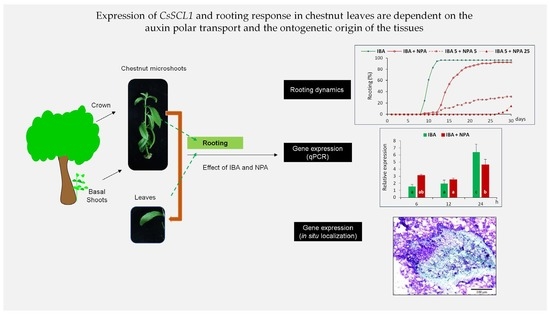
Expression of CsSCL1 and Rooting Response in Chestnut Leaves Are Dependent on the Auxin Polar Transport and the Ontogenetic Origin of the Tissues
Abstract
:1. Introduction
2. Results
2.1. Leaves Exhibited a Similar Rooting Response to the Donor Shoots
2.2. The Inhibitory Effect of NPA on Rooting Ability of Chestnut Depends on the Timing of Application
2.3. CsSCL1 Is Highly Expressed in Roots
2.4. CsSCL1 Is Induced by Auxin Only in Rooting-Competent Leaves
2.5. NPA Reduces Peak Expression of CsSCL1 during Induction of Adventitious Roots
2.6. Distribution of CsSCL1 Transcripts Is Affected by Maturation and by NPA during the Induction of Adventitious Roots
2.7. CsSCL1 Is Expressed in the Root Primordia and the Quiescent Center of the Root
2.8. Usefulness of the Leaf Rooting System in Different Woody Species
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Adventitious Roots Induction
4.3. RNA Extraction and Quantification
4.4. Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qPCR)
4.5. In Situ Hybridization and Histological Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchez, M.C.; Vieitez, A.M. In Vitro Morphogenetic Competence of Basal Sprouts and Crown Branches of Mature Chestnut. Tree Physiol. 1991, 8, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Day, M.E.; Greenwood, M.S.; Diaz-Sala, C. Age- and Size-Related Trends in Woody Plant Shoot Development: Regulatory Pathways and Evidence for Genetic Control. Tree Physiol. 2002, 22, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonga, J.M. Vegetative Propagation in Relation to Juvenility, Maturity, and Rejuvenation. In Tissue Culture in Forestry; Bonga, J.M., Durzan, D.J., Eds.; Springer: Dordrecht, The Netherlands, 1982; pp. 387–412. Available online: https://link.springer.com/chapter/10.1007/978-94-017-3538-4_13 (accessed on 7 July 2023).
- Sanchez, M.C.; San-Jose, M.C.; Ballester, A.; Vieitez, A.M. Requirements for in vitro Rooting of Quercus robur and Q. rubra Shoots Derived from Mature Trees. Tree Physiol. 1996, 16, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Vielba, J.M.; Vidal, N.; San Jose, M.C.; Rico, S.; Sanchez, C. Recent Advances in Adventitious Root Formation in Chestnut. Plants 2020, 9, 1543. [Google Scholar] [CrossRef]
- Berleth, T.; Sachs, T. Plant Morphogenesis: Long-Distance Coordination and Local Patterning. Curr. Opin. Plant Biol. 2001, 4, 57–62. [Google Scholar] [CrossRef]
- Casimiro, I.; Marchant, A.; Bhalerao, R.P.; Beeckman, T.; Dhooge, S.; Swarup, R.; Graham, N.; Inze, D.; Sandberg, G.; Casero, P.J.; et al. Auxin Transport Promotes Arabidopsis Lateral Root Initiation. Plant Cell 2001, 13, 843–852. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chen, Y.; Jiang, C.; Lu, M.Z.; Zhang, J. Exogenous Hormones Supplementation Improve Adventitious Root Formation in Woody Plants. Front. Bioeng. Biotechnol. 2022, 10, 1009531. [Google Scholar] [CrossRef]
- Paponov, I.A.; Paponov, M.; Teale, W.; Menges, M.; Chakrabortee, S.; Murray, J.A.; Palme, K. Comprehensive Transcriptome Analysis of Auxin Responses in Arabidopsis. Mol. Plant 2008, 1, 321–337. [Google Scholar] [CrossRef]
- Leyser, O. Auxin Signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef] [Green Version]
- Parry, G.; Calderon-Villalobos, L.I.; Prigge, M.; Peret, B.; Dharmasiri, S.; Itoh, H.; Lechner, E.; Gray, W.M.; Bennett, M.; Estelle, M. Complex Regulation of the TIR1/AFB Family of Auxin Receptors. Proc. Natl. Acad. Sci. USA 2009, 106, 22540–22545. [Google Scholar] [CrossRef]
- Li, S.B.; Xie, Z.Z.; Hu, C.G.; Zhang, J.Z. A Review of Auxin Response Factors (ARFs) in Plants. Front. Plant Sci. 2016, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Perez-Henriquez, P.; Yang, Z. Extranuclear Auxin Signaling: A New Insight into Auxin’s Versatility. New Phytol. 2023, 237, 1115–1121. [Google Scholar] [CrossRef]
- Gonin, M.; Bergougnoux, V.; Nguyen, T.D.; Gantet, P.; Champion, A. What Makes Adventitious Roots? Plants 2019, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Geisler, M.M. A Retro-Perspective on Auxin Transport. Front. Plant Sci. 2021, 12, 756968. [Google Scholar] [CrossRef]
- Kong, M.; Liu, X.; Sun, L.; Tan, S. Molecular Mechanisms of N-1-Naphthylphthalamic Acid, a Chemical Tool in Plant Biology and Agriculture. Mol. Hortic. 2022, 2, 22. [Google Scholar] [CrossRef]
- Woo, H.; Hackett, W.P.; Das, A. Differential Expression of a Chlorophyll a/b Binding Protein Gene and a Proline Rich Protein Gene in Juvenile and Mature Phase English Ivy (Hedera Helix). Physiol. Plant. 1994, 92, 69–78. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Smith, A.G.; Hackett, W.P. Localized Expression of a Proline-rich Protein Gene in Juvenile and Mature Ivy Petioles in Relation to Rooting Competence. Physiol. Plant. 1995, 93, 207–216. [Google Scholar] [CrossRef]
- Diaz-Sala, C.; Hutchison, K.W.; Goldfarb, B.; Greenwood, M.S. Maturation-related Loss in Rooting Competence by Loblolly Pine Stem Cuttings: The Role of Auxin Transport, Metabolism and Tissue Sensitivity. Physiol. Plant. 1996, 97, 481–490. [Google Scholar] [CrossRef]
- Sánchez, C.; Vielba, J.M.; Ferro, E.; Covelo, G.; Solé, A.; Abarca, D.; de Mier, B.S.; Díaz-Sala, C. Two SCARECROW-LIKE Genes Are Induced in Response to Exogenous Auxin in Rooting-Competent Cuttings of Distantly Related Forest Species. Tree Physiol. 2007, 27, 1459–1470. [Google Scholar] [CrossRef] [Green Version]
- Abarca, D.; Pizarro, A.; Hernandez, I.; Sanchez, C.; Solana, S.P.; Del Amo, A.; Carneros, E.; Diaz-Sala, C. The GRAS Gene Family in Pine: Transcript Expression Patterns Associated with the Maturation-Related Decline of Competence to Form Adventitious Roots. BMC Plant Biol. 2014, 14, 354. [Google Scholar] [CrossRef] [Green Version]
- Abu-abied, M.; Szwerdszarf, D.; Mordehaev, I.; Yaniv, Y.; Levinkron, S.; Rubinstein, M.; Riov, J.; Ophir, R.; Sadot, E. Gene Expression Profiling in Juvenile and Mature Cuttings of Eucalyptus grandis Reveals the Importance of Microtubule Remodeling during Adventitious Root Formation. BMC Genom. 2014, 15, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, M.E.; Woeste, K.E.; Pijut, P.M. Localized Gene Expression Changes during Adventitious Root Formation in Black Walnut (Juglans nigra L.). Tree Physiol. 2018, 38, 877–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, A.; Hosseini, S.A.; Hajirezaei, M.R.; Druege, U.; Geelen, D. Adventitious Rooting Declines with the Vegetative to Reproductive Switch and Involves a Changed Auxin Homeostasis. J. Exp. Bot. 2015, 66, 1437–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballester, A.; San-José, M.C.; Vidal, N.; Fernández-Lorenzo, J.L.; Vieitez, A.M. Anatomical and Biochemical Events During in Vitro rooting of Microcuttings from Juvenile and Mature Phases of Chestnut. Ann. Bot. 1999, 83, 619–629. [Google Scholar] [CrossRef]
- Gil, B.; Pastoriza, E.; Ballester, A.; Sánchez, C. Isolation and Characterization of a cDNA from Quercus Robur Differentially Expressed in Juvenile-like and Mature Shoots. Tree Physiol. 2003, 23, 633–640. [Google Scholar] [CrossRef] [Green Version]
- Vielba, J.M.; Díaz-Sala, C.; Ferro, E.; Rico, S.; Lamprecht, M.; Abarca, D.; Ballester, A.; Sánchez, C. CsSCL1 Is Differentially Regulated upon Maturation in Chestnut Microshoots and Is Specifically Expressed in Rooting-Competent Cells. Tree Physiol. 2011, 31, 1152–1160. [Google Scholar] [CrossRef]
- Valladares, S.; Varas, E.; Vielba, J.M.; Vidal, N.; Codesido, V.; Castro, R.; Sanchez, C. Expression of a Rap2. 12 like-1 ERF Gene during Adventitious Rooting of Chestnut and Oak Microshoots. Isr. J. Plant Sci. 2020, 67, 69–82. [Google Scholar] [CrossRef]
- Silverstone, A.L.; Ciampaglio, C.N.; Sun, T. The Arabidopsis RGA Gene Encodes a Transcriptional Regulator Repressing the Gibberellin Signal Transduction Pathway. Plant Cell 1998, 10, 155–169. [Google Scholar] [CrossRef] [Green Version]
- Stuurman, J.; Jaggi, F.; Kuhlemeier, C. Shoot Meristem Maintenance Is Controlled by a GRAS-Gene Mediated Signal from Differentiating Cells. Genes Dev. 2002, 16, 2213–2218. [Google Scholar] [CrossRef] [Green Version]
- Bolle, C. Structure and Evolution of Plant GRAS Family Proteins. In Plant Transcription Factors; Gonzalez, D.H., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 153–161. [Google Scholar] [CrossRef]
- Vidal, N.; Arellano, G.; San-Jose, M.C.; Vieitez, A.M.; Ballester, A. Developmental Stages during the Rooting of In-Vitro-Cultured Quercus robur Shoots from Material of Juvenile and Mature Origin. Tree Physiol. 2003, 23, 1247–1254. [Google Scholar] [CrossRef]
- Fortanier, E.J.; Jonkers, H. Juvenility and Maturity of Plants as Influenced by Their Ontogenetical and Physiological Ageing. In Symposium on Juvenility in Woody Perennials 56; ISHS: Leuven, Belgium, 1975; pp. 37–44. [Google Scholar]
- Hackett, W.P.; Murray, J.R. Maturation and Rejuvenation in Woody Species. In Micropropagation of Woody Plants; Ahuja, M.R., Ed.; Kluwer Academic Publishers: London, UK, 1993; pp. 93–105. [Google Scholar]
- Greenwood, M.S. Juvenility and Maturation in Conifers: Current Concepts. Tree Physiol. 1995, 15, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Vielba, J.M.; Varas, E.; Rico, S.; Covelo, P.; Sánchez, C. Auxin-Mediated Expression of a GH3 Gene in Relation to Ontogenic State in Chestnut. Trees 2016, 30, 2237–2252. [Google Scholar] [CrossRef]
- Vielba, J.M.; Rico, S.; Sevgin, N.; Castro-Camba, R.; Covelo, P.; Vidal, N.; Sanchez, C. Transcriptomics Analysis Reveals a Putative Role for Hormone Signaling and MADS-Box Genes in Mature Chestnut Shoots Rooting Recalcitrance. Plants 2022, 11, 3486. [Google Scholar] [CrossRef]
- Rose, R.J.; Wang, X.-D.; Nolan, K.E.; Rolfe, B.G. Root Meristems in Medicago truncatula Tissue Culture Arise from Vascular-Derived Procambial-like Cells in a Process Regulated by Ethylene. J. Exp. Bot. 2006, 57, 2227–2235. [Google Scholar] [CrossRef] [Green Version]
- Xu, L. De Novo Root Regeneration from Leaf Explants: Wounding, Auxin, and Cell Fate Transition. Curr. Opin. Plant Biol. 2018, 41, 39–45. [Google Scholar] [CrossRef]
- Chen, X.; Qu, Y.; Sheng, L.; Liu, J.; Huang, H.; Xu, L. A Simple Method Suitable to Study de Novo Root Organogenesis. Front. Plant Sci. 2014, 5, 208. [Google Scholar] [CrossRef] [Green Version]
- Maury, S.; Sow, M.D.; Le Gac, A.L.; Genitoni, J.; Lafon-Placette, C.; Mozgova, I. Phytohormone and Chromatin Crosstalk: The Missing Link for Developmental Plasticity? Front. Plant Sci. 2019, 10, 395. [Google Scholar] [CrossRef]
- Raihan, T.; Geneve, R.L.; Perry, S.E.; Rodriguez Lopez, C.M. The Regulation of Plant Vegetative Phase Transition and Rejuvenation: miRNAs, a Key Regulator. Epigenomes 2021, 5, 24. [Google Scholar] [CrossRef]
- Ayala, P.G.; Acevedo, R.M.; Luna, C.V.; Rivarola, M.; Acuña, C.; Marcucci Poltri, S.; Gonzalez, A.M.; Sansberro, P.A. Transcriptome Dynamics of Rooting Zone and Leaves during In Vitro Adventitious Root Formation in Eucalyptus nitens. Plants 2022, 11, 3301. [Google Scholar] [CrossRef]
- Di Laurenzio, L.; Wysocka-Diller, J.; Malamy, J.E.; Pysh, L.; Helariutta, Y.; Freshour, G.; Hahn, M.G.; Feldmann, K.A.; Benfey, P.N. The SCARECROW Gene Regulates an Asymmetric Cell Division That Is Essential for Generating the Radial Organization of the Arabidopsis Root. Cell 1996, 86, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Wysocka-Diller, J.W.; Helariutta, Y.; Fukaki, H.; Malamy, J.E.; Benfey, P.N. Molecular Analysis of SCARECROW Function Reveals a Radial Patterning Mechanism Common to Root and Shoot. Development 2000, 127, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.; Heidstra, R.; Wildwater, M.; Scheres, B. SCARECROW Is Involved in Positioning the Stem Cell Niche in the Arabidopsis Root Meristem. Genes Dev. 2003, 17, 354–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwood, M.S.; Cui, X.; Xu, F. Response to Auxin Changes during Maturation-Related Loss of Adventitious Rooting Competence in Loblolly Pine (Pinus taeda) Stem Cuttings. Physiol. Plant. 2001, 111, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.Y.; Zhang, K.; Yuan, Y.; Ni, P.; Ma, J.; Liu, H.; Gong, S.; Shun Yang, G.; Bai, M. A Simple, Rapid, and Quantifiable System for Studying Adventitious Root Formation in Grapevine. Plant Growth Regul. 2022, 98, 117–126. [Google Scholar] [CrossRef]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin Control of Root Development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001537. [Google Scholar] [CrossRef] [Green Version]
- Clark, N.M.; de Luis Balaguer, M.A.; Sozzani, R. Experimental Data and Computational Modeling Link Auxin Gradient and Development in the Arabidopsis Root. Front. Plant Sci. 2014, 5, 328. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhang, Y.; Fang, X.; Tran, S.; Zhai, N.; Yang, Z.; Guo, F.; Chen, L.; Yu, J.; Ison, M.S. Transcriptional Landscapes of de Novo Root Regeneration from Detached Arabidopsis Leaves Revealed by Time-Lapse and Single-Cell RNA Sequencing Analyses. Plant Commun. 2022, 3, 100306. [Google Scholar] [CrossRef]
- Gutierrez, L.; Bussell, J.D.; Pacurar, D.I.; Schwambach, J.; Pacurar, M.; Bellini, C. Phenotypic Plasticity of Adventitious Rooting in Arabidopsis Is Controlled by Complex Regulation of AUXIN RESPONSE FACTOR Transcripts and MicroRNA Abundance. Plant Cell 2009, 21, 3119–3132. [Google Scholar] [CrossRef] [Green Version]
- Sukumar, P.; Maloney, G.S.; Muday, G.K. Localized Induction of the ATP-Binding Cassette B19 Auxin Transporter Enhances Adventitious Root Formation in Arabidopsis. Plant Physiol. 2013, 162, 1392–1405. [Google Scholar] [CrossRef]
- Lakehal, A.; Bellini, C. Control of Adventitious Root Formation: Insights into Synergistic and Antagonistic Hormonal Interactions. Physiol. Plant. 2019, 165, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; David, A.; Bhatla, S.C. Nitric Oxide Modulates Specific Steps of Auxin-Induced Adventitious Rooting in Sunflower. Plant Signal. Behav. 2010, 5, 1163–1166. [Google Scholar] [CrossRef]
- Negishi, N.; Nakahama, K.; Urata, N.; Kojima, M.; Sakakibara, H.; Kawaoka, A. Hormone Level Analysis on Adventitious Root Formation in Eucalyptus globulus. New For. 2014, 45, 577–587. [Google Scholar] [CrossRef]
- Mauriat, M.; Petterle, A.; Bellini, C.; Moritz, T. Gibberellins Inhibit Adventitious Rooting in Hybrid Aspen and Arabidopsis by Affecting Auxin Transport. Plant J. 2014, 78, 372–384. [Google Scholar] [CrossRef] [Green Version]
- Ahkami, A.H.; Melzer, M.; Ghaffari, M.R.; Pollmann, S.; Ghorbani Javid, M.; Shahinnia, F.; Hajirezaei, M.R.; Druege, U. Distribution of Indole-3-Acetic Acid in Petunia Hybrida Shoot Tip Cuttings and Relationship between Auxin Transport, Carbohydrate Metabolism and Adventitious Root Formation. Planta 2013, 238, 499–517. [Google Scholar] [CrossRef] [Green Version]
- Teale, W.; Palme, K. Naphthylphthalamic Acid and the Mechanism of Polar Auxin Transport. J. Exp. Bot. 2018, 69, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Teale, W.D.; Pasternak, T.; Dal Bosco, C.; Dovzhenko, A.; Kratzat, K.; Bildl, W.; Schworer, M.; Falk, T.; Ruperti, B.; Schaefer, J.V.; et al. Flavonol-Mediated Stabilization of PIN Efflux Complexes Regulates Polar Auxin Transport. EMBO J. 2021, 40, e104416. [Google Scholar] [CrossRef]
- Abas, L.; Kolb, M.; Stadlmann, J.; Janacek, D.P.; Lukic, K.; Schwechheimer, C.; Sazanov, L.A.; Mach, L.; Friml, J.; Hammes, U.Z. Naphthylphthalamic Acid Associates with and Inhibits PIN Auxin Transporters. Proc. Natl. Acad. Sci. USA 2021, 118, e2020857118. [Google Scholar] [CrossRef]
- Guan, L.; Li, Y.; Huang, K.; Cheng, Z.M. Auxin Regulation and MdPIN Expression during Adventitious Root Initiation in Apple Cuttings. Hortic. Res. 2020, 7, 143. [Google Scholar] [CrossRef]
- Velada, I.; Cardoso, H.; Porfirio, S.; Peixe, A. Expression Profile of PIN-Formed Auxin Efflux Carrier Genes during IBA-Induced In Vitro Adventitious Rooting in Olea europaea L. Plants 2020, 9, 185. [Google Scholar] [CrossRef] [Green Version]
- Gresshoff, P.M.; Doy, C.H. Development and Differentiation of Haploid Lycopersicon esculentum (Tomato). Planta 1972, 107, 161–170. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]










| Explant | Rooting (%) | N° Roots | NBR (%) | LRL (cm) | MRT (days) |
|---|---|---|---|---|---|
| Shoot | 94.8 ± 3.2 (a) | 8.4 ± 0.9 (a) | 51.7 ± 19.8 (a) | 2.6 ± 1.8 (b) | 11.3 ± 0.7 (b) |
| Leaf | 96.3 ± 1.9 (a) | 5.8 ± 0.4 (b) | 8.7 ± 0.8 (b) | 3.7 ± 3.1 (a) | 9.3 ± 0.3 (a) |
| Leaf segment | 69.1 ± 5.0 (b) | 2.9 ± 0.2 (c) | 2.9 ± 1.5 (b) | 2.5 ± 0.8 (b) | 11.1 ± 0.2 (b) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varas, E.; Valladares, S.; Vielba, J.; Vidal, N.; Sánchez, C. Expression of CsSCL1 and Rooting Response in Chestnut Leaves Are Dependent on the Auxin Polar Transport and the Ontogenetic Origin of the Tissues. Plants 2023, 12, 2657. https://doi.org/10.3390/plants12142657
Varas E, Valladares S, Vielba J, Vidal N, Sánchez C. Expression of CsSCL1 and Rooting Response in Chestnut Leaves Are Dependent on the Auxin Polar Transport and the Ontogenetic Origin of the Tissues. Plants. 2023; 12(14):2657. https://doi.org/10.3390/plants12142657
Chicago/Turabian StyleVaras, Elena, Silvia Valladares, Jesús Vielba, Nieves Vidal, and Conchi Sánchez. 2023. "Expression of CsSCL1 and Rooting Response in Chestnut Leaves Are Dependent on the Auxin Polar Transport and the Ontogenetic Origin of the Tissues" Plants 12, no. 14: 2657. https://doi.org/10.3390/plants12142657
APA StyleVaras, E., Valladares, S., Vielba, J., Vidal, N., & Sánchez, C. (2023). Expression of CsSCL1 and Rooting Response in Chestnut Leaves Are Dependent on the Auxin Polar Transport and the Ontogenetic Origin of the Tissues. Plants, 12(14), 2657. https://doi.org/10.3390/plants12142657