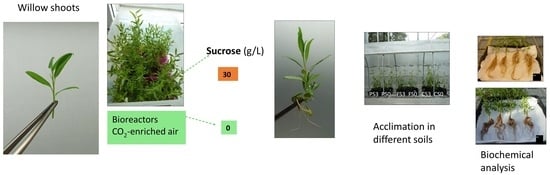
Effect of Soil Type and In Vitro Proliferation Conditions on Acclimation and Growth of Willow Shoots Micropropagated in Continuous Immersion Bioreactors
Abstract
:1. Introduction
2. Results
2.1. Effect of Support Material and Sucrose Supplementation on the Micropropagation of Willow Shoots in CIS
2.2. Effect of Soil Type on the Acclimation of Shoots Micropropagated with or without Sucrose
2.3. Main General Properties of the Soils Used in the Study
2.4. Enzymatic Activities of the Soil
3. Discussion
4. Materials and Methods
4.1. Plant Material and Micropropagation in Liquid Medium
4.2. Acclimation and Effect of Soil Type
4.3. Soil Analysis
4.4. Determination of Enzyme Activities
4.5. Data Recording and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Preece, J.E.; Sutter, E.G. Acclimatization of micropropagated plants to the greenhouse and field. In Micropropagation; Debergh, P.C., Zimmerman, R.H., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 71–93. [Google Scholar] [CrossRef]
- Xiao, Y.; Niu, G.; Kozai, T. Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Cult. 2011, 105, 149–158. [Google Scholar] [CrossRef]
- Grout, B.; Aston, M. Modified leaf anatomy of cauliflower plantlets regenerated from meristem culture. Ann. Bot. 1978, 180, 993–995. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Dhawan, V. Acclimatization of Tissue Culture-Raised Plants for Transplantation to the Field. In Applications of Biotechnology in Forestry and Horticulture; Dhawan, V., Ed.; Springer: Boston, MA, USA, 1989; pp. 249–256. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Alvard, D.; Côte, F.; Teisson, C. Comparison of methods of liquid medium culture for banana micropropagation. Effects of temporary immersion of explants. Plant Cell Tissue Organ Cult. 1993, 32, 55–60. [Google Scholar] [CrossRef]
- Watt, M.P. The status of temporary immersion system (TIS) technology for plant micropropagation. Afr. J. Biotechnol. 2012, 11, 14025–14035. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Mamun, N.H.A.; Egertsdotter, U.; Aidun, C.K. Bioreactor technology for clonal propagation of plants and metabolite production. Front. Biol. 2015, 10, 177–193. [Google Scholar] [CrossRef]
- Valdiani, A.; Hansen, O.K.; Nielsen, U.B.; Johannsen, V.K.; Shariat, M.; Georgiev, M.I.; Omidvar, V.; Ebrahimi, M.; Dinanai, E.T.; Abiri, R. Bioreactor-based advances in plant tissue and cell culture: Challenges and prospects. Crit. Rev. Biotechnol. 2019, 39, 20–34. [Google Scholar] [CrossRef]
- Vidal, N.; Sánchez, C. Use of bioreactor systems in the propagation of forest trees. Eng. Life Sci. 2019, 19, 896–915. [Google Scholar] [CrossRef] [Green Version]
- Cuenca, B.; Sánchez, C.; Aldrey, A.; Bogo, B.; Blanco, B.; Correa, B.; Vidal, N. Micropropagation of axillary shoots of hybrid chestnut (Castanea sativa × C. crenata) in liquid medium in a continuous immersion system. Plant Cell Tissue Organ Cult. 2017, 131, 307–320. [Google Scholar] [CrossRef]
- Gago, D.; Bernal, M.Á.; Sánchez, C.; Aldrey, A.; Cuenca, B.; Christie, C.B.; Vidal, N. Effect of Sucrose on Growth and Stress Status of Castanea sativa x C. crenata Shoots Cultured in Liquid Medium. Plants 2022, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- San José, M.C.; Blázquez, N.; Cernadas, M.J.; Cuenca, B.; Sánchez, C.; Vidal, N. Temporary immersion systems to improve alder micropropagation. Plant Cell Tissue Organ Cult. 2020, 143, 265–275. [Google Scholar] [CrossRef]
- Gago, D.; Sánchez, C.; Aldrey, A.; Christie, C.B.; Bernal, M.Á.; Vidal, N. Micropropagation of Plum (Prunus domestica L.) in Bioreactors Using Photomixotrophic and Photoautotrophic Conditions. Horticulturae 2022, 8, 286. [Google Scholar] [CrossRef]
- Rico, S.; Garrido, J.; Sánchez, C.; Ferreiro-Vera, C.; Codesido, V.; Vidal, N. A Temporary Immersion System to Improve Cannabis sativa Micropropagation. Front. Plant Sci. 2022, 13, 895971. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Blanco, B.; Cuenca, B. A temporary immersion system for micropropagation of axillary shoots of hybrid chestnut. Plant Cell Tissue Organ Cult. 2015, 123, 229–243. [Google Scholar] [CrossRef]
- Regueira, M.; Rial, E.; Blanco, B.; Bogo, B.; Aldrey, A.; Correa, B.; Varas, E.; Sánchez, C.; Vidal, N. Micropropagation of axillary shoots of Salix viminalis using a temporary immersion system. Trees 2018, 32, 61–71. [Google Scholar] [CrossRef]
- Gago, D.; Vilavert, S.; Bernal, M.Á.; Sánchez, C.; Aldrey, A.; Vidal, N. The effect of sucrose supplementation on the micropropagation of Salix viminalis L. shoots in semisolid medium and temporary immersion bioreactors. Forests 2021, 12, 1408. [Google Scholar] [CrossRef]
- Bhojwani, S.S. Micropropagation method for a hybrid willow (Salix matsudana × alba NZ-1002). N. Z. J. Bot. 1980, 18, 209–214. [Google Scholar] [CrossRef]
- Bergman, L.; von Arnold, S.; Eriksson, T. Effects of N6 benzyladenine on shoots of five willow clones (Salix spp.) cultured in vitro. Plant Cell Tissue Organ Cult. 1985, 4, 135–144. [Google Scholar] [CrossRef]
- Read, P.E.; Garton, S.; Tormala, T. Willows (Salix spp.). In Biotechnology in Agriculture and Forestry, Vol 5: Trees II; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 370–386. [Google Scholar]
- Amo-Marco, J.B.; Lledo, M.D. In vitro propagation of Salix tarraconensis Pau ex Font Quer, an endemic and threatened plant. In Vitro Cell. Dev. Biol–Plant 1996, 32, 42–46. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.W.; Moon, H.K.; Murthy, H.N.; Choi, Y.H.; Cho, H.M. Micropropagation of Salix pseudolasiogyne from nodal explants. Plant Cell Tissue Organ Cult. 2008, 93, 341–346. [Google Scholar] [CrossRef]
- Khan, M.; Ahmad, N.; Anis, M. The role of cytokinins on in vitro shoot production in Salix tetrasperma Roxb.: A tree of ecological importance. Trees 2011, 25, 577–584. [Google Scholar] [CrossRef]
- Palomo-Ríos, E.; Macalpine, E.; Shield, I.; Amey, J.; Karaoğlu, C.; West, J.; Hanley, S.; Krygier, R.; Karp, A.; Jones, H.D. Efficient method for rapid multiplication of clean and healthy willow clones via in vitro propagation with broad genotype applicability. Can. J. For. Res. 2015, 45, 1662–1667. [Google Scholar] [CrossRef] [Green Version]
- Debergh, P.C.; Maene, L.J. A scheme for the commercial propagation of ornamental plants by tissue culture. Sci. Hortic. 1981, 41, 335–345. [Google Scholar] [CrossRef]
- Sanchez, C.; San-Jose, M.C.; Ferro, E.; Ballester, A.; Vieitez, A.M. Improving micropropagation conditions for adult-phase shoots of chestnut. J. Hortic. Sci. 1997, 72, 433–443. [Google Scholar] [CrossRef]
- Newel, C.; Growns, D.; McComb, J. The influence of medium aeration on in vitro rooting of Australian plant microcuttings. Plant Cell Tissue Organ Cult. 2003, 75, 131–142. [Google Scholar] [CrossRef]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing Sustainability of Growing Media Constituents and Stand-Alone Substrates in Soilless Culture Systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Stuepp, C.A.; Fragoso, R.D.O.; Monteiro, P.H.R.; Kratz, D.; Wendling, I.; Zuffellato-Ribas, K.C. Use of renewable substrates for ex vitro production of Melaleuca alternifolia Cheel clonal plants by mini-cuttings technique. Cerne 2017, 23, 395–402. [Google Scholar] [CrossRef]
- Bignami, C.; Melegari, F.; Zaccardelli, M.; Pane, C.; Ronga, D. Composted solid digestate and vineyard winter prunings partially replace peat in growing substrates for micropropagated highbush blueberry in the nursery. Agronomy 2022, 12, 337. [Google Scholar] [CrossRef]
- Suárez, I.E.; Yepez, J.E.; López, C.M. Effect of different substrates on adaptation of arrow cane (Gynerium sagitatum Aubl.) micropropagated plants. Temas Agrar. 2020, 25, 77–84. [Google Scholar] [CrossRef]
- Ronga, D.; Francia, E.; Allesina, G.; Pedrazzi, S.; Zaccardelli, M.; Pane, C.; Tava, A.; Bignami, C. Valorization of Vineyard By-Products to Obtain Composted Digestate and Biochar Suitable for Nursery Grapevine (Vitis vinifera L.) Production. Agronomy 2019, 9, 420. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Li, J.; Zhang, J.; Fan, H.; Wu, L.; Li, Q. Improvement of the tissue culture technique for Melaleuca alternifolia. J. For. Res. 2016, 27, 1265–1269. [Google Scholar] [CrossRef]
- Brito, G.; Costa, A.; Coelho, C.; Santos, C. Large-scale field acclimatization of Olea maderensis micropropagated plants: Morphological and physiological survey. Trees 2009, 23, 1019–1031. [Google Scholar] [CrossRef]
- Moraes, R.M.; de Andrade, Z.; Bedir, E.; Dayan, F.E.; Lata, H.; Khan, I.; Pereira, A.M.S. Arbuscular mycorrhiza improves acclimatization and increases lignan content of micropropagated mayapple (Podophyllum peltatum L.). Plant Sci. 2004, 166, 23–29. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Anis, M. In vitro regeneration and the antioxidant enzymatic system on acclimatization of micropropagated Vitex trifolia L. Agrofor. Syst. 2014, 88, 437–447. [Google Scholar] [CrossRef]
- Frankenberger, W.T.; Dick, W.A. Relationship between enzyme activities and microbial growth and activity indices in soil. Soil Sci. Soc. Am. J. 1983, 47, 945–951. [Google Scholar] [CrossRef]
- Kandeler, E.; Kampichler, C.; Horak, O. Influence of heavy metals on the functional diversity of soil microbial communities. Biol. Fertil. Soils 1996, 23, 299–306. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S.; Gil-Sotres, F.G. Limitations of soil enzymes as indicators of soil pollution. Soil Biol. Biochem. 2000, 32, 1867–1875. [Google Scholar] [CrossRef]
- Dick, R.P. Soil enzyme activities as integrative indicators of soil health. In Biological Indicators of Soil Health; Pankhurst, C.E., Doube, B.M., Gupta, V.V.S.R., Eds.; CAB International: Wellingford, UK, 1997; pp. 121–156. [Google Scholar]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Casida, L.E., Jr.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Piao, X.C.; Chakrabarty, D.; Hahn, E.J.; Paek, K.Y. A simple method for mass production of potato microtubers using a bioreactor system. Curr. Sci. 2003, 84, 1129–1132. [Google Scholar]
- Dewir, Y.; Chakrabarty, D.; Ali, M.; Hahn, E.J.; Paek, K.Y. Effects of Hydroponic Solution EC, Substrates, PPF and Nutrient Scheduling on Growth and Photosynthetic Competence During Acclimatization of Micropropagated Spathiphyllum plantlets. Plant Growth Regul 2005, 46, 241–251. [Google Scholar] [CrossRef]
- Jo, E.A.; Tewari, R.K.; Hahn, E.J.; Paek, K.Y. In vitro sucrose concentration affects growth and acclimatization of Alocasia amazonica plantlets. Plant Cell Tissue Organ Cult. 2009, 96, 307–315. [Google Scholar] [CrossRef]
- Gao, R.; Wu, S.Q.; Piao, X.C.; Park, S.Y.; Lian, M.L. Micropropagation of Cymbidium sinense using continuous and temporary airlift bioreactor systems. Acta Physiol. Plant 2014, 36, 117–124. [Google Scholar] [CrossRef]
- Alawaadh, A.A.; Dewir, Y.H.; Alwihibi, M.S.; Aldubai, A.A.; El-Hendawy, S.; Naidoo, Y. Micropropagation of Lacy Tree Philodendron (Philodendron bipinnatifidum Schott ex Endl.). HortScience 2020, 55, 294–299. [Google Scholar] [CrossRef] [Green Version]
- Manokari, M.; Priyadharshini, S.; Jogam, P.; Dey, A.; Shekhawat, M.S. Meta-topolin and liquid medium mediated enhanced micropropagation via ex vitro rooting in Vanilla planifolia Jacks. ex Andrews. Plant Cell Tissue Organ Cult. 2021, 146, 69–82. [Google Scholar] [CrossRef]
- Debnath, S.C. Temporary immersion and stationary bioreactors for mass propagation of true-to-type highbush, half-high, and hybrid blueberries (Vaccinium spp.). J. Hortic. Sci. Biotechnol. 2017, 92, 72–80. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Hahn, E.J.; Yoon, Y.S.; Paek, K.Y. Micropropagation of apple root stock ‘M9 EMLA’ using bioreactor. J. Hortic. Sci. Biotechnol. 2003, 78, 605–609. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Dewir, Y.H.; Hahn, E.J.; Datta, S.K.; Paek, K.Y. The dynamics of nutrient utilization and growth of apple root stock ‘M9 EMLA’ in temporary versus continuous immersion bioreactors. Plant Growth Regul. 2007, 51, 11–19. [Google Scholar] [CrossRef]
- Shaik, S.; Dewir, Y.H.; Singh, N.; Nicholas, A. Micropropagation and bioreactor studies of the medicinally important plant Lessertia (Sutherlandia) frutescens L. S. Afr. J. Bot. 2010, 76, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Mendonça, E.G.; Stein, V.C.; Carvalho, H.H.; de Santos, B.R.; Beijo, L.A.; Paiva, L.V. The use of continuous, temporary immersion bioreactor system and semisolid culture medium for the production of Eucalyptus camaldulensis clones. Ciência Florest. 2016, 26, 1211–1224. [Google Scholar] [CrossRef] [Green Version]
- Preece, J.E. Micropropagation in stationary liquid media. Propag. Ornam. Plants 2010, 10, 183–187. [Google Scholar]
- Shukla, M.R.; Piunno, K.; Saxena, P.K.; Jones, A.M.P. Improved in vitro rooting in liquid culture using a two piece scaffold system. Eng. Life Sci. 2020, 20, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ševčíková, H.; Lhotáková, Z.; Hamet, J.; Lipavská, H. Mixotrophic in vitro cultivations: The way to go astray in plant physiology. Physiol. Plant. 2019, 167, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Lucchesini, M.; Monteforti, G.; Mensuali-Sodi, A.; Serra, G. Leaf ultrastructure, photosynthetic rate and growth of myrtle plantlets under different in vitro culture conditions. Biol. Plant 2006, 50, 161–168. [Google Scholar] [CrossRef]
- Sáez, P.; Bravo, L.; Latsague, M.; Sánchez-Olate, M.; Ríos, D. Increased light intensity during in vitro culture improves water loss control and photosynthetic performance of Castanea sativa grown in ventilated vessels. Sci. Hortic. 2012, 130, 7–16. [Google Scholar] [CrossRef]
- Kim, N.-Y.; Hwang, H.-D.; Kim, J.-H.; Kwon, B.-M.; Kim, D.; Park, S.-Y. Efficient production of virus-free apple plantlets using the temporary immersion bioreactor system. Hortic. Environ. Biotechnol. 2020, 61, 779–785. [Google Scholar] [CrossRef]
- Fortini, E.A.; Batista, D.S.; Memedes-Rodrigues, T.C.; Felipe, S.H.S.; Corriera, L.N.F.; Chagas, K.; Silva, P.O.; Rocha, D.I.; Otoni, W.C. Gas exchange rates and sucrose concentrations affect plant growth and production of flavonoids in Vernonia condensate grown in vitro. Plant Cell Tissue Organ Cult. 2021, 144, 593–605. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Factors affecting glucosidase and galactosidase activities in soils. Soil Biol. Biochem. 1990, 22, 891–897. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Factors Affecting Soil Arylsulfatase Activity. Soil Sci. Soc. Am. J. 1970, 34, 427–429. [Google Scholar] [CrossRef]
- Trevors, J.T. Dehydrogenase activity in soil: A comparison between the INT and TTC assay. Soil Biol. Biochem. 1984, 16, 673–674. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Guitián Ojea, F.; Carballas Fernández, T. Técnicas de Análisis de Suelos; Pico Sacro Editorial: Santiago de Compostela, Spain, 1976. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chem. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, Z.; Chen, C. Effect of mulching on labile soil organic matter pools, microbial community functional diversity and nitrogen transformations in two hardwood plantations of subtropical Australia. Appl. Soil Ecol. 2008, 40, 229–239. [Google Scholar] [CrossRef]
- Bremner, J.M. Inorganic forms of nitrogen. In Methods of Soil Analysis. Part 2; Black, C.A., Evans, D.D., White, J.L., Ensminger, L.L., Clark, F.E., Eds.; Soil Science Society of America-American Society of Agronomy: Madison, WI, USA, 1965; pp. 1179–1237. [Google Scholar]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubation. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Davidescu, D.; Davidescu, V. Evaluation of Fertility by Plant and Soil Analysis; Abacus Press: London, UK, 1982. [Google Scholar]
- Camiña, F.; Trasar-Cepeda, C.; Gil-Sotres, F.; Leirós, M.C. Measurement of dehydrogenase activity in acid soils rich in organic matter. Soil Biol. Biochem. 1998, 30, 1005–1011. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ceccanti, B.; Cervelli, S.; Matarese, E. Extraction of phosphatase, urease, proteases, organic carbon and nitrogen from soil. Soil Sci. Am. J. 1980, 44, 1011–1016. [Google Scholar] [CrossRef]
- Schinner, F.; von Mersi, W. Xylanase-, CM-cellulase- and invertase activity in soil: An improved method. Soil Biol. Biochem. 1990, 22, 511–515. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Trasar, M.C.; Gil-Sotres, F.; Guitián, F. Determinacion de la actividad fosfatasa en suelos gallegos: Precisiones al método de Sarathchandra y Perrott. An. Edafol. Agrobiol. 1985, 44, 987–999. [Google Scholar]
- Saá, A.; Trasar-Cepeda, C.; Gil-Sotres, F.; Carballas, T. Changes in soil phosphorus and acid phosphatase activity immediately following forest fires. Soil Biol. Biochem. 1993, 22, 511–515. [Google Scholar]
- Vuorinen, A.H. Requirement of p-nitrophenol standard for each soil. Soil Biol. Biochem. 1993, 25, 295–296. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]









| Soil Type | ||||
|---|---|---|---|---|
| Peat | Forest | Crop | ||
| pH H2O | 5.4 ± 0.06 | 4.5 ± 0.05 | 5.2 ± 0.11 | |
| pH KCl | 4.9 ± 0.01 | 3.5 ± 0.01 | 4.0 ± 0.01 | |
| % total C | 43.2 ± 0.9 | 12.6 ± 0.8 | 2.5 ± 0.2 | |
| % total N | 1.2 ± 0.02 | 0.6 ± 0.02 | 0.2 ± 0.00 | |
| C/N | 38 | 21 | 15 | |
| Total P | 505 ± 30 | 452 ± 31 | 591 ± 31 | |
| Available P # | Inorganic P | 298.4 ± 13.5 | 6.6 ± 2.3 | 52.9 ± 0.7 |
| Organic P | 26.5 ± 16.3 | 36.6 ± 2.3 | 25.8 ± 1.0 | |
| Inorganic N # | N-NH4+ | 346.9 ± 2.9 | 33.5 ± 1.4 | 19.3 ± 1.4 |
| N-NO3− | 257.6 ± 6.4 | 9.1 ± 3.2 | 9.1 ± 3.2 | |
| Soluble C # (hot water) | 6621 ± 107 | 4256 ± 234 | 899 ± 39 | |
| Enzyme | Peat | Forest | Crop | |
|---|---|---|---|---|
| Urease (µmol NH3 g−1 h−1) | Control t 0 | 4.46 ± 0.27 | 11.78 ± 0.80 | 5.40 ± 0.07 |
| Control t 6 weeks | 2.83 ± 0.05 | 17.15 ± 1.26 | 9.36 ± 0.87 | |
| Plants S3 | 5.33 ± 0.47 | 19.75 ± 2.33 | 10.95 ± 0.87 | |
| Plants S0 | 5.39 ± 0.65 | 17.23 ± 1.24 | 11.10 ± 5.12 | |
| Dehydrogenase (µmol INTF g−1 h−1) | Control t 0 | 0.54 ± 0.02 | 0.49 ± 0.01 | 0.38 ± 0.03 |
| Control t 6 weeks | 0.52 ± 0.01 | 0.43 ± 0.01 | 0.34 ± 0.02 | |
| Plants S3 | 0.63 ± 0.06 | 0.45 ± 0.04 | 0.33 ± 0.01 | |
| Plants S0 | 0.75 ± 0.04 | 0.48 ± 0.03 | 0.33 ± 0.00 | |
| Acid phosphomonoesterase (µmol PNP g−1 h−1) | Control t 0 | 5.04 ± 0.79 | 6.96 ± 0.08 | 2.93 ± 0.07 |
| Control t 6 weeks | 2.75 ± 0.10 | 7.88 ± 0.37 | 2.51 ± 0.18 | |
| Plants S3 | 3.12 ± 0.64 | 7.24 ± 0.90 | 2.31 ± 0.14 | |
| Plants S0 | 3.46 ± 0.44 | 8.19 ± 0.30 | 2.44 ± 0.14 | |
| ß-glucosidase (µmol PNG g−1 h−1) | Control t 0 | 1.53 ± 0.06 | 1.86 ± 0.09 | 0.43 ± 0.03 |
| Control t 6 weeks | 4.18 ± 0.11 | 1.53 ± 0.08 | 0.55 ± 0.02 | |
| Plants S3 | 3.97 ± 0.24 | 1.59 ± 0.13 | 0.53 ± 0.06 | |
| Plants S0 | 4.25 ± 0.19 | 1.60 ± 0.08 | 0.51 ± 0.03 | |
| Invertase (µmol Glu g−1 h−1) | Control t 0 | 1.68 ± 0.09 | 11.19 ± 0.33 | 4.01 ± 0.01 |
| Control t 6 weeks | 1.86 ± 0.12 | 8.32 ± 0.30 | 3.51 ± 0.36 | |
| Plants S3 | 2.54 ± 0.28 | 8.61 ± 0.63 | 3.68 ± 0.35 | |
| Plants S0 | 2.18 ± 0.51 | 8.07 ± 0.53 | 3.81 ± 0.10 | |
| Arylsulfatase (µmol PNS g−1 h−1) | Control t 0 | 0.09 ± 0.06 | 0.35 ± 0.02 | 0.16 ± 0.01 |
| Control t 6 weeks | 0.04 ± 0.00 | 0.46 ± 0.02 | 0.17 ± 0.01 | |
| Plants S3 | 0.09 ± 0.04 | 0.42 ± 0.06 | 0.19 ± 0.01 | |
| Plants S0 | 0.09 ± 0.02 | 0.43 ± 0.02 | 0.19 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trasar-Cepeda, C.; Sánchez, C.; Casalderrey, M.; Bello, D.; Vielba, J.M.; Rico, S.; Aldrey, A.; Vidal, N. Effect of Soil Type and In Vitro Proliferation Conditions on Acclimation and Growth of Willow Shoots Micropropagated in Continuous Immersion Bioreactors. Plants 2023, 12, 132. https://doi.org/10.3390/plants12010132
Trasar-Cepeda C, Sánchez C, Casalderrey M, Bello D, Vielba JM, Rico S, Aldrey A, Vidal N. Effect of Soil Type and In Vitro Proliferation Conditions on Acclimation and Growth of Willow Shoots Micropropagated in Continuous Immersion Bioreactors. Plants. 2023; 12(1):132. https://doi.org/10.3390/plants12010132
Chicago/Turabian StyleTrasar-Cepeda, Carmen, Conchi Sánchez, Mar Casalderrey, Diana Bello, Jesús María Vielba, Saleta Rico, Anxela Aldrey, and Nieves Vidal. 2023. "Effect of Soil Type and In Vitro Proliferation Conditions on Acclimation and Growth of Willow Shoots Micropropagated in Continuous Immersion Bioreactors" Plants 12, no. 1: 132. https://doi.org/10.3390/plants12010132
APA StyleTrasar-Cepeda, C., Sánchez, C., Casalderrey, M., Bello, D., Vielba, J. M., Rico, S., Aldrey, A., & Vidal, N. (2023). Effect of Soil Type and In Vitro Proliferation Conditions on Acclimation and Growth of Willow Shoots Micropropagated in Continuous Immersion Bioreactors. Plants, 12(1), 132. https://doi.org/10.3390/plants12010132