Phytochemicals from Pterocarpus angolensis DC and Their Cytotoxic Activities against Breast Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolated Compounds
2.2. Physical Properties of the Isolated Compounds
2.3. Structural Analysis
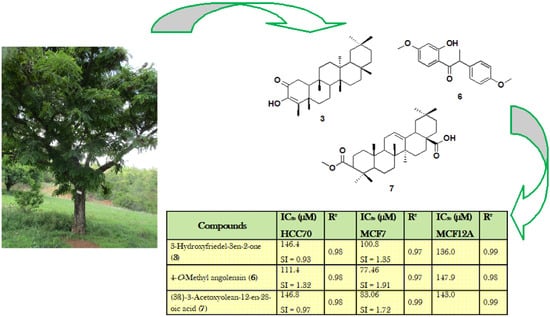
2.4. Cytotoxicity Activity
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Plant Material
3.3. Sequential Extraction
3.4. Nuclear Magnetic Resonance Spectroscopy
3.5. Infrared Spectroscopy
3.6. Mass Spectroscopy
3.7. In Vitro Cytotoxicity Assay
The Resazurin Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Govaerts, R.; Nic Lughadha, E.; Black, N.; Turner, R.; Paton, A. The World Checklist of Vascular Plants is a continuously updated resource for exploring global plant diversity. Sci. Data 2021, 8, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Abouelela, M.E.; Abdelhamid, R.A.; Orabi, M.A.A. Phytochemical constituents of plant species of Pterocarpus (F: Leguminosae): A review. Int. J. Pharmacol. Phytochem. Res. 2019, 11, 264–281. [Google Scholar] [CrossRef]
- van Wyk, B. Field Guide to Trees of Southern Africa; Struik Publishers (Pty) Ltd.: Cape Town, South Africa, 1997; pp. 462–464. ISBN 9781868259229. [Google Scholar]
- Banda, T.; Schwartz, M.W.; Caro, T. Effects of fire on germination of Pterocarpus angolensis. For. Ecol. Manag 2006, 233, 116–120. [Google Scholar] [CrossRef]
- Palgrave, M.C. Keith Coates Palgrave Trees of Southern Africa; Struik Publishers: Cape Town, South Africa, 2002; pp. 469–470. ISBN 978-1-86872-389-8. [Google Scholar]
- Maroyi, A. Traditional use of medicinal plants in south-central Zimbabwe: Review and perspectives. J. Ethnobiol. Ethnomed. 2013, 9, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.C.; Uetimane, E., Jr.; Lhate, I.A.; Terziev, N. Anatomical characteristics, properties and use of traditionally used and lesser-known wood species from Mozambique: A literature review. Wood Sci. Technol. 2008, 42, 453–472. [Google Scholar] [CrossRef]
- King, F.E.; King, T.J.; Warwick, A.J. The Chemistry of Extractives from Hardwoods. Part V I P Constituents of Muninga, the Heartwood of Pterocarpus angolensis. B: 2: 4Dihydroxyphenyl l-p-Methoxyphenylethyl Ketone (Angolensin). J. Chem. Soc. 1952, 354, 1920–1924. [Google Scholar] [CrossRef]
- Samie, A.; Housein, A.; Lall, N.; Meyer, J.J. Crude extracts of, and purified compounds from, Pterocarpus angolensis, and the essential oil of Lippia javanica: Their in vitro cytotoxicities and activities against selected bacteria and Entamoeba histolytica. Ann. Trop. Med. Parasitol. 2009, 103, 427–439. [Google Scholar] [CrossRef]
- Abubakar, M.N.; Majinda, R.R.T. GC-MS analysis and preliminary antimicrobial activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines 2016, 3, 3. [Google Scholar] [CrossRef]
- King, F.; Jurd, L. The chemistry of extractives from hardwoods. Part VIII. The isolation of 5: 4′-dihydroxy-7-methoxyisoflavone (prunetin) from the heartwood of Pterocarpus angolensis and a synthesis of 7: 4′-dihydroxy-5-methoxyisoflavone hitherto known as prunusetin. J. Chem. Soc. 1952, 3211–3215. [Google Scholar] [CrossRef]
- Bezuidenhoudt, B.C.; Brandt, E.V.; Roux, D.G.; VAN Rooyen, P.H. Novel α-methyldeoxybenzoins from the heartwood of Pterocarpus angolensis DC: Absolute configuration and conformation of the first sesquiterpenylangolensis, and X-ray crystal structure of 4-O-α-cadinylangolensin. J. Chem. Soc. Perkin Tran. 1 1980, 2179–2183. [Google Scholar] [CrossRef]
- Chipinga, J.V.; Kamanula, J.F.; Ben moyo, P.B. Efficacy of Pterocarpus angolensis crude extracts against Candida krusei, Staphylococcus aureus, Streptococcus agalactiae and Escherichia coli. J. Med. Asso. Malawi 2018, 30, 219–224. [Google Scholar] [CrossRef]
- Zininga, T.; Anokwuru, C.; Sigidi, M.; Tshisikhawe, M.; Ramaite, I.; Traoré, A.; Potgieter, N. Extracts obtained from Pterocarpus angolensis DC and Ziziphus mucronata exhibit antiplasmodial activity and inhibit heat shock protein 70 (Hsp70) function. Molecules 2017, 22, 1224. [Google Scholar] [CrossRef] [PubMed]
- Ssemakalu, C.C.; Razwinani, M.; Maepa, M.J.; Motaung, K.S. Pterocarpus angolensis crude extracts induce the expression of collagen type ii in articular cartilage in vitro. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 76–84. [Google Scholar] [CrossRef]
- Karimi, A.; Krähmer, A.; Herwig, N.; Schulz, H.; Hadian, J.; Meiners, T. Variation of Secondary Metabolite Profile of Zataria multiflora Boiss. Populations Linked to Geographic, Climatic, and Edaphic Factors. Front. Plant Sci. 2020, 11, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Klass, J.; Tinto, W.F.; Mclean, S.; Reynolds, W.F. Friedelane triterpenoids from Perztassa compta: Complete 1H and 13C assignments by 2D NMR spectroscopy. J. Nat. Prod. 1992, 55, 1626–1630. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.; Bryce, D.L. Spectrometric Identification of Organic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 150–195. ISBN 139780470926222. [Google Scholar]
- Guo, W.J.; Guo, S.X.; Yang, J.S.; Chen, X.M.; Xiao, P.G. Triterpenes and steroids from Armillaria mellea Vahl. ex Fr. Biochem. Syst. Eco. 2007, 35, 790–793. [Google Scholar] [CrossRef]
- Nazemi, M.; Khaledi, M.; Golshan, M.; Ghorbani, M.; Amiran, M.R.; Darvishi, A.; Rahmanian, O. Cytotoxicity activity and drug ability studies of stigmasterol Isolated from Marine Sponge Dysidea avara against oral epithelial cancer cell (KB/C152) and T-Lymphocytic leukemia cell line (Jurkat/E6-1). Asian Pac. J. Cancer Prev. 2020, 21, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Ahmad, V.U. Handbook of Natural Products, Volume 2 Pentacyclic Triterpenoids; Elsevier Science: Amsterdam, The Netherlands, 1994; pp. 1025–1065. ISBN 9780444882004. [Google Scholar]
- Blair, J.A.; Ongley, P.A.; Chiswell, J.; Griffiths, M.H.G. The isolation of lupeol from the bark of Heritiera utilis (Tarrietia utilis). Phytochemistry 1970, 9, 463–686. [Google Scholar] [CrossRef]
- Wahyuono, S.; Hoffmann, J.J.; Jolad, S.D.; Dentali, S.J. Triterpenoids of Amsonia grandiflora. Phytochemistry 1987, 26, 1213. [Google Scholar] [CrossRef]
- Erwin; Pusparohmana, W.R.; Safitry, R.D.; Marliana, E.; Usman; Kusuma, I.W. Isolation and characterization of stigmasterol and β-sitosterol from wood bark extract of Baccaurea macrocarpa Miq. Mull. Arg. Rasayan J. Chem. 2020, 13, 2552–2558. [Google Scholar] [CrossRef]
- Clark-Lewis, J.W.; Ramsay, G.C. The absolute configurations of (-)-angolensin and some related 1,2-diarylpropanes. Aust. J. Chem. 1965, 18, 1791–1797. [Google Scholar] [CrossRef]
- Tringali, C. Identification of bioactive metabolites from the bark of Pericopsis (A frormosia) laxiflora. Phytochem. Anal. 1995, 6, 289–291. Available online: https://fdocuments.net/document/identification-of-bioactive-metabolites-from-the-bark-of-pericopsis-afrormosia.html?page=1 (accessed on 12 August 2023). [CrossRef]
- Endo, M.; ShigetomI, K.; Mitsuhashi, S.; Igarashi, M.; Ubukata, M. Isolation, structure determination and structure–activity relationship of anti-toxoplasma triterpenoids from Quercus crispula Blume outer bark. J. Wood Sci. 2019, 65, 3–11. [Google Scholar] [CrossRef]
- Xiang, W.S.; Wang, J.D.; Wang, X.J.; Zhang, J. Two new components from Gnetum pendulum. J. Asian Nat. Prod. Res. 2008, 10, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Kashinatham, A. Studies on phytochemicals: Part XVII-Phenolics from the root Jatropha gosspifolia. Indian J. Chem. 1997, 36B, 1077–1078. Available online: http://nopr.niscpr.res.in/handle/123456789/57522 (accessed on 12 August 2023).
- Subash-Babu, P.; Li, D.K.; Alshatwi, A.A. In vitro cytotoxic potential of friedelin in human MCF-7 breast cancer cell: Regulate early expression of Cdkn2a and pRb1, neutralize mdm2-p53 amalgamation and functional stabilization of p53. J. Exp. Toxicol. Pathol. 2017, 69, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, H.; Zhaoa, Q. In vitro inhibitory effects of Friedelin on human liver cytochrome P450 enzymes. Pharm. Bio. 2018, 56, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Moiteiro, C.; Curto, M.M.; Mohamed, N.; Baileä, M.N.; Martinez-Diaz, R.; Lez-Coloma, A.G. Biovalorization of friedelane triterpenes derived from cork processing industry byproducts. J. Agri. Food Chem. 2006, 54, 3566–3571. [Google Scholar] [CrossRef]
- NyabokE, H.O.; Moraa, M.; Omosa, L.K.; Mbaveng, A.T.; Vaderament-Alexe, N.-N.; Masila, V.; Okemwa, E.; Heydenreich, M.; Efferth, T.; Kuete, V. Cytotoxicity of lupeol from the stem bark of Zanthoxylum gilletii against multi-factorial drug-resistant cancer cell lines. Invest. Med. Chem. Pharmacol. 2018, 1, 10–16. Available online: https://investchempharm.com/ (accessed on 10 August 2023).
- Tanaka, R.; Nakata, T.; Yamaguchi, C.; Wada, S.; Yamada, T.; Tokuda, H. Potential Anti-Tumor-Promoting Activity of 3α-Hydroxy-D:A-friedooleanan-2-one from the Stem Bark of Mallotus philippensis. Planta Med. 2008, 74, 413–416. [Google Scholar] [CrossRef]
- Kurniasih, N.; Supriadin, A.; Harneti, D.; Abdulah, R.; Mohamad Taib, M.N.A.; Unang Supratman, U. Ergosterol peroxide and stigmasterol from the stembark of Aglaia simplicifolia (Meliaceae) and their cytotoxic against HeLa cervical cancer cell lines. J. Kim. Val. 2021, 7, 46–51. [Google Scholar] [CrossRef]
- Jo, J.H.; Park, D.W.; Lee, C.; Min, C.K. Oleanolic acid 3-acetate, a minor element of ginsenosides, induces apoptotic cell death in ovarian carcinoma and endometrial carcinoma cells via the involvement of a reactive oxygen species–independent mitochondrial pathway. J. Ginseng Res. 2020, 44, 96–104. [Google Scholar] [CrossRef]
- De la Mare, J.-A.; Sterrenberg, J.N.; Sukhthankar, M.G.; Chiwakata, M.T.; Beukes, D.R.; Blatch, G.L.; Edkins, A.L. Assessment of potential anti-cancer stem cell activity of marine algal compounds using an in vitro mammosphere assay. Cancer Cell Int. 2013, 13, 39–55. [Google Scholar] [CrossRef] [PubMed]

| Compound Name | [α]D 19.9° | MP (°C) | IR (cm−1) | [M + H]+ |
|---|---|---|---|---|
| Friedelan-3-one (1) | −42.75 | 261–263 | 2928 (C-H), 1715 (C=O) | 427.3805 |
| 3α-Hydroxyfriedel-2-one (2) | −31.65 | 248–251 | 3444 (O-H), 2939 (C-H), 1662.6 (C=O) | 443.3447 |
| 3-Hydroxyfriedel-3-en-2-one (3) | −31.65 | 265–268 | 3377.5 (O-H), 2942.7 (C-H), 1709 (C=O), 1464 (C=C), 1385.6 (C-O) | 441.2928 |
| (3β)-lup-20(29)-en-3-ol (4) | +42.44 | 123–125 | 3314.4 (O-H), 2949.7 (C-H), 1605 (C=O) | 427.3677 |
| Stigmasta-5-22-dien-3-ol (5) | +42.44 | 169–170 | 3416.3 (OH), 2939 (C-H), 1683.4 (C=C), 1045.8 (C-O) | 413.3239 |
| (±)-4-O-Methylangolensin (6) | −62.71 | 70–71 | 3455 (O-H), 1608 (C=O) 1451 (C=C) 1230 (C-O) | 287.1341 |
| (3β)-3-Acetoxyolean-12-en-28-oic acid (7) | +57.49 | 119–122 | 2934 (C-H), 1732, 1697 (C=O), 1462 (C=C), 1246 (C-O) | 499.3795 |
| Tetradecyl (E)-ferulate (8) | +1.06 | 68–70 | 3381 (OH), 1709 (C=O) at 1635 | 391.2914 |
| Compound | IC50 (µM) HCC70 | R2 | IC50 (µM) MCF7 | R2 | IC50 (µM) MCF12A | R2 |
|---|---|---|---|---|---|---|
| Friedelan-3-one (1) | Non-Toxic | Non-Toxic | 43.86 | 0.52 | ||
| 3α-Hydroxyfriedel-2-one (2) | Non-Toxic | 157.0 SI = 0.53 | 0.91 | 84.20 | 0.76 | |
| 3-Hydroxyfriedel-3en-2-one (3) | 146.4 SI = 0.93 | 0.98 | 100.8 SI = 1.35 | 0.97 | 136.0 | 0.99 |
| (3β)-lup-20(29)-en-3-ol (4) | Non-Toxic | 148.7 SI = 0.25 | 0.61 | 36.60 | 0.65 | |
| Stigmasta-5-22-dien-3-ol (5) | 109.4 SI = 0.40 | 0.80 | 226.9 SI = 0.19 | 0.86 | 44.14 | 0.49 |
| (±)-4-O-methylangolensin (6) | 111.4 SI = 1.32 | 0.98 | 77.46 SI = 1.91 | 0.97 | 147.9 | 0.98 |
| (3β)-3-Acetoxyolean-12-en-28-oic acid (7) | 146.8 SI = 0.97 | 0.98 | 83.06 SI = 1.72 | 0.99 | 143.0 | 0.99 |
| Tetradecyl (E)-ferulate (8) | 128.2 SI = 0.05 | 0.56 | 223.1 SI = 0.03 | 0.86 | 6.95 | 0.54 |
| Etoposide | 44.83 SI = 0.24 | 0.93 | 15.38 SI = 0.71 | 0.91 | 10.94 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teclegeorgish, Z.W.; Mokgalaka, N.S.; Kemboi, D.; Krause, R.W.M.; Siwe-Noundou, X.; Nyemba, G.R.; Davison, C.; de la Mare, J.-A.; Tembu, V.J. Phytochemicals from Pterocarpus angolensis DC and Their Cytotoxic Activities against Breast Cancer Cells. Plants 2024, 13, 301. https://doi.org/10.3390/plants13020301
Teclegeorgish ZW, Mokgalaka NS, Kemboi D, Krause RWM, Siwe-Noundou X, Nyemba GR, Davison C, de la Mare J-A, Tembu VJ. Phytochemicals from Pterocarpus angolensis DC and Their Cytotoxic Activities against Breast Cancer Cells. Plants. 2024; 13(2):301. https://doi.org/10.3390/plants13020301
Chicago/Turabian StyleTeclegeorgish, Zecarias W., Ntebogeng S. Mokgalaka, Douglas Kemboi, Rui W. M. Krause, Xavier Siwe-Noundou, Getrude R. Nyemba, Candace Davison, Jo-Anne de la Mare, and Vuyelwa J. Tembu. 2024. "Phytochemicals from Pterocarpus angolensis DC and Their Cytotoxic Activities against Breast Cancer Cells" Plants 13, no. 2: 301. https://doi.org/10.3390/plants13020301
APA StyleTeclegeorgish, Z. W., Mokgalaka, N. S., Kemboi, D., Krause, R. W. M., Siwe-Noundou, X., Nyemba, G. R., Davison, C., de la Mare, J. -A., & Tembu, V. J. (2024). Phytochemicals from Pterocarpus angolensis DC and Their Cytotoxic Activities against Breast Cancer Cells. Plants, 13(2), 301. https://doi.org/10.3390/plants13020301