Essential Oil Biodiversity of Achillea ligustica All. Obtained from Mainland and Island Populations
Abstract
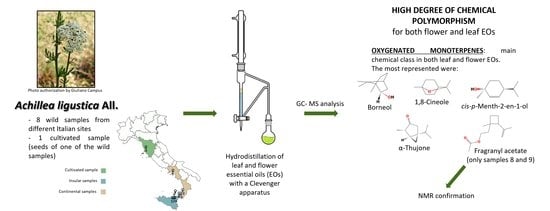
:1. Introduction
2. Results
2.1. Essential Oil (EO) Compositions
2.2. Statistical Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Plant Cultivation
4.3. Essential Oil Hydrodistillation
4.4. Gas Chromatography with Electron Impact Mass Spectrometry (GC-EIMS)
4.5. Compound Isolation
4.6. Nuclear Magnetic Resonance Spectroscopy (NMR) of Fragranyl Acetate
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asres, K.; Tei, A.; Moges, G.; Sporer, F.; Wink, M. Terpenoid composition of the wound-induced bark exudate of Commiphora tenuis from Ethiopia. Planta Med. 1998, 64, 473–475. [Google Scholar] [CrossRef] [PubMed]
- İşcan, G. Antibacterial and Anticandidal Activities of Common Essential Oil Constituents. Rec. Nat. Prod. 2017, 11, 374–388. [Google Scholar]
- Buckle, J. Chapter 4-Essential Oil Toxicity and Contraindications. In Clinical Aromatherapy, 3rd ed.; Buckle, J., Ed.; Churchill Livingstone: St. Louis, MI, USA, 2015; pp. 73–94. [Google Scholar]
- Edris, A.E. Pharmaceutical and therapeutic Potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- El-Said, H.; Ashgar, S.S.; Bader, A.; AlQathama, A.; Halwani, M.; Ascrizzi, R.; Flamini, G. Essential oil analysis and antimicrobial evaluation of three aromatic plant species growing in Saudi Arabia. Molecules 2021, 26, 959. [Google Scholar] [CrossRef]
- Maggi, F.; Giuliani, C.; Fico, G.; Ricciutelli, M.; Bramucci, M.; Quassinti, L.; Petrelli, D.; Vitali, L.A.; Cianfaglione, K.; Tirillini, B. Secondary metabolites, secretory structures and biological activity of water celery (Apium nodiflorum (L.) Lag.) growing in central Italy. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2019, 153, 325–335. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Giordani, C.; Casettari, L.; Curzi, G.; Cappellacci, L.; Petrelli, R.; Maggi, F. Acute and sub-lethal toxicity of eight essential oils of commercial interest against the filariasis mosquito Culex quinquefasciatus and the housefly Musca domestica. Ind. Crops Prod. 2018, 112, 668–680. [Google Scholar] [CrossRef]
- Abdalla, A.N.; Shaheen, U.; Abdallah, Q.; Flamini, G.; Bkhaitan, M.M.; Abdelhady, M.I.; Ascrizzi, R.; Bader, A. Proapoptotic activity of Achillea membranacea essential oil and its major constituent 1, 8-cineole against A2780 ovarian cancer cells. Molecules 2020, 25, 1582. [Google Scholar] [CrossRef] [Green Version]
- Maggi, F.; Bramucci, M.; Cecchini, C.; Coman, M.M.; Cresci, A.; Cristalli, G.; Lupidi, G.; Papa, F.; Quassinti, L.; Sagratini, G.; et al. Composition and biological activity of essential oil of Achillea ligustica All. (Asteraceae) naturalized in central Italy: Ideal candidate for anti-cariogenic formulations. Fitoterapia 2009, 80, 313–319. [Google Scholar] [CrossRef]
- Conforti, F.; Loizzo, M.R.; Statti, G.A.; Menichini, F. Comparative Radical Scavenging and Antidiabetic Activities of Methanolic Extract and Fractions from Achillea ligustica. Biol. Pharm. Bull. 2005, 28, 1791–1794. [Google Scholar] [CrossRef] [Green Version]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982. [Google Scholar]
- Tuttolomondo, T.; Licata, M.; Leto, C.; Bonsangue, G.; Gargano, M.L.; Venturella, G.; La Bella, S. Popular uses of wild plant species for medicinal purposes in the Nebrodi Regional Park (North-Eastern Sicily, Italy). J. Ethnopharmacol. 2014, 157, 21–37. [Google Scholar] [CrossRef]
- Bruni, A.; Ballero, M.; Poli, F. Quantitative ethnopharmacological study of the Campidano Valley and Urzulei district, Sardinia, Italy. J. Ethnopharmacol. 1997, 57, 97–124. [Google Scholar] [CrossRef]
- Filippi, J.-J.; Lanfranchi, D.-A.; Prado, S.; Baldovini, N.; Meierhenrich, U.J. Composition, Enantiomeric Distribution, and Antibacterial Activity of the Essential Oil of Achillea ligustica All. from Corsica. J. Agric. Food Chem. 2006, 54, 6308–6313. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Guarcini, L.; Bianco, A.; Rosselli, S.; Bruno, M.; Senatore, F. Phytochemical analysis of Achillea ligustica All. from Lipari Island (Aeolian Islands). Nat. Prod. Res. 2016, 30, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Tzakou, O.; Loukis, A.; Verykokidou, E.; Roussis, V. Chemical Constituents of the Essential Oil of Achillea ligustica All. from Greece. J. Essent. Oil Res. 1995, 7, 549–550. [Google Scholar] [CrossRef]
- Maffei, M.; Germano, F.; Doglia, G.; Chialva, F. Essential Oils, Chromosome Numbers and Karyotypes from Italian Achillea Species. Part II. J. Essent. Oil Res. 1993, 5, 61–70. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Kowalczyk, A.; Coroneo, V.; Russo, M.T.; Dessì, S.; Cabras, P. Chemical Composition and Antioxidant, Antimicrobial, and Antifungal Activities of the Essential Oil of Achillea ligustica All. J. Agric. Food Chem. 2005, 53, 10148–10153. [Google Scholar] [CrossRef]
- Bader, A.; Panizzi, L.; Cioni, P.; Flamini, G. Achillea ligustica: Composition and antimicrobial activity of essential oils from the leaves, flowers and some pure constituents. Cent. Eur. J. Biol. 2007, 2, 206–212. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Montoro, P.; Piacente, S.; Corona, G.; Deiana, M.; Dessì, M.A.; Pizza, C.; Cabras, P. Flavonoid characterization and antioxidant activity of hydroalcoholic extracts from Achillea ligustica All. J. Pharm. Biomed. Anal. 2009, 50, 440–448. [Google Scholar] [CrossRef]
- Greger, H.; Zdero, C.; Bohlmann, F. Pyrrolidine and piperidine amides from Achillea. Phytochemistry 1984, 23, 1503–1505. [Google Scholar] [CrossRef]
- Bruno, M.; Herz, W. Guaianolides and other constituents of Achillea ligustica. Phytochemistry 1988, 27, 1871–1872. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Gáti, T.; Hussein, T.A.; Ali, A.T.; Tzakou, O.A.; Couladis, M.A.; Mabry, T.J.; Tóth, G. Ligustolide A and B, two novel sesquiterpenes with rare skeletons and three 1,10-seco-guaianolide derivatives from Achillea ligustica. Tetrahedron 2003, 59, 3729–3735. [Google Scholar] [CrossRef]
- Muselli, A.; Pau, M.; Desjobert, J.-M.; Foddai, M.; Usai, M.; Costa, J. Volatile Constituents of Achillea ligustica All. by HS-SPME/GC/GC-MS. Comparison with Essential Oils Obtained by Hydrodistillation from Corsica and Sardinia. Chromatographia 2009, 69, 575–585. [Google Scholar] [CrossRef]
- Giuliani, C.; Ascrizzi, R.; Lupi, D.; Tassera, G.; Santagostini, L.; Giovanetti, M.; Flamini, G.; Fico, G. Salvia verticillata: Linking glandular trichomes, volatiles and pollinators. Phytochemistry 2018, 155, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F.; Zdero, C.; Faass, U. Naturally occurring terpene derivatives. XXVI. Constituents of Artemisia fragrans. Chem. Ber. 1973, 106, 2904–2909. [Google Scholar] [CrossRef]
- Bernard, A.M.; Frongia, A.; Secci, F.; Delogu, G.; Ollivier, J.; Piras, P.P.; Salaün, J. Stereospecific palladium(0)-catalyzed reduction of 2-cyclobutylidenepropyl esters. A versatile preparation of diastereomeric monoterpenoids: (±)-fragranol and (±)-grandisol. Tetrahedron 2003, 59, 9433–9440. [Google Scholar] [CrossRef]
- Graham, T.J.A.; Gray, E.E.; Burgess, J.M.; Goess, B.C. An efficient synthesis of (+/-)-grandisol featuring 1,5-enyne metathesis. J. Org. Chem. 2010, 75, 226–228. [Google Scholar] [CrossRef] [Green Version]
- Martín, T.; Rodríguez, C.M.; Martín, V.S. A new approach to functionalized cyclobutanes: Stereoselective synthesis of the enantiomers of grandisol and fraganol. Tetrahedron Asymmetry 1995, 6, 1151–1164. [Google Scholar] [CrossRef]
- Stevenson, P.C. For antagonists and mutualists: The paradox of insect toxic secondary metabolites in nectar and pollen. Phytochem. Rev. 2020, 19, 603–614. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, C.; Bottoni, M.; Ascrizzi, R.; Santagostini, L.; Papini, A.; Flamini, G.; Fico, G. A novel study approach on Scutellaria altissima L. cultivated at the Ghirardi Botanic Garden (Lombardy, Italy). Plant Biol. 2020, 22, 1013–1021. [Google Scholar] [CrossRef]
- Giamperi, L.; Bucchini, A.; Ricci, D.; Papa, F.; Maggi, F. Essential Oil of Achillea ligustica (Asteraceae) as an antifungal agent against phytopathogenic fungi. Nat. Prod. Commun. 2018, 13, 1171–1174. [Google Scholar] [CrossRef] [Green Version]
- Grandi, R.; Messerotti, W.; Pagnoni, U.M. Monoterpene variation in two Achillea ageratum chemotypes. Phytochemistry 1976, 15, 1770–1771. [Google Scholar] [CrossRef]
- Saeidi, K.; Moosavi, M.; Lorigooini, Z.; Maggi, F. Chemical characterization of the essential oil compositions and antioxi-dant activity from Iranian populations of Achillea wilhelmsii K.Koch. Ind. Crops Prod. 2018, 112, 274–280. [Google Scholar] [CrossRef]
- Gudaitytė, O.; Venskutonis, P.R. Chemotypes of Achillea millefolium transferred from 14 different locations in Lithuania to the controlled environment. Biochem. Syst. Ecol. 2007, 35, 582–592. [Google Scholar] [CrossRef]
- Gudaitytė, O.; Venskutonis, P.; Maždžierienė, R. Distribution and chemical polymorphism of the essential oils of Achillea cartilaginea growing wild in Lithuania. Nat. Prod. Res. 2012, 26, 722–730. [Google Scholar] [CrossRef]
- Mirahmadi, S.F.; Hassandokht, M.R.; Sefidkon, F.; Hassani, M.E. Variability of the essential oil content and composition among the wild populations of Achillea biebersteinii Afan. from Iran: Occurrence of new nepetalactones chemotypes. J. Essent. Oil Res. 2012, 24, 523–531. [Google Scholar] [CrossRef]
- Németh, É.; Bernáth, J.; Héthelyi, É. Chemotypes and their stability in Achillea crithmifolia W. et K. populations. J. Essent. Oil Res. 2000, 12, 53–58. [Google Scholar] [CrossRef]
- Vogel, H.; Razmilic, I.; Muñoz, M.; Doll, U.; Martin, J.S. Studies of Genetic Variation of Essential Oil and Alkaloid Content in Boldo (Peumus boldus). Planta Med. 1999, 65, 90–91. [Google Scholar] [CrossRef]
- Orav, A.; Arak, E.; Raal, A. Phytochemical analysis of the essential oil of Achillea millefolium L. from various European Countries. Nat. Prod. Res. 2006, 20, 1082–1088. [Google Scholar] [CrossRef]
- Afsharypuor, S.; Asgary, S.; Lockwood, G. Constituents of the essential oil of Achillea wilhelmsii from Iran. Planta Med. 1996, 62, 77–78. [Google Scholar] [CrossRef]
- Çakır, A.; Özer, H.; Aydın, T.; Kordali, Ş.; Çavuşoglu, A.T.; Akçin, T.; Mete, E.; Akçin, A. Phytotoxic and Insecticidal Properties of Essential Oils and Extracts of Four Achillea Species. Rec. Nat. Prod. 2016, 10, 154–167. [Google Scholar]
- Nenaah, G.E. Chemical composition, insecticidal and repellence activities of essential oils of three Achillea species against the Khapra beetle (Coleoptera: Dermestidae). J. Pest Sci. 2014, 87, 273–283. [Google Scholar] [CrossRef]
- Radulović, N.S.; Dekić, M.S.; Ranđelović, P.J.; Stojanović, N.M.; Zarubica, A.R.; Stojanović-Radić, Z.Z. Toxic essential oils: Anxiolytic, antinociceptive and antimicrobial properties of the yarrow Achillea umbellata Sibth. et Sm.(Asteraceae) volatiles. Food Chem. Toxicol. 2012, 50, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.; Flamini, G.; Cioni, P.L.; Morelli, I. The composition of the root oil of Salvadora persica L. J. Essent. Oil Res. 2002, 14, 128–129. [Google Scholar] [CrossRef]
- Bader, A.; Caponi, C.; Cioni, P.L.; Flamini, G.; Morelli, I. Acorenone in the essential oil of flowering aerial parts of Seseli tortuosum L. Flavour Fragr. J. 2003, 18, 57–58. [Google Scholar] [CrossRef]
- NIST; Wiley Technology. NIST/EPA/NIH Mass Spectral Library 2014, 1st ed.; Wiley: Hoboken, NJ, USA, 2014.
- Adams, R. Identification of Essential Oil Components by Gas Chromatography–Mass Spectroscopy; Allured Publishing Corp.: Carol Stream, IL, USA, 1995. [Google Scholar]




| Compound | l.r.i a | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| (E)-2-Hexenal | 845 | - | - | - | - | - | - | - | - | 0.1 |
| 1-Nonene | 892 | - | - | - | tr | - | - | - | - | 0.1 |
| Santolina triene | 910 | - | - | - | - | - | - | - | - | 0.3 |
| α-Thujene | 933 | 0.3 | 0.2 | - | 0.1 | 0.2 | - | 0.2 | 0.1 | 0.2 |
| α-Pinene | 940 | 2.5 | 0.9 | 0.8 | 0.6 | 2.7 | 0.2 | 1.2 | 0.2 | 0.9 |
| Camphene | 955 | 3.7 | 0.1 | 0.5 | 0.2 | 0.6 | - | 0.7 | - | 0.4 |
| Benzaldehyde | 963 | - | - | - | - | - | - | - | - | 0.1 |
| Sabinene | 978 | 3.4 | 5.7 | 0.9 | 1.8 | 4.6 | 1.3 | 3.4 | 0.2 | 0.7 |
| β-Pinene | 981 | 12.0 | 8.4 | 6.4 | 1.9 | 17.1 | 0.8 | 1.9 | - | 2.3 |
| 2,3-Dehydro-1,8-cineole | 993 | 0.2 | 0.2 | 0.2 | 0.2 | 0.4 | 0.1 | - | 0.2 | 0.3 |
| Yomogi alcohol | 998 | - | - | - | - | - | - | - | 0.2 | 0.3 |
| α-Phellandrene | 1007 | - | - | - | 0.3 | - | - | - | - | - |
| α-Terpinene | 1020 | 0.4 | 0.4 | 0.2 | 0.7 | 0.3 | 0.2 | 0.7 | 0.5 | 1.5 |
| p-Cymene | 1028 | 0.9 | 1.3 | 0.5 | 0.7 | 1.7 | 0.5 | 1.1 | 0.7 | 2.1 |
| Limonene | 1033 | 0.6 | 0.4 | 0.2 | 0.3 | 0.7 | - | 0.3 | - | 0.2 |
| Santolina alcohol | 1037 | - | - | - | - | - | 8.3 | - | - | - |
| 1,8-Cineole | 1039 | 4.6 | 11.8 | 10.4 | 8.7 | 34.8 | 8.5 | 5.7 | 0.1 | 1.6 |
| γ-Terpinene | 1064 | 0.8 | 1.1 | 0.5 | 1.1 | 0.9 | 0.5 | 1.3 | 0.9 | 2.7 |
| cis-Sabinene hydrate | 1070 | 4.1 | 5.8 | 10.0 | 9.7 | 2.1 | 3.6 | 5.6 | 2.5 | 11.1 |
| Terpinolene | 1089 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.4 | 0.2 | 0.6 |
| Linalool | 1101 | 0.1 | 0.2 | 0.4 | tr | tr | 0.7 | - | tr | - |
| trans-Sabinene hydrate | 1102 | 1.1 | 3.0 | 2.2 | 2.1 | 0.8 | 1.3 | 1.6 | 0.8 | 3.0 |
| α-Thujone | 1105 | - | - | - | - | - | - | 21.0 | - | - |
| β-Thujone | 1116 | - | - | - | - | - | - | 3.0 | - | - |
| cis-p-Menth-2-en-1-ol | 1123 | 0.2 | 0.2 | 2.9 | 16.7 | tr | 0.1 | 0.4 | 0.2 | 1.1 |
| trans-Pinocarveol | 1141 | - | 3.6 | - | - | - | 0.3 | - | - | 0.3 |
| trans-p-Menth-2-en-1-ol | 1147 | 0.4 | - | 1.7 | 9.6 | - | - | 0.4 | 0.1 | 0.4 |
| Camphor | 1148 | 0.2 | 0.7 | 1.4 | 1.1 | 0.2 | 9.4 | 9.3 | - | 2.8 |
| Pinocarvone | 1165 | 1.2 | 1.7 | 4.9 | 0.1 | 0.2 | 0.5 | 0.5 | - | 0.5 |
| δ-Terpineol | 1166 | - | - | - | 0.1 | - | - | - | - | - |
| Borneol | 1167 | 10.2 | 2.0 | 7.7 | 0.2 | 2.2 | 0.3 | 12.1 | 0.1 | 0.2 |
| Isopinocamphone | 1175 | - | - | - | - | - | - | - | - | 0.1 |
| 4-Terpineol | 1182 | 1.4 | 3.1 | 1.8 | 3.0 | 0.8 | 0.8 | 3.2 | 2.1 | 7.5 |
| Dill ether | 1186 | - | - | - | 0.2 | - | - | - | - | - |
| α-Terpineol | 1192 | 3.2 | 6.2 | 5.5 | 5.3 | 4.6 | 2.3 | 1.6 | 0.1 | 1.0 |
| trans-Piperitol | 1207 | 0.2 | - | 1.0 | 6.3 | - | - | 0.3 | 0.1 | 0.2 |
| Fragranol | 1217 | - | - | - | - | - | - | - | 8.5 | 3.6 |
| trans-Carveol | 1219 | - | tr | - | - | - | 0.1 | 0.5 | - | - |
| Piperitone | 1254 | 0.8 | - | - | 0.4 | - | - | 1.0 | - | - |
| cis-Chrysanthenyl acetate | 1265 | - | - | 0.9 | - | - | - | - | - | - |
| Isobornyl acetate | 1285 | 9.4 | 1.2 | 5.2 | 0.7 | tr | - | 0.3 | - | - |
| Lavandulyl acetate | 1389 | - | - | - | - | - | - | - | - | 0.5 |
| Thymol | 1292 | 0.3 | 0.2 | 0.2 | 0.1 | 0.4 | 0.2 | 0.2 | 0.3 | 0.3 |
| trans-Pinocarvyl acetate | 1297 | - | 1.0 | 0.2 | - | - | - | 0.6 | - | - |
| Carvacrol | 1300 | - | - | - | - | - | - | - | 0.4 | - |
| iso-Dihydrocarveol acetate | 1297 | - | - | - | - | - | - | - | 0.7 | - |
| trans-Carvyl acetate | 1337 | - | - | 0.4 | - | - | - | - | - | - |
| Fragranyl acetate | 1346 | - | - | - | - | - | - | - | 54.3 | 24.0 |
| α-Longipinene | 1351 | 0.1 | - | - | - | - | - | - | - | - |
| Eugenol | 1357 | tr | tr | - | - | - | - | - | - | 0.1 |
| cis-Carvyl acetate | 1362 | - | - | 0.5 | - | - | - | - | - | - |
| Neryl acetate | 1365 | - | - | - | - | - | - | - | 0.1 | - |
| Cyclosativene | 1370 | 0.3 | tr | - | - | 0.1 | 0.1 | - | 0.2 | 0.1 |
| α-Copaene | 1377 | 0.2 | 0.2 | 0.2 | - | 0.3 | 0.3 | - | 0.3 | 0.3 |
| trans-Myrtanol acetate | 1381 | - | - | 0.2 | - | - | - | - | - | - |
| Geranyl acetate | 1383 | - | - | 3.3 | - | - | - | - | - | - |
| β-Bourbonene | 1384 | - | - | - | - | - | 0.1 | - | - | - |
| β-Cubebene | 1390 | - | - | - | 0.1 | - | - | - | - | - |
| β-Elemene | 1391 | - | - | - | - | - | - | - | 0.1 | - |
| Methyl eugenol | 1402 | - | - | 0.2 | - | - | - | - | - | - |
| α-Gurjunene | 1409 | - | - | - | - | tr | - | - | - | - |
| β-Caryophyllene | 1420 | 1.6 | 3.7 | 0.2 | 0.1 | 0.4 | 0.4 | 1.1 | 0.7 | 1.1 |
| α-Himachalene | 1449 | 0.4 | - | 0.2 | - | - | - | - | - | - |
| α-Humulene | 1456 | 0.1 | 0.3 | - | - | tr | - | - | 0.1 | - |
| Alloaromadendrene | 1461 | 0.3 | 0.4 | 0.3 | 0.1 | 0.8 | 0.7 | 0.3 | 0.2 | 0.2 |
| β-Chamigrene | 1475 | 0.2 | - | - | - | - | - | - | - | - |
| γ-Muurolene | 1475 | - | 0.2 | - | 0.1 | - | - | - | 0.1 | - |
| Germacrene D | 1482 | 1.3 | 0.9 | 0.2 | 1.8 | 1.0 | 9.5 | 1.6 | 5.7 | 1.5 |
| β-Selinene | 1493 | 1.0 | - | - | - | - | - | 0.5 | - | - |
| Bicyclogermacrene | 1496 | 0.2 | 0.1 | 0.2 | 0.2 | - | 0.8 | 0.2 | 0.7 | 0.2 |
| α-Muurolene | 1499 | - | - | - | - | - | 0.1 | - | - | - |
| β-Himachalene | 1499 | 0.2 | - | - | - | - | - | - | - | - |
| (E,E)-α-Farnesene | 1508 | - | 0.1 | - | 0.3 | - | 0.1 | - | 0.1 | - |
| cis-γ-Cadinene | 1511 | 0.2 | 0.1 | - | - | - | - | - | 0.1 | 0.1 |
| δ-Cadinene | 1525 | 0.7 | 0.3 | - | 0.1 | tr | 0.2 | 0.2 | 0.1 | - |
| 8,14-Cedranoxide | 1542 | - | - | - | - | - | - | - | 0.5 | - |
| α-Elemol | 1549 | - | - | 1.1 | - | - | - | - | - | 0.4 |
| trans-Nerolidol | 1564 | 0.5 | 6.4 | 0.4 | - | - | - | - | 0.5 | 3.0 |
| Spathulenol | 1578 | 0.4 | 0.4 | 0.2 | 0.1 | 0.1 | 0.4 | 0.3 | 0.4 | 1.0 |
| Caryophyllene oxide | 1583 | 2.2 | 2.4 | 0.8 | - | - | 0.1 | 1.5 | 1.0 | 1.4 |
| Viridiflorol | 1592 | 3.3 | 6.3 | 7.0 | 2.4 | 10.5 | 9.1 | 5.3 | 3.7 | 3.8 |
| Guaiol | 1597 | - | - | - | - | - | - | - | 1.1 | - |
| Cedrol | 1598 | - | - | - | 0.3 | - | - | - | - | - |
| 1-epi-Cubenol | 1629 | - | - | - | 1.5 | - | - | - | - | - |
| γ-Eudesmol | 1632 | 3.5 | - | 1.6 | - | 0.1 | 1.6 | - | - | - |
| Caryophylla-4(14),8(15)-dien-5-α-ol | 1641 | - | - | - | - | - | - | - | 0.5 | - |
| T-Cadinol | 1642 | 1.2 | 0.5 | 0.7 | - | - | - | - | - | - |
| T-Muurolol | 1643 | - | - | - | 0.3 | - | - | - | - | - |
| β-Eudesmol | 1651 | 3.5 | 2.8 | - | - | 2.4 | 30.8 | - | - | 0.9 |
| α-Eudesmol | 1654 | - | - | 0.2 | - | - | - | - | - | 0.8 |
| Kongol | 1655 | - | 5.4 | - | 1.7 | - | - | 5.6 | - | - |
| α-Santalol | 1680 | - | - | - | 0.3 | - | - | - | - | - |
| α-Bisabolol | 1685 | - | 1.6 | - | - | 2.8 | - | - | - | - |
| Chamazulene | 1727 | 0.4 | 0.1 | 0.3 | 0.1 | - | - | - | 0.1 | - |
| Monoterpene hydrocarbons | 24.8 | 18.7 | 10.2 | 7.9 | 28.9 | 3.6 | 11.2 | 2.8 | 11.9 | |
| Oxygenated monoterpenes | 37.6 | 40.9 | 61.0 | 64.5 | 46.5 | 37.5 | 67.3 | 70.6 | 58.5 | |
| Sesquiterpene hydrocarbons | 6.8 | 7.2 | 1.3 | 2.8 | 2.6 | 12.3 | 4.0 | 8.4 | 3.4 | |
| Oxygenated sesquiterpenes | 14.6 | 25.8 | 12.0 | 6.6 | 15.9 | 42.0 | 12.7 | 7.9 | 11.3 | |
| Phenylpropanoids | tr | tr | 0.2 | - | - | - | - | - | 0.1 | |
| Nor-terpenes | 0.4 | 0.1 | 0.3 | 0.1 | - | - | - | 0.1 | - | |
| Non-terpene derivatives | - | - | - | 0.1 | - | - | - | - | 0.3 | |
| Total identified | 84.25% | 92.7% | 85% | 81.9% | 93.9% | 95.4% | 95.2% | 89.9% | 80.5% |
| Compound | l.r.i a | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Tricyclene | 928 | 0.4 | - | - | - | - | - | - | - | - |
| α-Thujene | 933 | - | 0.1 | - | 0.1 | - | 0.1 | 0.2 | - | - |
| α-Pinene | 940 | 2.6 | 0.3 | 0.9 | 0.6 | 0.1 | 0.4 | 1.2 | 0.1 | - |
| Camphene | 955 | 4.9 | 0.1 | 0.5 | 0.4 | 0.2 | 0.1 | 0.7 | - | - |
| Benzaldehyde | 963 | 0.1 | - | 0.2 | - | 0.1 | 0.1 | 0.1 | 0.2 | tr |
| Sabinene | 978 | 4.0 | 2.4 | 0.8 | 1.2 | 0.2 | 0.4 | 6.0 | 0.1 | - |
| β-Pinene | 981 | 6.7 | 2.7 | 5.8 | 2.5 | 0.9 | 0.9 | 1.6 | tr | tr |
| Myrcene | 992 | 0.1 | - | - | - | - | - | 0.2 | - | - |
| 2,3-Dehydro-1,8-cineole | 993 | - | - | 0.4 | - | - | 0.3 | 0.1 | - | - |
| α-Phellandrene | 1007 | - | - | - | 0.5 | - | - | - | - | - |
| α-Terpinene | 1020 | 0.4 | 0.3 | 0.1 | 0.9 | 0.1 | 0.3 | 0.3 | 0.1 | - |
| p-Cymene | 1028 | 0.9 | 0.4 | 0.5 | 1.1 | 0.5 | 0.1 | 0.7 | 0.7 | 0.2 |
| Limonene | 1033 | 0.7 | 0.3 | 0.2 | 0.3 | 0.2 | - | 0.2 | - | - |
| Santolina alcohol | 1037 | - | - | - | - | - | 19.3 | - | - | - |
| 1,8-Cineole | 1038 | 8.2 | 10.2 | 21.0 | 8.1 | 5.2 | 11.9 | 6.0 | 0.2 | 0.1 |
| γ-Terpinene | 1064 | 1.3 | 0.7 | 0.3 | 1.3 | 0.5 | 0.6 | 0.9 | 0.3 | 0.2 |
| cis-Sabinene hydrate | 1070 | 2.0 | 3.3 | 2.5 | 5.0 | 1.7 | 0.8 | 4.2 | 1.6 | 0.3 |
| Terpinolene | 1089 | 0.2 | 0.2 | - | 0.2 | tr | 0.1 | 0.2 | 0.1 | - |
| trans-Linalool oxide | 1090 | - | - | - | - | - | 0.2 | - | - | - |
| Linalool | 1101 | 10.7 | 23.1 | 11.4 | 13.1 | 26.0 | 8.0 | 3.8 | 0.7 | 3.0 |
| trans-Sabinene hydrate | 1102 | - | 0.9 | 2.2 | 1.7 | - | 0.3 | - | 1.0 | 0.6 |
| Isoamylisovalerate | 1103 | 0.4 | 0.4 | 0.7 | - | - | - | - | - | - |
| α-Thujone | 1105 | - | - | - | - | - | - | 22.6 | - | - |
| β-Thujone | 1116 | - | - | - | - | - | tr | 3.3 | - | - |
| cis-p-Menth-2-en-1-ol | 1123 | 0.1 | 0.1 | - | 14.4 | 0.1 | - | 0.1 | 0.1 | 0.2 |
| α-Campholenal | 1127 | - | 0.1 | - | - | - | - | - | - | - |
| trans-Pinocarveol | 1141 | 0.2 | 2.3 | 1.0 | - | 0.2 | 0.4 | - | - | 0.4 |
| cis-Sabinol | 1145 | - | - | - | - | - | - | 1.0 | - | - |
| trans-p-Menth-2-en-1-ol | 1147 | - | - | - | 9.5 | - | - | 0.2 | - | - |
| Camphor | 1148 | 8.2 | 1.2 | 2.5 | 2.0 | 2.9 | 13.5 | 8.8 | - | 0.2 |
| Pinocarvone | 1165 | 0.7 | - | 4.0 | 0.1 | 0.2 | - | 0.5 | - | - |
| Borneol | 1167 | 16.5 | 5.7 | 12.7 | 1.1 | 16.3 | 1.3 | 13.9 | 0.1 | 0.4 |
| Terpinen-4-ol | 1182 | 1.6 | 2.1 | 1.0 | 3.4 | 1.3 | 2.0 | 1.5 | 0.7 | 2.4 |
| α-Thujenal | 1184 | - | 0.1 | - | - | - | - | - | - | - |
| Dill ether | 1186 | - | 0.2 | - | - | - | - | - | - | - |
| α-Terpineol | 1192 | 2.9 | 5.6 | 3.8 | 4.5 | 4.4 | 1.7 | 1.8 | 0.1 | 0.4 |
| trans-Piperitol | 1207 | - | - | - | 8.0 | tr | tr | 0.3 | - | - |
| Fragranol | 1217 | - | - | - | - | - | - | - | 16.9 | 4.0 |
| trans-Carveol | 1219 | - | - | - | - | tr | 0.3 | 0.7 | - | - |
| cis-Carveol | 1231 | - | - | - | - | - | - | 0.1 | - | - |
| Cuminaldehyde | 1242 | - | - | - | 0.1 | - | - | - | - | - |
| Carvone | 1244 | - | - | - | - | tr | - | 1.0 | - | - |
| Piperitone | 1254 | 0.5 | - | - | 0.4 | - | - | 0.8 | - | - |
| cis-Chrysanthenyl acetate | 1265 | - | - | 1.3 | - | - | 0.1 | - | - | - |
| Isobornyl acetate | 1285 | 7.2 | 2.3 | 5.7 | 1.8 | 0.2 | 0.2 | 0.3 | 0.1 | 0.4 |
| Thymol | 1292 | 0.9 | 0.2 | 0.3 | 0.1 | 4.1 | 0.7 | 0.1 | 1.1 | 0.3 |
| trans-Pinocarvyl acetate | 1297 | - | 0.9 | 0.2 | - | - | - | tr | - | - |
| Carvacrol | 1300 | - | - | 0.5 | - | - | - | - | - | - |
| Myrtenyl acetate | 1327 | - | 0.1 | 0.2 | - | - | - | tr | - | - |
| trans-Carvyl acetate | 1337 | - | - | 0.2 | - | - | - | - | - | - |
| Fragranyl acetate | 1346 | - | - | - | - | - | - | - | 59.7 | 71.4 |
| α-Longipinene | 1351 | 0.1 | - | - | - | - | - | - | - | - |
| Eugenol | 1357 | - | - | - | - | tr | - | tr | - | - |
| cis-Carvyl acetate | 1362 | - | - | 0.5 | - | - | - | - | - | - |
| Cyclosativene | 1370 | 0.3 | 0.1 | 0.5 | - | 0.1 | 0.1 | 0.1 | 0.3 | 0.2 |
| α-Ylangene | 1372 | - | - | 0.5 | - | - | - | - | - | - |
| α-Copaene | 1377 | 0.1 | 0.1 | 0.2 | tr | 0.2 | 0.1 | 0.1 | 0.4 | 0.2 |
| β-Elemene | 1391 | - | - | - | - | 0.1 | - | - | - | - |
| α-Gurjunene | 1409 | - | - | - | - | 0.1 | tr | 0.1 | - | - |
| β-Caryophyllene | 1420 | 0.6 | 0.9 | 0.2 | - | 0.1 | - | 1.7 | 1.0 | 0.4 |
| α-Himachalene | 1449 | 0.2 | - | 0.1 | - | - | - | - | - | - |
| (E)-β-Farnesene | 1455 | - | 0.1 | - | - | - | - | 0.2 | - | - |
| α-Humulene | 1456 | 0.1 | 0.1 | - | - | - | - | 0.2 | - | - |
| Alloaromadendrene | 1461 | 0.3 | 0.7 | 0.6 | 0.3 | 1.4 | 0.9 | 0.5 | 0.4 | - |
| β-Chamigrene | 1475 | - | - | - | - | - | - | 0.2 | - | - |
| γ-Muurolene | 1475 | - | 0.1 | - | 0.1 | 0.3 | - | 0.1 | 0.1 | - |
| Germacrene D | 1482 | - | 0.4 | 0.2 | - | 0.2 | - | 0.9 | 0.8 | - |
| ar-Curcumene | 1483 | - | - | - | 0.1 | - | - | - | - | - |
| β-Selinene | 1493 | - | 0.5 | - | - | - | - | - | - | - |
| α-Zingiberene | 1494 | - | - | - | 0.2 | - | - | - | - | - |
| Bicyclogermacrene | 1496 | - | 0.1 | - | 0.1 | 0.1 | - | 0.1 | 0.1 | - |
| α-Muurolene | 1499 | - | - | - | - | 0.1 | - | 0.1 | 0.1 | - |
| cis-γ-Cadinene | 1511 | 0.3 | 0.2 | 0.3 | - | - | - | 0.6 | - | - |
| δ-Cadinene | 1525 | 1.3 | 0.2 | - | 0.1 | 0.1 | - | - | 0.1 | - |
| trans-Nerolidol | 1564 | 1.1 | 4.4 | - | - | - | - | 0.1 | - | 1.2 |
| Spathulenol | 1578 | - | 0.4 | - | - | 0.2 | 0.1 | 0.1 | 0.4 | 0.1 |
| Caryophyllene oxide | 1583 | 0.5 | 1.3 | 0.3 | - | 0.2 | - | 0.7 | 0.3 | 1.0 |
| Viridiflorol | 1592 | 1.7 | 6.4 | 5.7 | 5.1 | 22.4 | 12.7 | 4.8 | 6.0 | 2.4 |
| Caryophylla-4(14),8(15)-dien-5-α-ol | 1641 | - | - | - | - | - | - | - | - | 0.1 |
| T-Cadinol | 1642 | 0.1 | 1.8 | 0.7 | - | - | - | - | - | - |
| Cubenol | 1644 | 1.3 | - | - | - | - | - | - | - | - |
| β-Eudesmol | 1651 | - | 2.9 | - | - | 1.2 | 11.4 | - | - | - |
| Kongol | 1655 | - | - | - | 1.7 | - | - | 2.2 | - | - |
| α-Bisabolol | 1685 | - | 0.4 | - | - | 3.0 | - | - | - | - |
| Chamazulene | 1727 | - | 0.1 | tr | tr | 0.1 | 0.1 | 0.1 | - | - |
| Monoterpene hydrocarbons | 22.2 | 7.5 | 9.1 | 9.1 | 2.7 | 3.0 | 12.2 | 1.4 | 0.4 | |
| Oxygenated monoterpenes | 60.0 | 58.4 | 71.4 | 73.3 | 62.6 | 61.0 | 71.1 | 82.3 | 84.1 | |
| Sesquiterpene hydrocarbons | 3.3 | 3.5 | 2.6 | 0.9 | 2.8 | 1.1 | 4.9 | 3.3 | 0.8 | |
| Oxygenated sesquiterpenes | 4.7 | 17.9 | 6.7 | 6.8 | 27.0 | 24.2 | 7.9 | 6.7 | 4.8 | |
| Phenylpropanoids | tr | tr | 0.2 | - | tr | - | tr | - | - | |
| Nor-terpenes | - | 0.1 | tr | tr | tr | 0.1 | 0.1 | - | - | |
| Non-terpene derivatives | 0.5 | 0.4 | 0.9 | - | 0.1 | 0.1 | 0.1 | 0.2 | - | |
| Total identified | 90.7% | 87.8% | 90.7% | 90.1% | 95.3% | 89.5% | 96.3% | 93.9% | 90.1% |
 |  |  |
| β-eudesmol | Viridiflorol | Germacrene D |
 |  |  |
| β-Caryophyllene | alloaromadendrene | δ-cadinene |
 |  |  |
| α-thujone | Camphor | Santolina alcohol |
| Site of Collection | Sample Code | Origin | Altitude (m) | Soil pH |
|---|---|---|---|---|
| Penta di Fisciano | Sample 1 | Mainland | 320 | 7.2 |
| Vibo Valentia | Sample 2 | Mainland | 476 | 6.8 |
| Villa San Giovanni | Sample 3 | Mainland | sea level | 7.2 |
| Vulcano Island | Sample 4 | Island | sea level | 5.4 |
| Montalbano | Sample 5 | Island | 908 | 7.2 |
| Novara di Sicilia | Sample 6 | Island | 650 | 6.6 |
| Sella Mandrazzi | Sample 7 | Island | 1125 | 6.9 |
| Messina | Sample 8 | Island | 100 | 7.1 |
| Rottaia (PI) | Sample 9 | Cultivated | 50 | 7.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bader, A.; AlQathama, A.; Cioni, P.L.; Ceccarini, L.; Abdelhady, M.I.S.; Al-Shareef, W.; Ascrizzi, R.; Flamini, G. Essential Oil Biodiversity of Achillea ligustica All. Obtained from Mainland and Island Populations. Plants 2022, 11, 1054. https://doi.org/10.3390/plants11081054
Bader A, AlQathama A, Cioni PL, Ceccarini L, Abdelhady MIS, Al-Shareef W, Ascrizzi R, Flamini G. Essential Oil Biodiversity of Achillea ligustica All. Obtained from Mainland and Island Populations. Plants. 2022; 11(8):1054. https://doi.org/10.3390/plants11081054
Chicago/Turabian StyleBader, Ammar, Aljawharah AlQathama, Pier Luigi Cioni, Lucia Ceccarini, Mohamed I. S. Abdelhady, Wajih Al-Shareef, Roberta Ascrizzi, and Guido Flamini. 2022. "Essential Oil Biodiversity of Achillea ligustica All. Obtained from Mainland and Island Populations" Plants 11, no. 8: 1054. https://doi.org/10.3390/plants11081054
APA StyleBader, A., AlQathama, A., Cioni, P. L., Ceccarini, L., Abdelhady, M. I. S., Al-Shareef, W., Ascrizzi, R., & Flamini, G. (2022). Essential Oil Biodiversity of Achillea ligustica All. Obtained from Mainland and Island Populations. Plants, 11(8), 1054. https://doi.org/10.3390/plants11081054