 0.002 | 0.079 ± 0.002 | n.d. | 1.092 ± 0.023 | 0.712 ± 0.015 |
| Ole 11 | 2.229 ± 0.06 | 3.513 ± 0.11 | 6.834 ± 0.022 | 4.288 ± 0.15 | 1.9281 ± 0.005 | 1.969 ± 0.041 | 2.303 ± 0.048 | 2 ± 0.042 | 0.0041 ± 0.0001 | 13.603 ± 0.286 | 8.357 ± 0.18 |
| Ols 12 | 0.324 ± 0.009 | 0.393 ± 0.012 | 0.627 ± 0.001 | 0.392 ± 0.014 | 0.20211 ± 0.0003 | 0.164 ± 0.003 | 0.181 ± 0.004 | 0.145 ± 0.003 | 0.0031 ± 0.0001 | 1.059 ± 0.022 | 0.625 ± 0.013 |
| Sec 13 | n.d. | 0 | 0.037 ± 0.017 | 0.017 ± 0.001 | 0.0121 ± 0.002 | 0.0092 ± 0.0002 | 0.0141 ± 0.0003 | 0.0150 ± 0.0003 | n.d. | 0.090 ± 0.002 | 0.065 ± 0.001 |
| TyrOH 14 | 0.23 ± 0.006 | 0.372 ± 0.011 | 0.488 ± 0.005 | 0.305 ± 0.011 | 0.0871 ± 0.002 | 0.0272 ± 0.0006 | 0.0202 ± 0.0004 | 0.012 ± 0.0002 | n.d. | 0.078 ± 0.002 | 0.034 ± 0.001 |
| Ver 15 | 0.029 ± 0.001 | 0.172 ± 0.005 | 0.146 ± 0.239 | 0.08 ± 0.003 | 0.0621 ± 0.05 | 0.0234 ± 0.0005 | 0.0122 ± 0.0003 | 0.0091 ± 0.0002 | n.d. | 0.176 ± 0.004 | 0.107 ± 0.002 |
| Type | Leccino | ||
|---|---|---|---|
| Air Drying, Room T | Air Drying, T = 105 °C | Freeze Drying | |
| Sample ID | SC_5 | SC_10 | SC_15 |
| Time SCE (min) | 120 | 120 | 120 |
| Compound | mgc/g d.w. | ||
| HO-Tyr-O-Glu 1 | 0.119 ± 0.002 | 0.032 ± 0.001 | 0.079 ± 0.002 |
| 2-MeO-OLE a 2 | n.d. 16 | n.d. | 0.065 |
| 2-MeO-OLE b 3 | n.d. | 0.0241 ± 0.0005 | n.d. |
| Ap 4 | 0.046 ± 0.001 | 0.0050 ± 0.0001 | 1.330 ± 0.028 |
| Api-7-O-Glu 5 | 0.100 ± 0.002 | 0.0260 ± 0.0005 | 0.860 ±0.0005 |
| Glu-O-OLE-O-Glu 6 | 0.070 ± 0.001 | 0.013 ± 0.0003 | 0.271 ± 0.0057 |
| HO-Ole 7 | n.d. | n.d. | 0.266 ± 0.0060 |
| Lig 8 | 0.080 ± 0.002 | 0.018 ± 0.0004 | 0.078 ± 0.0004 |
| Lu 9 | 0.026 ± 0.001 | 0.0101 ±0.0002 | 0.439 ± 0.0002 |
| Lu-7-O-Glu 10 | 0.058 ± 0.012 | 0.0111 ± 0.0002 | 1.418 ± 0.0002 |
| Ole 11 | 3.522 ± 0.074 | 0.707 ± 0.015 | 1.886 ± 0.039 |
| Ols 12 | 0.517 ± 0.011 | 0.093 ± 0.002 | 0.494 ± 0.01 |
| Sec 13 | 0.066 ± 0.001 | 0.009 ± 0.0002 | n.d. |
| TyrOH 14 | 0.814 ± 0.017 | 0.025 ± 0.0005 | 0.098 ± 0.002 |
| Ver 15 | 0.155 ±0.003 | 0.021 ± 0.0004 | 0.117 ± 0.002 |
| Olive Cultivar | Location | Harvesting Month | Sampling Air Temperature | Age of Trees | Drying Method |
|---|---|---|---|---|---|
| Istrska belica | 45°31′22.8″ N Izola, Slovenia | February | 11 °C | 6 years | air at room temperature dryer at T = 105 °C lyophilization |
| Leccino | 13°39′47.2″ E Izola, Slovenia |
| Active Compound | Confirmation |
|---|---|
| Oleuropein (Ole) | [M-H−] 539, MS/MS (539/275) |
| Verbascoside (Ver) | [M-H−] 623, MS/MS (623/161) |
| Oleuroside (Ols) | [M-H−] 539, MS/MS (539/275) |
| Ligstroside (Lig) | [M-H−] 523 |
| Hydroxy oleuropein (HO-Ole) | [M-H−] 555 |
| Hydroxytyrosol glucoside [HO-Tyr-O-Glu] | [M-H−] 315 |
| Hydroxytyrosol (TyrOH) | [M-H−] 153, MS/MS (153/123) |
| Luteolin-7-Glucoside | [M-H−] 447, MS/MS (447/285) |
| Apigenin-7-Glucoside (Api-7-O-Glu) | [M-H−] 431, MS/MS (431/268) |
| Secologanoside (Sec) | [M-H−] 389 |
| Luteolin (Lu) | [M-H−] 285, MS/MS (285/133) |
| Apigenin (Api) | [M-H−] 269 (269/151) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cör Andrejč, D.; Butinar, B.; Knez, Ž.; Tomažič, K.; Knez Marevci, M. The Effect of Drying Methods and Extraction Techniques on Oleuropein Content in Olive Leaves. Plants 2022, 11, 865. https://doi.org/10.3390/plants11070865
Cör Andrejč D, Butinar B, Knez Ž, Tomažič K, Knez Marevci M. The Effect of Drying Methods and Extraction Techniques on Oleuropein Content in Olive Leaves. Plants. 2022; 11(7):865. https://doi.org/10.3390/plants11070865
Chicago/Turabian StyleCör Andrejč, Darija, Bojan Butinar, Željko Knez, Kaja Tomažič, and Maša Knez Marevci. 2022. "The Effect of Drying Methods and Extraction Techniques on Oleuropein Content in Olive Leaves" Plants 11, no. 7: 865. https://doi.org/10.3390/plants11070865
APA StyleCör Andrejč, D., Butinar, B., Knez, Ž., Tomažič, K., & Knez Marevci, M. (2022). The Effect of Drying Methods and Extraction Techniques on Oleuropein Content in Olive Leaves. Plants, 11(7), 865. https://doi.org/10.3390/plants11070865