Characterization of the Mechanism of Action of Serratia rubidaea Mar61-01 against Botrytis cinerea in Strawberries
Abstract
:1. Introduction
2. Results
2.1. Antifungal Activity of Serratia rubidaea Mar61-01 against Mycelial Growth of Botrytis cinerea
2.2. Effects of Volatile Organic Compounds of Serratia rubidaea Mar61-01 on Botrytis cinerea Mycelial Growth and Conidia Germination
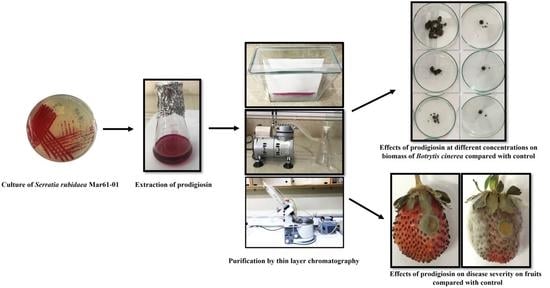
2.3. Effects of Prodigiosin on Mycelial Growth and Biomass of Botrytis cinerea
2.4. Protection of Strawberry Fruits against Botrytis cinerea by Serratia rubidaea MarR61-01 Cells
2.5. Effects of Prodigiosin Pigment on Activities of Defense-Related Enzymes
3. Discussion
4. Materials and Methods
4.1. Bacterial and Fungal Strains and Plant Material
4.2. Antifungal Activity of Serratia rubidaea Mar61-01 against Mycelial Growth of Botrytis cinerea
4.3. Effects of Volatile Organic Compounds of Serratia rubidaea Mar61-01 on Botrytis cinerea Mycelial Growth and Conidia Germination
4.4. Extraction of Prodigiosin
4.5. Effects of Prodigiosin on Mycelial Growth and Biomass of Botrytis cinerea
4.6. Protection of Strawberry Fruits against Botrytis cinerea by Serratia rubidaea MarR61-01 Cells
4.7. Protection of Strawberry Fruits against Botrytis cinerea by Volatile Organic Compounds Emitted by Serratia rubidaea MarR61-01
4.8. Effects of Prodigiosin on Activities of Plant Defense-Related Enzymes
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grimont, F.; Grimont, P.A. The genus serratia. In The Prokaryotes: Volume 6: Proteobacteria: Gamma Subclass; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 219–244. [Google Scholar]
- Soenens, A.; Imperial, J. Biocontrol capabilities of the genus Serratia. Phytochem. Rev. 2020, 19, 577–587. [Google Scholar] [CrossRef]
- Okamoto, H.; Sato, Z.; Sato, M.; Koiso, Y.; Iwasaki, S.; Isaka, M. Identification of antibiotic red pigments of Serratia marcescens F-1-1 a biocontrol agent of damping off of cucumber and antimicrobial activity against other plant pathogens. JPN J. Phytopathol. 1998, 64, 294–298. [Google Scholar] [CrossRef]
- Someya, N.; Kataoka, N.; Komagata, T.; Hirayae, K.; Hibi, T.; Akutsu, K. Biological control of cyclamen soilborne diseases by Serratia marcescens strain B2. Plant Dis. 2000, 84, 334–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Román, M.I.; Holguín-Meléndez, F.; Dunn, M.F.; Guillén-Navarro, K.; Huerta-Palacios, G. Antifungal activity of Serratia marcescens CFFSUR-B2 purified chitinolytic enzymes and prodigioSin. against Mycosphaerella fijiensis, causal agent of black Sigatoka in banana (Musa spp.). BioControl 2015, 60, 565–572. [Google Scholar] [CrossRef]
- Gutiérrez-Román, M.I.; Holguín-Meléndez, F.; Bello-Mendoza, R.; Guillén-Navarro, K.; Dunn, M.F.; Huerta-Palacios, G. Production of prodigioSin. and chitinases by tropical Serratia marcescens strains with potential to control plant pathogens. World J. Microbiol. Biotechnol. 2012, 28, 145–153. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Fillinger, S.; Elad, Y. Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer International Publishing: Cham, Switzerland, 2016; 488p. [Google Scholar]
- Saito, S.; Michailides, T.J.; Xiao, C.L. Fungicide-resistant phenotypes in Botrytis cinerea populations and their impact on control of gray mold on stored table grapes in California. Eur. J. Plant Pathol. 2019, 154, 203–213. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (u cinerea)–prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 241–262. [Google Scholar] [CrossRef]
- Singh, M.; Meenakshi, S.; Kumar, A.; Singh, A.K.; Pandey, K.D. Endophytic bacteria in plant disease management. In Microbial Endophytes: Prospects for Sustainable Agriculture, Woodhead Publishing Series in Food Science; Kumar, A., Singh, V.K., Eds.; Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2020; pp. 61–89. [Google Scholar]
- Yang, P.; Sun, Z.X.; Liu, S.Y.; Lu, H.X.; Zhou, Y.; Sun, M. Combining antagonistic bacteria in different growth stages of cotton for control of Verticillium wilt. Crop Prot. 2013, 47, 17–23. [Google Scholar] [CrossRef]
- Hidayati, U.; Chaniago, I.A.; Munif, A.; Santosa, D.A. Potency of plant growth promoting endophytic bacteria from rubber plants (Hevea brasiliensis). J. Agron. 2014, 13, 147–152. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Z.; Tian, C.; Liang, Y.; You, C.; Chen, L. Identification and characterization of the endophytic bacterium Bacillus atrophaeus XW2, antagonistic towards Colletotrichum gloeosporioides. Ann. Microbiol. 2015, 65, 1361–1371. [Google Scholar] [CrossRef]
- Ben Abdallah, R.A.; Mejdoub-Trabelsi, B.; Nefzi, A.; Jabnoun-Khiareddin, H.; Daami-Remadi, M. Isolation of endophytic bacteria from Withania somnifera and assessment of their ability to suppress fusarium wilt disease in tomato and to promote plant growth. J. Plant Pathol. Microbiol. 2016, 7, 352. [Google Scholar] [CrossRef]
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.F.; Kloepper, J.W. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Berg, G.; Krechel, A.; Ditz, M.; Sikora, R.A.; Ulrich, A.; Hallmann, J. Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microbiol. Ecol. 2005, 51, 215–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [Green Version]
- Van Kan, J.A.L.; Shaw, M.W.; Grant-Downton, R.T. Botrytis species: Relentless necrotrophic thugs or endophytes gone rogue? Mol. Plant Pathol. 2014, 15, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Duffy, B.; Nowak, J.; Clement, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [Green Version]
- Nair, D.N.; Padmavathy, S. Impact of endophytic microorganisms on plants, environment and humans. Sci. World J. 2014, 2014, 250693. [Google Scholar] [CrossRef] [Green Version]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Kamou, N.N.; Dubey, M.; Tzelepis, G.; Menexes, G.; Papadakis, E.N.; Karlsson, M.; Lagopodi, A.L.; Jensen, D.F. Investigating the compatibility of the biocontrol agent Clonostachys rosea IK726 with prodigiosin-producing Serratia rubidaea S55 and phenazine-producing Pseudomonas chlororaphis ToZa7. Arch. Microbiol. 2016, 198, 369–377. [Google Scholar] [CrossRef]
- Alijani, Z.; Amini, J.; Ashengroph, M.; Bahramnejad, B. Antifungal activity of Serratia rubidaea Mar61-01 purified prodigioSin. against Colletotrichum nymphaeae, the causal agent of strawberry anthracnose. J. Plant Growth Regul. 2022, 41, 585–595. [Google Scholar] [CrossRef]
- Tamandegani, P.R.; Sharifnabi, B.; Massah, A.; Zahravi, M. Induced reprogramming of oxidative stress responses in cucumber by Trichoderma asperellum (Iran 3062C) enhances defense against cucumber mosaic virus. Biol. Control 2021, 164, 104779. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Banerjee, S.; Acharya, U.; Mitra, A.; Mallick, I.; Haldar, A.; Haldar, S.; Ghosh, A.; Ghosh, A. Evaluation of plant growth promotion properties and induction of antioxidative defense mechanism by tea rhizobacteria of Darjeeling, India. Sci. Rep. 2020, 10, 15536. [Google Scholar] [CrossRef]
- Pratap, S.S.; Keswani, C.; Sansinenea, E.; Xuan, T.H. Trichoderma spp. mediated induction of systemic defense response in brinjal against Sclerotinia sclerotiorum. Curr. Res. Microbial. Sci. 2021, 2, 100051. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 2009, 81, 1001–1012. [Google Scholar] [CrossRef]
- Terry, L.A.; Joyce, D.C. Elicitors of induced disease resistance in postharvest horticultural crops: A brief review. Postharvest Biol. Technol. 2004, 32, 1–13. [Google Scholar] [CrossRef]
- Dawoud, T.M.; Alharbi, N.S.; Theruvinthalakal, A.M.; Thekkangil, A.; Kadaikunnan, S.; Khaled, J.M.; Rajaram, S.K. Characterization and antifungal activity of the yellow pigment produced by a Bacillus sp. DBS4 isolated from the lichen Dirinaria agealita. Saudi J. Biol. Sci. 2020, 27, 1403–1411. [Google Scholar] [CrossRef]
- Schmidt, R.; de Jager, V.; Zuhlke, V. Fungal volatile compounds induce production of the secondary metabolite Sodorifen in Serratia plymuthica PRI-2C. Sci. Rep. 2017, 7, 862. [Google Scholar] [CrossRef]
- Elad, Y. The use of antioxidants (free radical scavengers) to control grey mould (Botrytis cinerea) and white mold (Sclerotinia sclerotiorum) in various crops. Plant Pathol. 1992, 41, 417–426. [Google Scholar] [CrossRef]
- El-Korany, A.E.; Mohamed, R.A. The use of antioxidants to control grey mould and to enhance yield and quality of strawberry. J. Agric. Environ. Sci. Alex Univ. Egypt 2008, 7, 1–30. [Google Scholar]
- Amil-Ruiz, F.; Blanco-Portales, R.; Muñoz-Blanco, J.; Caballero, J.L. The strawberry plant defense mechanism: A molecular review. Plant Cell Physiol. 2011, 52, 1873–1903. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Hu, Z.; Zheng, Y.; Yang, Z.; Lu, B. Effect of BTH on antioxidant enzymes, radical-scavenging activity and decay in strawberry fruit. Food Chem. 2011, 125, 145–149. [Google Scholar] [CrossRef]
- Vanti, G.L.; Leshem, Y.; Masaphy, S. Resistance response enhancement and reduction of Botrytis cinerea infection in strawberry fruit by Morchella conica mycelial extract. Postharvest Biol. Technol. 2021, 175, 111470. [Google Scholar] [CrossRef]
- Martinez-Tellez, C.; Montilet, J.L.; Bresson, E.; Agnel, J.J. Apoplastic peroxidase generates, superoxides anions in cells of cotton cotyledons undergoing the hypersensitivite reaction to Xanthomonas campestris pv. malvacearum race 18. Mol. Plant Microbe Interact. 1998, 11, 1038–1047. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Mathioudakis, M.M.; Veiga, S.R.L.; Canto, T.; Medina, V.; Mossialos, D.; Makris, A.M.; Livieratos, I. Pepino mosaic virus triple gene block protein 1 (TGBp1) interacts with and increases tomato catalase 1 activity to enhance virus accumulation. Mol. Plant Pathol. 2013, 14, 589–601. [Google Scholar] [CrossRef]
- Ye, W.Q.; Sun, Y.F.; Tang, Y.J.; Zhou, W.W. Biocontrol potential of a broad-spectrum antifungal strain Bacillus amyloliquefaciens B4 for postharvest loquat fruit storage. Postharvest. Biol. Technol. 2021, 174, 111439. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Gu, N.; Yan, X.; Wang, K.; Dhanasekaran, S.; Gu, X.; Zhao, L.; Zhang, H. Postharvest biological control of Rhizopus rot and the mechanisms involved in induced disease resistance of peaches by Pichia membranefaciens. Postharvest Biol. Technol. 2020, 163, 111146. [Google Scholar] [CrossRef]
- Rais, A.; Jabeen, Z.; Shair, F.; Hafeez, F.Y.; Hassan, M.N. Bacillus spp., a bio-control agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryzae. PloS ONE 2017, 12, e0187412. [Google Scholar] [CrossRef] [Green Version]
- Alamri, S.A. Enhancing the efficiency of the bioagent Bacillus subtilis JF419701 against soil-borne phytopathogens by increasing the productivity of fungal cell wall degrading enzymes. Arch. Phytopathol. Plant Prot. 2015, 48, 159–170. [Google Scholar] [CrossRef]
- Jangir, M.; Sharma, S.; Sharma, S. Development of next-generation formulation against Fusarium oxysporum and unraveling bioactive antifungal metabolites of biocontrol agents. Sci. Rep. 2021, 11, 22895. [Google Scholar] [CrossRef]
- Devi, K.A.; Pandey, P.; Sharma, G.D. Plant growth-promoting endophyte Serratia marcescens AL2-16 enhances the growth of Achyranthes aspera L., a medicinal plant. Hayati J. Biosci. 2016, 23, 173–180. [Google Scholar] [CrossRef]
- Eisendle, M.; Oberegger, H.; Buttinger, R.; Illmer, P.; Haas, H. Biosynthesis and, uptake of siderophoRes. is controlled by the PacC- mediated ambient-pH regulatory system in Aspergillus nidulans. Eukaryot. Cell 2004, 3, 561–563. [Google Scholar] [CrossRef] [Green Version]
- Tanimoto, E. Regulation of root growth by plant hormones-roles for auxin and gibberellin. CRC Crit. Rev. Plant Sci. 2005, 24, 249–265. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 4, 1327–1350. [Google Scholar] [CrossRef]
- Peralta, K.D.; Araya, T.; Valenzuela, S.; Sossa, K.; Martínez, M.; Peña-Cortés, H.; Sanfuentes, E. Production of phytohormones, siderophoRes. and population fluctuation of two root-promoting rhizobacteria in Eucalyptus globulus cuttings. World J. Microbiol. Biotechnol. 2012, 28, 2003–2014. [Google Scholar] [CrossRef]
- Mates, A.D.P.K.; de Carvalho Pontes, N.; de Almeida Halfeld-Vieira, B. Bacillus velezensis GF267 as a multi-site antagonist for the control of tomato bacterial spot. Biol. Control 2019, 137, 104–113. [Google Scholar]
- Moreira, R.R.; Nesi, C.N.; De Mio, L.L.M. Bacillus spp. and pseudomonas putida as inhibitors of the Colletotrichum acutatum group and potential to control Glomerella leaf spot. Biol. Control 2014, 72, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Dennis, C.; Webbster, J. Antagonistic properties of species-groups of Trichoderma. Trans. Br. Mycol. Soc. 1971, 57, 41–48. [Google Scholar] [CrossRef]
- Huang, R.; Li, G.Q.; Zhang, J.; Yang, L.; Che, H.J.; Jiang, D.H.; Huang, H.C. Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology 2011, 101, 859–869. [Google Scholar] [CrossRef] [Green Version]
- Jangir, M.; Pathak, R.; Sharma, S.; Sharma, S. Biocontrol mechanisms of Bacillus sp., isolated from tomato rhizosphere, against Fusarium oxysporum f. sp. lycopersici. Biol. Control 2018, 123, 60–70. [Google Scholar] [CrossRef]
- Kim, J.D. Antifungal Activity of Lactic Acid Bacteria Isolated from Kimchi against Aspergillus Fumigatus. Mycobiology 2005, 33, 210–214. [Google Scholar] [CrossRef] [Green Version]
- Essghaier, B.; Fardeau, M.C.; Cayol, J.L.; Hajlaoui, M.R.; Boudabous, A.; Jijakli, H.; Sadfi-Zouaoui, N. Biological control of grey mold in strawberry fruits by halophilic bacteria. J. Appl. Microbiol. 2009, 106, 833–846. [Google Scholar] [CrossRef] [Green Version]
- Alijani, Z.; Amini, J.; Ashengroph, M.; Bahramnejad, B. Antifungal activity of volatile compounds produced by Staphylococcus sciuri strain MarR44 and its potential for the biocontrol of Colletotrichum nymphaeae, causal agent strawberry anthracnose. Int. J. Food Microbiol. 2019, 307, 108276. [Google Scholar] [CrossRef]
- Naeem-Abadi, T.; Keshavarzi, M. Involvement of protective enzymes and phenols in decay (Penicillium expansum) resistance in apple. J. Crop Prot. 2016, 5, 349–357. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beers, R.F.J.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 2002, 215, 1022–1030. [Google Scholar] [CrossRef]
- Wang, S.H.; Yang, Z.M.; Yang, H.; Lu, B.; Li, S.Q.; Lu, Y.P. Copper-induced stress and antioxidative responses in roots of Brassica juncea L. Bot. Bull. Acad. Sin. 2004, 45, 203–212. [Google Scholar]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]

| Treatments | Dual-Culture Test | Paper-Disc Test | ||||
|---|---|---|---|---|---|---|
| Inhibition Zone (cm) | Inhibition Im (%) | t-Value | Colony Diameter (cm2) | Inhibition Im (%) | t-Value | |
| MarR61-01 | 1.4 ± 0.29 | - | −8.828 ** | 17.19 ± 1.50 | 71.72 | 36.63 ** |
| Control | 0.0 ± 0.00 | - | 60.80 ± 1.41 | - | ||
| Treatments | Mycelial Growth Test | Conidia Germination Test | ||||
|---|---|---|---|---|---|---|
| Colony Diameter (cm2) | Inhibition Im (%) | t-Value | Germinated Conidia (n) | Inhibition Ic (%) | t-Value | |
| MarR61-01 | 17.14 ± 5.80 | 65.01 | 5.36 | 31 ± 12.00 | 71.63 | 8.34 ** |
| Control | 48.99 ± 8.40 | - | 109.3 ± 10.90 | - | ||
| Concentration of Pigment (µg/mL) | Biomass Weight (gr) | Inhibition Ib (%) |
|---|---|---|
| 0 | 3.08 ± 0.56 a | - |
| 20 | 0.62± 0.11 bc | 59.74 |
| 120 | 0.35 ± 0.037 cd | 88.63 |
| 220 | 0.19 ± 0.026 d | 93.83 |
| 320 | 0.196 ± 0.032 d | 93.63 |
| 420 | 0.18 ± 0.02 d | 94.15 |
| Treatments | Living Cells | Volatile Compounds | ||||
|---|---|---|---|---|---|---|
| Disease Severity | Biocontrol Efficacy (%) | t-Value | Disease Severity | Biocontrol Efficacy (%) | t-Value | |
| MarR61-01 | 0.10 ± 0.04 | 64.28 | 7.3 ** | 0.11 ± 0.04 | 63.33 | 3.94 ** |
| Control | 0.28 ± 0.06 | 0 | 0.3 ± 0.11 | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alijani, Z.; Amini, J.; Karimi, K.; Pertot, I. Characterization of the Mechanism of Action of Serratia rubidaea Mar61-01 against Botrytis cinerea in Strawberries. Plants 2023, 12, 154. https://doi.org/10.3390/plants12010154
Alijani Z, Amini J, Karimi K, Pertot I. Characterization of the Mechanism of Action of Serratia rubidaea Mar61-01 against Botrytis cinerea in Strawberries. Plants. 2023; 12(1):154. https://doi.org/10.3390/plants12010154
Chicago/Turabian StyleAlijani, Zahra, Jahanshir Amini, Kaivan Karimi, and Ilaria Pertot. 2023. "Characterization of the Mechanism of Action of Serratia rubidaea Mar61-01 against Botrytis cinerea in Strawberries" Plants 12, no. 1: 154. https://doi.org/10.3390/plants12010154
APA StyleAlijani, Z., Amini, J., Karimi, K., & Pertot, I. (2023). Characterization of the Mechanism of Action of Serratia rubidaea Mar61-01 against Botrytis cinerea in Strawberries. Plants, 12(1), 154. https://doi.org/10.3390/plants12010154