Nutritive Value and Bioactivities of a Halophyte Edible Plant: Crithmum maritimum L. (Sea Fennel)
Abstract
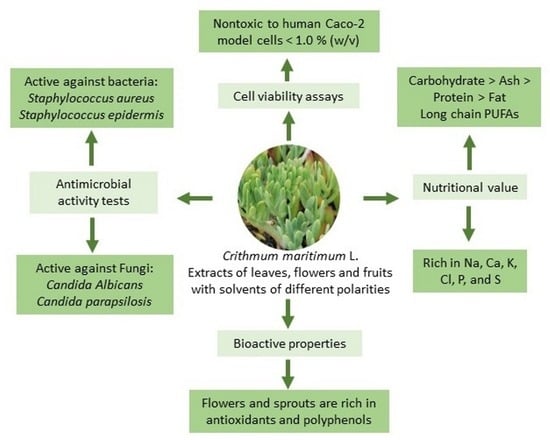
:1. Introduction
2. Results and Discussion
2.1. Centesimal Composition and Mineral Content
2.2. Photosynthetic Pigments and Antioxidant Capacity
2.3. Phenolic Content
2.4. Fatty Acid Content
2.5. Antibacterial Activity
2.6. Antifungal Activity
2.7. Caco-2 Cells Viability
2.8. Prebiotic Effect on Lactobacilli
3. Materials and Methods
3.1. Plant Material and Analytical Reagents
3.2. Centesimal Composition and Pigments
3.3. Profile of Minerals
3.4. Profile of Fatty Acids
3.5. Total Phenolic Content and Antioxidant Capacity
3.6. NMR Experiments
3.7. Antimicrobial Activity
3.7.1. Antibacterial Activity
3.7.2. Antifungal Activity
3.8. MTT Cell Viability Tests
3.9. Prebiotic Effect on Lactobacillus bulgaricus
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martins-Noguerol, R.; Matías, L.; Pérez-Ramos, I.M.; Moreira, X.; Francisco, M.; Pedroche, J.; DeAndrés-Gil, C.; Gutiérrez, E.; Salas, J.J.; Moreno-Pérez, A.J.; et al. Soil physicochemical properties associated with the yield and phytochemical composition of the edible halophyte Crithmum maritimum. Sci. Total Environ. 2023, 869, 161806. [Google Scholar] [CrossRef]
- Ventura, Y.; Myrzabayeva, M.; Alikulov, Z.; Omarov, R.; Khozin-Goldberg, I.; Sagi, M. Effects of salinity on flowering, morphology, biomass accumulation and leaf metabolites in an edible halophyte. AoB Plants 2014, 6, plu053. [Google Scholar] [CrossRef]
- Giungato, P.; Renna, M.; Rana, R.; Licen, S.; Barbieri, P. Characterization of dried and freeze-dried sea fennel (Crithmum maritimum L.) samples with headspace gas-chromatography/mass spectrometry and evaluation of an electronic nose discrimination potential. Food Res. Int. 2019, 115, 65–72. [Google Scholar] [CrossRef]
- Hulkko, L.S.S.; Turcios, A.E.; Kohnen, S.; Chaturvedi, T.; Papenbrock, J.; Thomsen, M.H. Cultivation and characterisation of Salicornia europaea, Tripolium pannonicum and Crithmum maritimum biomass for green biorefinery applications. Sci. Rep. 2022, 12, 20507. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, M.; Wang, S.; Cai, J.; Zhou, X.; Zhu, C. Nutritional characterization and changes in quality of Salicornia bigelovii Torr. during storage. LWT Food Sci. Technol. 2010, 43, 519–524. [Google Scholar] [CrossRef]
- Maciel, E.; Domingues, P.; Domingues, M.R.M.; Calado, R.; Lillebø, A. Halophyte plants from sustainable marine aquaponics are a valuable source of omega-3 polar lipids. Food Chem. 2020, 320, 126560. [Google Scholar] [CrossRef]
- Buhmann, A.; Papenbrock, J. An economic point of view of secondary compounds in halophytes. Funct. Plant Biol. 2013, 40, 952–967. [Google Scholar] [CrossRef]
- Parracho, T.; Vaz, D.C.; Veríssimo, P.; Ribeiro, V. Sand-Dune Plants from the Atlantic Coast of the Iberian Peninsula: Features and Applications. In Proceedings of the 1st International Conference on Water Energy Food and Sustainability ICoWEFS2021, Leiria, Portugal, 10–12 May 2021; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Pereira, C.G.; Barreira, L.; da Rosa Neng, N.; Nogueira, J.M.F.; Marques, C.; Santos, T.F.; Varela, J.; Custódio, L. Searching for new sources of innovative products for the food industry within halophyte aromatic plants: In vitro antioxidant activity and phenolic and mineral contents of infusions and decoctions of Crithmum maritimum L. Food Chem. Toxicol. 2017, 107 Pt B, 581–589. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fraga-Corral, M.; García-Oliveira, P.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Carpena, M.; Otero, P.; Gullón, P.; Prieto, M.A.; Simal-Gandara, J. Culinary and nutritional value of edible wild plants from northern Spain rich in phenolic compounds with potential health benefits. Food Funct. 2020, 11, 8493–8515. [Google Scholar] [CrossRef]
- Kadoglidou, K.; Irakli, M.; Boutsika, A.; Mellidou, I.; Maninis, N.; Sarrou, E.; Georgiadou, V.; Tourvas, N.; Krigas, N.; Moysiadis, T.; et al. Metabolomic Fingerprinting and Molecular Characterization of the Rock Samphire Germplasm Collection from the Balkan Botanic Garden of Kroussia, Northern Greece. Plants 2022, 11, 573. [Google Scholar] [CrossRef]
- Mekinić, G.I.; Šimat, V.; Ljubenkov, I.; Burčul, F.; Grga, M.; Mihajlovski, M.; Lončar, R.; Katalinić, V.; Skroza, D. Influence of the vegetation period on sea fennel, Crithmum maritimum L. (Apiaceae), phenolic composition, antioxidant, and anticholinesterase activities. Ind. Crops Prod. 2018, 124, 947–953. [Google Scholar] [CrossRef]
- Pedreiro, S.; Figueirinha, A.; Cavaleiro, C.; Cardoso, O.; Donato, M.M.; Salgueiro, L.; Ramos, F. Exploiting the Crithmum maritimum L. Aqueous Extracts and Essential Oil as Potential Preservatives in Food, Feed, Pharmaceutical and Cosmetic Industries. Antioxidants 2023, 12, 252. [Google Scholar] [CrossRef]
- Politeo, O.; Popović, M.; Veršić Bratinčević, M.; Kovačević, K.; Urlić, B.; Generalić Mekinić, I. Chemical Profiling of Sea Fennel (Crithmum maritimum L., Apiaceae) Essential Oils and Their Isolation Residual Waste-Waters. Plants 2023, 12, 214. [Google Scholar] [CrossRef]
- Jallali, I.; Zaouali, Y.; Missaoui, I.; Smeoui, A.; Abdelly, C.; Ksouri, R. Variability of antioxidant and antibacterial effects of essential oils and acetonic extracts of two edible halophytes: Crithmum maritimum L. and Inula crithmoїdes L. Food Chem. 2014, 145, 1031–1038. [Google Scholar] [CrossRef]
- Marongi, B.; Maxia, A.; Piras, A.; Porcedda, S.; Tuveri, E.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L. Isolation of Crithmum maritimum L. volatile oil by supercritical carbon dioxide extraction and biological assays. Nat. Prod. Res. 2007, 21, 1145–1150. [Google Scholar] [CrossRef]
- Pasias, I.N.; Ntakoulas, D.D.; Raptopoulou, K.; Gardeli, C.; Proestos, C. Chemical Composition of Essential Oils of Aromatic and Medicinal Herbs Cultivated in Greece-Benefits and Drawbacks. Foods 2021, 10, 2354. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T.; Deans, S.G.; Dorman, H.J. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Medica 2000, 66, 687–693. [Google Scholar] [CrossRef]
- Kamte, S.L.; Ranjbarian, F.; Cianfaglione, K.; Sut, S.; Dall’Acqua, S.; Bruno, M.; Afshar, F.H.; Iannarelli, R.; Benelli, G.; Cappellacci, L.; et al. Identification of highly effective antitrypanosomal compounds in essential oils from the Apiaceae family. Ecotoxicol. Environ. Saf. 2018, 156, 154–165. [Google Scholar] [CrossRef]
- Pereira, C.G.; Moraes, C.B.; Franco, C.H.; Feltrin, C.; Grougnet, R.; Barbosa, E.G.; Panciera, M.; Correia, C.R.D.; Rodrigues, M.J.; Custódio, L. In Vitro Anti-Trypanosoma cruzi Activity of Halophytes from Southern Portugal Reloaded: A Special Focus on Sea Fennel (Crithmum maritimum L.). Plants 2021, 10, 2235. [Google Scholar] [CrossRef]
- Tabari, M.A.; Rostami, A.; Khodashenas, A.; Maggi, F.; Petrelli, R.; Giordani, C.; Tapondjou, L.A.; Papa, F.; Zuo, Y.; Cianfaglione, K.; et al. Acaricidal activity, mode of action, and persistent efficacy of selected essential oils on the poultry red mite (Dermanyssus gallinae). Food Chem. Toxicol. 2020, 138, 111207. [Google Scholar] [CrossRef]
- Mustapha, M.B.; Zardi-Bergaoui, A.; Chaieb, I.; Flamini, G.; Ascrizzi, R.; Jannet, H.B. Chemical Composition and Insecticidal Activity of Crithmum Maritimum L. Essential Oil against Stored-Product Beetle Tribolium Castaneum. Chem. Biodivers. 2020, 17, e1900552. [Google Scholar] [CrossRef]
- Tsoukatou, M.; Tsitsimpikou, C.; Vagias, C.; Roussis, V. Chemical intra-Mediterranean variation and insecticidal activity of Crithmum maritimum. Z. Naturforsch. C J. Biosci. 2001, 56, 211–215. [Google Scholar] [CrossRef]
- Barbosa, P.; Lima, A.S.; Vieira, P.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Nematicidal activity of essential oils and volatiles derived from Portuguese aromatic flora against the pinewood nematode, Bursaphelenchus xylophilus. J. Nematol. 2010, 42, 8–16. [Google Scholar]
- Coiffard, L.; Piron-Frenet, M.; Amicel, L. Geographical variations of the constituents of the essential oil of Crithmum maritimum L., Apiaceae. Int. J. Cosmet. Sci. 1993, 15, 15–21. [Google Scholar] [CrossRef]
- Ozcan, M.M.; Pedro, L.G.; Figueiredo, A.C.; Barroso, J.G. Constituents of the essential oil of sea fennel (Crithmum maritimum L.) growing wild in Turkey. J. Med. Food 2006, 9, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Meot-Duros, L.; Cérantola, S.; Talarmin, H.; Le Meur, C.; Le Floch, G.; Magné, C. New antibacterial and cytotoxic activities of falcarindiol isolated in Crithmum maritimum L. leaf extract. Food Chem. Toxicol. 2010, 48, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Maoloni, A.; Cardinali, F.; Milanović, V.; Garofalo, C.; Osimani, A.; Mozzon, M.; Aquilanti, L. Microbiological safety and stability of novel green sauces made with sea fennel (Crithmum maritimum L.). Food Res. Int. 2022, 157, 111463. [Google Scholar] [CrossRef] [PubMed]
- Maoloni, A.; Cardinali, F.; Milanović, V.; Osimani, A.; Verdenelli, M.C.; Coman, M.M.; Aquilanti, L. Exploratory Study for Probiotic Enrichment of a Sea Fennel (Crithmum maritimum L.) Preserve in Brine. Foods 2022, 11, 2219. [Google Scholar] [CrossRef]
- Campana, R.; Tiboni, M.; Maggi, F.; Cappellacci, L.; Cianfaglione, K.; Morshedloo, M.R.; Frangipani, E.; Casettari, L. Comparative Analysis of the Antimicrobial Activity of Essential Oils and Their Formulated Microemulsions against Foodborne Pathogens and Spoilage Bacteria. Antibiotics 2022, 11, 447. [Google Scholar] [CrossRef]
- Sánchez-Faure, A.; Calvo, M.M.; Pérez-Jiménez, J.; Martín-Diana, A.B.; Rico, D.; Montero, M.P.; Gómez-Guillén, M.D.C.; López-Caballero, M.E.; Martínez-Alvarez, O. Exploring the potential of common iceplant, seaside arrowgrass and sea fennel as edible halophytic plants. Food Res. Int. 2020, 137, 109613. [Google Scholar] [CrossRef]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.; Gama, F.; Salazar, M.; Neng, N.R.; Nogueira, J.; Varela, J.; et al. Wild vs. cultivated halophytes: Nutritional and functional differences. Food Chem. 2020, 333, 127536. [Google Scholar] [CrossRef]
- Barreira, L.; Reseka, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Primitivo, M.J.; Neves, M.; Pires, C.L.; Cruz, P.F.; Brito, C.; Rodrigues, A.C.; de Carvalho, C.C.C.R.; Mortimer, M.M.; Moreno, M.J.; Brito, R.M.M.; et al. Edible flowers of Helichrysum italicum: Composition, nutritive value, and bioactivities. Food Res. Int. 2022, 157, 111399. [Google Scholar] [CrossRef] [PubMed]
- Sarrou, E.; Siomos, A.S.; Riccadona, S.; Aktsoglou, D.C.; Tsouvaltzis, P.; Angeli, A.; Franceschi, P.; Chatzopoulou, P.; Vrhovsek, U.; Martens, S. Improvement of sea fennel (Crithmum maritimum L.) nutritional value through iodine biofortification in a hydroponic floating system. Food Chem. 2019, 296, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Bertotti, T.; Primitivo, M.J.; Neves, M.; Pires, C.L.; Cruz, P.F.; Martins, P.A.T.; Rodrigues, A.C.; Moreno, M.J.; Brito, R.M.M.; et al. Corema album spp: Edible wild crowberries with a high content in minerals and organic acids. Food Chem. 2021, 345, 128732. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Rezzouk, F.Z.; Thushar, S.; Shahid, M.; Elouafi, I.A.; Bort, J.; Serret, M.D. Effect of irrigation salinity and ecotype on the growth, physiological indicators and seed yield and quality of Salicornia europaea. Plant Sci. 2021, 304, 110819. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, Y.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Screening seeds of Scottish plants for antibacterial activity. J. Ethnopharmacol. 2002, 83, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Meot-Duros, L.; Le Floch, G.; Magné, C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J. Ethnopharmacol. 2008, 116, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Souid, A.; Della Croce, C.M.; Frassinetti, S.; Gabriele, M.; Pozzo, L.; Ciardi, M.; Abdelly, C.; Hamed, K.B.; Magné, C.; Longo, V. Nutraceutical Potential of Leaf Hydro-Ethanolic Extract of the Edible Halophyte Crithmum maritimum L. Molecules 2021, 26, 5380. [Google Scholar] [CrossRef]
- Houta, O.; Akrout, A.; Neffati, M.; Amri, H. Phenolic contents, antioxidant and antimicrobial potentials of Crithmum maritimum cultivated in Tunisia arid zones. J. Biol. Act. Prod. Nat. 2011, 1, 138–143. [Google Scholar]
- Messina, C.; Bono, G.; Renda, G.; Barbera, L.; Santulli, A. Effect of natural antioxidants and modified atmosphere packaging in preventing lipid oxidation and increasing the shelf-life of common dolphinfish (Coryphaena hippurus) fillets. LWT Food Sci. Technol. 2015, 62, 271–277. [Google Scholar] [CrossRef]
- Alemán, A.; Marín, D.; Taladrid, D.; Montero, P.; Gómez-Guillén, M. Encapsulation of antioxidant sea fennel (Crithmum maritimum) aqueous and ethanolic extracts in freeze-dried soy phosphatidylcholine liposomes. Food Res. Int. 2019, 119, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.; Alves, M.I.; Neves, M.; Tecelão, C.; Ferreira-Dias, S. Enrichment of Sunflower Oil with Ultrasound-Assisted Extracted Bioactive Compounds from Crithmum maritimum L. Foods 2022, 11, 439. [Google Scholar] [CrossRef]
- Caucanas, M.; Montastier, C.; Piérard, G.E.; Quatresooz, P. Dynamics of skin barrier repair following preconditioning by a biotechnology-driven extract from samphire (Crithmum maritimum) stem cells. J. Cosmet. Dermatol. 2011, 10, 288–293. [Google Scholar] [CrossRef]
- Carlsen, M.; Blomhoff, R.; Andersen, L. Intakes of culinary herbs and spices from a food frequency questionnaire evaluated against 28-days estimated records. Nutr. J. 2011, 10, 50. [Google Scholar] [CrossRef]
- Gnocchi, D.; Del Coco, L.; Girelli, C.R.; Castellaneta, F.; Cesari, G.; Sabbà, C.; Fanizzi, F.P.; Mazzocca, A. 1H-NMR metabolomics reveals a multitarget action of Crithmum maritimum ethyl acetate extract in inhibiting hepatocellular carcinoma cell growth. Sci. Rep. 2021, 11, 1259. [Google Scholar] [CrossRef] [PubMed]
- Coman, M.M.; Cecchini, C.; Verdenelli, M.C.; Silvi, S.; Orpianesi, C.; Cresci, A. Functional foods as carriers for SYNBIO®, a probiotic bacteria combination. Int. J. Food Microbiol. 2012, 157, 346–352. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC (Association of Official Analytical Chemists) International, 20th ed.; AOAC Int. Ed.: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Ulbricht, T.L.; Southgate, D.A. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Trabelsi, N.; Megdiche, W.; Ksouri, R.; Falleh, H.; Oueslati, S.; Soumaya, B.; Hajlaoui, H.; Abdelly, C. Solvent effects on phenolic contents and biological activities of the halophyte Limoniastrum monopetalum leaves. LWT Food Sci. Technol. 2010, 43, 632–639. [Google Scholar] [CrossRef]
- Neves, M.; Antunes, M.; Fernandes, W.; Campos, M.J.; Azevedo, Z.A.; Freitas, V.; Rocha, J.; Tecelão, C. Physicochemical and nutritional profile of leaves, flowers, and fruits of the edible halophyte chorão-da-praia (Carpobrotus edulis) on Portuguese west shores. Food Biosci. 2021, 43, 101288. [Google Scholar] [CrossRef]
- Antunes, M.; Barreto, I.; Faria, A.; Silva, S.; Tecelão, C.; Campos, M.J.; Neves, M. Antioxidant Activity and Nutritional Composition of Fine Grounds Obtained in the Production of Sour Cherry Liqueur: A By-Product Valorization. In Proceedings of the 2nd International Conference on Water Energy Food and Sustainability—ICoWEFS 2022, Leiria, Portugal, 10–12 May 2022; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Mogana, R.; Adhikari, A.; Tzar, M.N.; Ramliza, R.; Wiart, C. Antibacterial activities of the extracts, fractions, and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement. Med. Ther. 2020, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tudela, J.L.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Denning, D.; Donnelly, J.P.; Dupont, B.; Fegeler, W.; Moore, C.; et al. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Eucast Discuss. Doc. 2003, 9, I–III. [Google Scholar] [CrossRef]


| Crithmum maritimum L.—Sea Fennel | Leaves | Flowers | Schizocarps | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Moisture (%) | 88.4 ± 0.7 a | 85.0 ± 0.1 b | 86.8 ± 0.3 c | ||||||
| Centesimal composition (% dw) | Ash | 24.9 ± 0.8 a | 15.9 ± 0.3 b | 19.6 ± 0.5 c | |||||
| Crude fat | 4.0 ± 0.1 a | 4.2 ± 0.1 a | 6.5 ± 0.5 b | ||||||
| Proteins | 4.6 ± 0.1 a | 8.3 ± 0.2 b | 7.1 ± 0.3 c | ||||||
| Carbohydrates | 66.5 ± 0.7 a | 71.7 ± 0.4 b | 66.7 ± 0.4 a | ||||||
| Pigments (µg/g dw) | Chlorophyll a | 855.8 ± 6.0 a | 197.5 ± 4.6 b | 132.0 ± 1.6 c | |||||
| Chlorophyll b | 236.5 ± 2.1 a | 56.8 ± 2.4 b | 43.1 ± 1.0 c | ||||||
| Xanthophylls and Carotenes | 258.0 ± 2.0 a | 86.1 ± 1.1 b | 63.6 ± 0.7 c | ||||||
| Ca | Cl | Cu | Fe | K | Mn | Na | P | S | Zn |
| 1180 ± 10 | 2980 ± 20 | 1.30 ± 0.24 | 43.9 ± 1.72 | 1600 ± 10 | 16.7 ± 1.24 | 36,500 ± 5000 | 335 ± 49.0 | 983 ± 55.2 | 5.02 ± 0.42 |
| Extract | Petroleum Ether | Acetone | Ethanol | 50% Ethanol | Water |
|---|---|---|---|---|---|
| TPC (µg GAE/mg) | |||||
| Leaves | 13.6 ± 2.3 a | 44.0 ± 4.3 b | 164 ± 15 c | 119 ± 15 d | 83.2 ± 5.3 e |
| Flowers | 16.2 ± 2.6 f | 88.4 ± 6.1 g | 254 ± 15 h | 118.4 ± 8.2 i | 64.2 ± 3.8 j |
| Schizocarps | 11.7 ± 2.3 k | 44.2 ± 3.4 l | 111 ± 20 m | 84 ± 16 n | 44.3 ± 5.3 o |
| ABTS (µg Trolox Eq/mg) | |||||
| Leaves | 16.0 ± 10.3 a | 23.2 ± 2.9 b | 164.4 ± 6.6 c | 119.2 ± 7.0 d | 78.6 ± 4.4 e |
| Flowers | 2.5 ± 4.6 f | 69.7 ± 3.9 g | 184 ± 11 h | 121.2 ± 6.1 i | 62.7 ± 3.8 j |
| Schizocarps | 1.0 ± 2.7 k | 32.8 ± 3.2 l | 109 ± 17 m | 87.8 ± 7.8 n | 44.7 ± 2.8 o |
| FRAP (µg Trolox Eq/mg) | |||||
| Leaves | 12.6 ± 2.8 a | 36.2 ± 4.9 b | 307.4 ± 8.2 c | 230.8 ± 5.2 d | 147.2 ± 7.4 e |
| Flowers | 7.4 ± 2.7 f | 114.3 ± 6.1 g | 359.8 ± 7.7 h | 232 ± 21 i | 121.7 ± 7.6 j |
| Schizocarps | 5.2 ± 1.6 k | 55.9 ± 4.3 l | 204 ± 27 m | 165 ± 13 n | 83.1 ± 4.0 o |
| DPPH (µg Trolox Eq/mg) | |||||
| Leaves | 22.1 ± 6.4 a | 24.4 ± 9.1 b | 194.6 ± 12.0 c | 132.2 ± 10.8 d | 80.9 ± 9.3 e |
| Flowers | 7.5 ± 4.6 f | 69.3 ± 7.5 g | 254.5 ± 9.9 h | 127.7 ± 4.2 i | 59.8 ± 9.2 j |
| Schizocarps | 5.9 ± 4.7 k | 29.9 ± 7.5 l | 106 ± 22 m | 86 ± 10 n | 38.4 ± 7.8 o |
| Leaves | Flowers | Schizocarps | ||||
|---|---|---|---|---|---|---|
| % Total FA | µg/mg dw | % Total FA | µg/mg dw | % Total FA | µg/mg dw | |
| SFA | ||||||
| C12:0 | 0.45 ± 0.29 | 0.077 ± 0.064 | 0.066 ± 0.03 | 0.011 ± 0.005 | 0.02 ± 0.01 | 0.013 ± 0.008 |
| C14:0 | 2.49 ± 0.09 | 0.429 ± 0.019 | 0.547 ± 0.003 | 0.142 ± 0.008 | 0.27 ± 0.01 | 0.131 ± 0.042 |
| C15:0 | 0.22 ± 0.06 | 0.030 ± 0.004 | 0.18 ± 0.01 | 0.048 ± 0.005 | 0.11 ± 0.00 | 0.055 ± 0.019 |
| C16:0 | 18.53 ± 0.18 | 3.210 ± 0.130 | 17.57 ± 0.10 | 4.570 ± 0.270 | 8.89 ± 0.12 | 4.359 ± 1.440 |
| C17:0 | 0.25 ± 0.01 | 0.043 ± 0.001 | 0.225 ± 0.003 | 0.059 ± 0.004 | 0.12 ± 0.00 | 0.058 ± 0.020 |
| C18:0 | 7.97 ± 0.08 | 1.377 ± 0.062 | 3.54 ± 0.02 | 0.918 ± 0.051 | 0.42 ± 0.03 | 0.200 ± 0.080 |
| C20:0 | 1.43 ± 0.02 | 0.246 ± 0.011 | 0.72 ± 0.01 | 0.186 ± 0.014 | 0.28 ± 0.01 | 0.134 ± 0.044 |
| C24:0 | 2.12 ± 0.07 | 0.364 ± 0.006 | 2.64 ± 0.02 | 0.684 ± 0.038 | 1.00 ± 0.02 | 0.49 ± 0.16 |
| MUFA | ||||||
| C15:1 | 0.32 ± 0.05 | 0.059 ± 0.006 | 0.17 ± 0.02 | 0.043 ± 0.008 | 0.07 ± 0.01 | 0.035 ± 0.016 |
| C16:1 | 1.43 ± 0.26 | 0.220 ± 0.018 | 0.27 ± 0.01 | 0.067 ± 0.004 | 0.16 ± 0.01 | 0.076 ± 0.026 |
| C17:1 | 0.22 ± 0.01 | 0.039 ± 0.002 | 0.53 ± 0.01 | 0.138 ± 0.010 | 0.07 ± 0.02 | 0.040 ± 0.012 |
| C18:1 n-9 | 2.03 ± 0.07 | 0.355 ± 0.013 | 3.43 ± 0.03 | 0.889 ± 0.043 | 50.1 ± 1.00 | 29.7 ± 2.00 |
| C18:1 n-7 | 0.44 ± 0.05 | 0.072 ± 0.008 | 0.52 ± 0.01 | 0.134 ± 0.007 | 0.48 ± 0.06 | 0.24 ± 0.06 |
| C22:1 n-9 | 6.62 ± 0.07 | 1.139 ± 0.052 | 10.04 ± 0.17 | 2.610 ± 0.160 | 7.05 ± 0.30 | 3.43 ± 1.05 |
| C24:1 n-9 | 2.63 ± 0.07 | 0.456 ± 0.015 | 4.12 ± 0.06 | 1.074 ± 0.076 | 3.07 ± 0.03 | 1.51 ± 0.51 |
| PUFA | ||||||
| C16:3 n-4 | 0.33 ± 0.01 | 0.057 ± 0.002 | 0.07 ± 0.01 | 0.015 ± 0.003 | 0.07 ± 0.00 | 0.033 ± 0.009 |
| C18:2 n-6 | 29.47 ± 0.39 | 5.11 ± 0.33 | 45.25 ± 0.24 | 11.77 ± 0.64 | 23.70 ± 0.50 | 11.6 ± 3.8 |
| C18:3 n-3 (ALA) | 22.50 ± 0.36 | 3.90 ± 0.24 | 8.54 ± 0.04 | 2.22 ± 0.12 | 2.67 ± 0.10 | 1.30 ± 0.41 |
| C20:5 n-3 (EPA) | 0.56 ± 0.04 | 0.091 ± 0.001 | 1.58 ± 0.02 | 0.406 ± 0.025 | 1.51 ± 0.02 | 0.74 ± 0.25 |
| SFA | 33.45 ± 0.44 | 25.48 ± 0.11 | 11.10 ± 0.14 | |||
| MUFA | 13.69 ± 0.30 | 19.08 ± 0.20 | 60.95 ± 0.76 | |||
| PUFA | 52.86 ± 0.63 | 55.44 ± 0.27 | 27.95 ± 0.62 | |||
| n-3 | 23.07 ± 0.35 | 10.12 ± 0.04 | 4.18 ± 0.12 | |||
| n-6 | 29.47 ± 0.39 | 45.25 ± 0.24 | 23.70 ± 0.50 | |||
| n-3/n-6 | 0.78 ± 0.01 | 0.22 ± 0.00 | 0.176 ± 0.001 | |||
| H/H | 2.57 ± 0.06 | 3.16 ± 0.03 | 8.35 ± 0.16 | |||
| AI | 1.247 ± 0.020 | 0.992 ± 0.007 | 0.405 ± 0.006 | |||
| TI | 0.317 ± 0.005 | 0.345 ± 0.002 | 0.174 ± 0.002 | |||
| MIC (µg/mL) | |||||||
| Leaves | Flowers | Schizocarps | |||||
| Bacteria | acetone | petrol. ether | acetone | ethanol | petrol. ether | acetone | ethanol |
| E. coli | >600 | >600 | >600 | >600 | >600 | >600 | >600 |
| K. pneumoniae | >600 | >600 | >600 | >600 | >600 | >600 | >600 |
| S. aureus | >600 | 75 | 75 | 300 | 75 | 75 | 300 |
| S. epidermidis | >600 | 150 | >600 | 300 | 150 | >600 | 600 |
| Fungi | |||||||
| C. albicans | 300 | 150 | 75 | >600 | 75 | 75 | 600 |
| C. parapsilosis | >600 | 300 | 300 | >600 | 150 | 300 | >600 |
| K. phaffii | >600 | 150 | >600 | >600 | 300 | 150 | >600 |
| S. cerevisiae | >600 | >600 | >600 | >600 | 300 | 300 | >600 |
| MBC (µg/mL) | |||||||
| Leaves | Flowers | Schizocarps | |||||
| Bacteria | acetone | petrol. ether | acetone | ethanol | petrol. ether | acetone | ethanol |
| E. coli | >600 | >600 | >600 | >600 | >600 | >600 | >600 |
| K. pneumoniae | >600 | >600 | >600 | >600 | >600 | >600 | >600 |
| S. aureus | 300 | 300 | 150 | >600 | 150 | 150 | >600 |
| S. epidermidis | 600 | 300 | 300 | >600 | 300 | 300 | >600 |
| MFC (µg/mL) | |||||||
| Fungi | |||||||
| C. albicans | >600 | 300 | 150 | >600 | 150 | 150 | >600 |
| C. parapsilosis | >600 | 300 | 300 | >600 | 300 | 300 | >600 |
| K. phaffii | 600 | 150 | 150 | >600 | 150 | 150 | 600 |
| S. cerevisiae | >600 | 600 | >600 | >600 | 600 | 300 | >600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, I.; Antunes, M.; Tecelão, C.; Neves, M.; Pires, C.L.; Cruz, P.F.; Rodrigues, M.; Peralta, C.C.; Pereira, C.D.; Reboredo, F.; et al. Nutritive Value and Bioactivities of a Halophyte Edible Plant: Crithmum maritimum L. (Sea Fennel). Plants 2024, 13, 427. https://doi.org/10.3390/plants13030427
Correia I, Antunes M, Tecelão C, Neves M, Pires CL, Cruz PF, Rodrigues M, Peralta CC, Pereira CD, Reboredo F, et al. Nutritive Value and Bioactivities of a Halophyte Edible Plant: Crithmum maritimum L. (Sea Fennel). Plants. 2024; 13(3):427. https://doi.org/10.3390/plants13030427
Chicago/Turabian StyleCorreia, Iris, Madalena Antunes, Carla Tecelão, Marta Neves, Cristiana L. Pires, Pedro F. Cruz, Maria Rodrigues, Claúdia C. Peralta, Cidália D. Pereira, Fernando Reboredo, and et al. 2024. "Nutritive Value and Bioactivities of a Halophyte Edible Plant: Crithmum maritimum L. (Sea Fennel)" Plants 13, no. 3: 427. https://doi.org/10.3390/plants13030427
APA StyleCorreia, I., Antunes, M., Tecelão, C., Neves, M., Pires, C. L., Cruz, P. F., Rodrigues, M., Peralta, C. C., Pereira, C. D., Reboredo, F., Moreno, M. J., Brito, R. M. M., Ribeiro, V. S., Vaz, D. C., & Campos, M. J. (2024). Nutritive Value and Bioactivities of a Halophyte Edible Plant: Crithmum maritimum L. (Sea Fennel). Plants, 13(3), 427. https://doi.org/10.3390/plants13030427