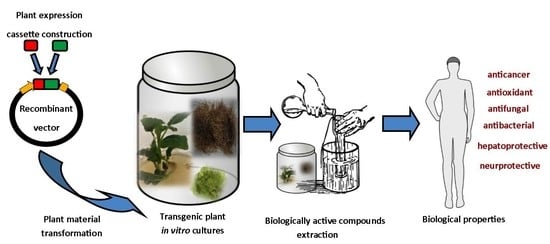
Transgenesis as a Tool for the Efficient Production of Selected Secondary Metabolites from Plant in Vitro Cultures
Abstract
:1. Introduction
2. In Vitro Plant Cells and Organ Cultures as an Alternative Source of Secondary Metabolites
3. From Natural Gene Transfer to Plant Metabolic Engineering
4. Binary Vectors as a Basic Tool in Plant Genetic Transformation
5. Calli and Cell Suspension Cultures
6. Hairy Roots
7. Selected Secondary Metabolites in Medical Use Obtained by in Vitro Transgenic Plant Culture
7.1. Anticancer Compounds
7.2. Paclitaxel
7.3. Camptothecin
7.4. Vincristine
7.5. Vinblastine
8. Overproduction of Other Secondary Metabolites in Transgenic in Vitro Cell Culture
9. In Vitro Transgenic Plant Cultures and Societal Implication
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pham, T.T.N.; Nguyen, T.N.L.; Bui, T.H.; Nguyen, H.Q.; Nguyen, T.T.; Le, V.S.; Chu, H.M. Agrobacterium-mediated transformation of the CrDAT gene and selection of transgenic periwinkle lines with a high vincristine accumulation. J. Hortic. Sci. Biotechnol. 2019, 94, 591–598. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Kumar, S.; Thomas, T.D.; Ramchiary, N.; Swamy, M.K.; Ahmad, I. Linking Omics Approaches to Medicinal Plants and Human Health. In Natural Bio-Active Compounds; Springer: Berlin/Heidelberg, Germany, 2019; pp. 31–57. [Google Scholar]
- Lu, X.; Tang, K.; Li, P. Plant metabolic engineering strategies for the production of pharmaceutical terpenoids. Front. Plant Sci. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Bednarek, P.; Schulze-Lefert, P. Role of Plant Secondary Metabolites at the Host-Pathogen Interface. In Molecular Aspects of Plant Disease Resistance 2018; Parker, J., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; Volume 34, pp. 220–260. [Google Scholar]
- Köhler, H.; Contreras, R.A.; Pizarro, M.; Cortés-Antíquera, R.; Zúñiga, G.E. Antioxidant responses induced by UVB radiation in Deschampsia Antarctica Desv. Front. Plant Sci. 2017, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Takshak, S.; Agrawal, S.B. Defense potential of secondary metabolites in medicinal plants under UV-B stress. J. Photochem. Photobiol. B Biol. 2019, 193, 51–88. [Google Scholar] [CrossRef]
- Huang, W.; Gfeller, V.; Erb, M. Root volatiles in plant–plant interactions II: Root volatiles alter root chemistry and plant–herbivore interactions of neighbouring plants. Plant Cell Environ. 2019, 42, 1964–1973. [Google Scholar] [CrossRef] [Green Version]
- Huber, M.; Epping, J.; Schulze Gronover, C.; Fricke, J.; Aziz, Z.; Brillatz, T.; Swyers, M.; Köllner, T.G.; Vogel, H.; Hammerbacher, A.; et al. A Latex Metabolite Benefits Plant Fitness under Root Herbivore Attack. PLoS Biol. 2016, 14, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Miró, Ò.; Gil, V.; Peacock, W.F. Morphine in acute heart failure: Good in relieving symptoms, bad in improving outcomes. J. Thorac. Dis. 2017, 9, E871–E874. [Google Scholar] [CrossRef] [Green Version]
- Holan, V.; Cechova, K.; Zajicova, A.; Kossl, J.; Hermankova, B.; Bohacova, P.; Hajkova, M.; Krulova, M.; Svoboda, P.; Javorkova, E. The Impact of Morphine on the Characteristics and Function Properties of Human Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2018, 14, 801–811. [Google Scholar] [CrossRef]
- Tuerxun, H.; Cui, J. The dual effect of morphine on tumor development. Clin. Transl. Oncol. 2019, 21, 695–701. [Google Scholar] [CrossRef] [Green Version]
- Nishiwada, T.; Kawaraguchi, Y.; Uemura, K.; Kawaguchi, M. Morphine inhibits cell viability and growth via suppression of vascular endothelial growth factor in human oral cancer HSC-3 cells. J. Anesth. 2019, 33, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Zlatić, N.; Jakovljević, D.; Stanković, M. Temporal, plant part, and interpopulation variability of secondary metabolites and antioxidant activity of Inula helenium L. Plants 2019, 8, 179. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, S.; Mansinhos, I.; Rodríguez-Solana, R.; Pérez-Santín, E.; Coelho, N.; Romano, A. Elicitation improves rosmarinic acid content and antioxidant activity in Thymus lotocephalus shoot cultures. Ind. Crops Prod. 2019, 137, 214–220. [Google Scholar] [CrossRef]
- Bernstein, N.; Akram, M.; Daniyal, M.; Koltai, H.; Fridlender, M.; Gorelick, J. Antiinflammatory Potential of Medicinal Plants: A Source for Therapeutic Secondary Metabolites, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 150. [Google Scholar]
- Flores-Sánchez, K.; Cruz-Sosa, F.; Zamilpa-Alvarez, A.; Nicasio-Torres, P. Active compounds and anti-inflammatory activity of the methanolic extracts of the leaves and callus from Tilia americana var. mexicana propagated plants. Plant Cell Tissue Organ Cult. 2019, 137, 55–64. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Reichling, J. Plant-Microbe Interactions and Secondary Metabolites with Antibacterial, Antifungal and Antiviral Properties, 2nd ed.; Functions and Biotechnology of Plant Secondary Metabolites, Chapter 4; Annual Plant Reviews; Wink, M., Ed.; Wiley: Oxford, UK, 2010; Volume 39. [Google Scholar]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The antifungal activity of Moroccan plants and the mechanism of action of secondary metabolites from plants. Journal de Mycologie Medicale 2017, 27, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Epifano, F.; Molinaro, G.; Genovese, S.; Ngomba, R.T.; Nicoletti, F.; Curini, M. Neuroprotective effect of prenyloxycoumarins from edible vegetables. Neurosci. Lett. 2008, 443, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Barros, L.; Ferreira, I.C. Extraction, identification, fractionation and isolation of phenolic compounds in plants with hepatoprotective effects. J. Sci. Food Agric. 2016, 96, 1068–1084. [Google Scholar] [CrossRef]
- Zhou, M.; Memelink, J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol. Adv. 2016, 34, 441–449. [Google Scholar] [CrossRef]
- Nielsen, E.; Temporiti, M.E.E.; Cella, R. Improvement of phytochemical production by plant cells and organ culture and by genetic engineering. Plant Cell Rep. 2019, 38, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhao, M.; Xu, Z.; Zhao, L.; Han, X. RrGT2, a key gene associated with anthocyanin biosynthesis in Rosa rugosa, was identified via virus-induced gene silencing and overexpression. Int. J. Mol. Sci. 2018, 19, 4057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catania, T.M.; Branigan, C.A.; Stawniak, N.; Hodson, J.; Harvey, D.; Larson, T.R.; Czechowski, T.; Graham, I.A. Silencing amorpha-4,11-diene synthase genes in Artemisia annua leads to FPP accumulation. Front. Plant Sci. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Bahramnejad, B.; Naji, M.; Bose, R.; Jha, S. A critical review on use of Agrobacterium rhizogenes and their associated binary vectors for plant transformation. Biotechnol. Adv. 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulos, A.; Sutikovic, Z.; Wenzl, C.; Maegele, I.; Lohmann, J.U.; Forner, J. GreenGate—A novel, versatile, and efficient cloning system for plant transgenesis. PLoS ONE 2013, 8, e83043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dafny-Yelin, M.; Tzfira, T. Delivery of multiple transgenes to plant cells. Plant Physiol. 2007, 145, 1118–1128. [Google Scholar] [CrossRef] [Green Version]
- Ncube, B.; van Staden, J. Tilting plant metabolism for improved metabolite biosynthesis and enhanced human benefit. Molecules 2015, 20, 12698–12731. [Google Scholar] [CrossRef] [Green Version]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Luo, X.; Ju, G.; Li, L.; Huang, S.; Zhang, T.; Wang, H.; Kai, G. Enhanced Diterpene Tanshinone Accumulation and Bioactivity of Transgenic Salvia miltiorrhiza Hairy Roots by Pathway Engineering. J. Agric. Food Chem. 2016, 64, 2523–2530. [Google Scholar] [CrossRef]
- Hemanthakumar, A.S.; Preetha, T.S.; Pillai, P.P.; Seeni, S. Embryogenesis followed by enhanced micro-multiplication and eco-restoration of Calamus thwaitesii Becc.: An economic non-wood forest produce for strengthening agroforestry system. Agrofor. Syst. 2019, 93, 1093–1105. [Google Scholar] [CrossRef]
- Kado, C.I. Historical account on gaining insights on the mechanism of crown gall tumorigenesis induced by Agrobacterium tumefaciens. Front. Microbiol. 2014, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nester, E.W. Agrobacterium: Nature’s Genetic Engineer. Front. Plant Sci. 2015, 5, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar, J.; Zupan, J.; Cameron, T.A.; Zambryski, P.C. Agrobacterium type IV secretion system and its substrates form helical arrays around the circumference of virulence-induced cells. Proc. Natl. Acad. Sci. USA 2010, 107, 3758–3763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevan, M.W.; Flavell, R.B.; Chilton, M.D. A chimaeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature 1983, 304, 184–187. [Google Scholar] [CrossRef]
- Pitzschke, A. Agrobacterium infection and plant defense-transformation success hangs by a thread. Front. Plant Sci. 2013, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Ye, J.; Gao, D.; Xu, N.; Yang, J. Agrobacterium-mediated horizontal gene transfer: Mechanism, biotechnological application, potential risk and forestalling strategy. Biotechnol. Adv. 2019, 37, 259–270. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Iwase, A.; Rymen, B.; Lambolez, A.; Kojima, M.; Takebayashi, Y.; Heyman, J.; Watanabe, S.; Seo, M.; de Veylder, L.; et al. Wounding triggers callus formation via dynamic hormonal and transcriptional changes. Plant Physiol. 2017, 175, 1158–1174. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Masuoka, S.; Nishimura, Y.; Sakai, T.; Takahata, Y.; Xu, C. Attempt to Establish Direct Gene Transformation System to Seeds of Sweet Potato (Ipomoea batatas) Using Electroporation Method. Biotechnol. J. Int. 2017, 19, 1–8. [Google Scholar] [CrossRef]
- Gao, S.; Yang, Y.; Xu, L.; Guo, J.; Su, Y.; Wu, Q.; Wang, C.; Que, Y. Particle bombardment of the cry2A gene cassette induces stem borer resistance in sugarcane. Int. J. Mol. Sci. 2018, 19, 1692. [Google Scholar] [CrossRef] [Green Version]
- Buyel, J.F. Plants as sources of natural and recombinant anti-cancer agents. Biotechnol. Adv. 2018, 36, 506–520. [Google Scholar] [CrossRef]
- Erpen, L.; Devi, H.S.; Grosser, J.W.; Dutt, M. Potential use of the DREB/ERF, MYB, NAC and WRKY transcription factors to improve abiotic and biotic stress in transgenic plants. Plant Cell Tissue Organ Cult. 2018, 132, 1–25. [Google Scholar] [CrossRef]
- Reddy, C.S.; Vijayalakshmi, M.; Kaul, T.; Islam, T.; Reddy, M.K. Improving Flavour and Quality of Tomatoes by Expression of Synthetic Gene Encoding Sweet Protein Monellin. Mol. Biotechnol. 2015, 57, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Satish, L.; Rency, A.S.; Muthubharathi, B.C.; Shamili, S.; Rameshkumar, R.; Swamy, M.K.; Ramesh, M. Transgenic Plant Cell Cultures: A Promising Approach for Secondary Metabolite Production. In Natural Bio-Active Compounds; Akhtar, M., Swamy, M., Eds.; Springer: Singapore, 2019. [Google Scholar]
- Jensen, P.E.; Scharff, L.B. Engineering of plastids to optimize the production of high-value metabolites and proteins. Curr. Opin. Biotechnol. 2019, 59, 8–15. [Google Scholar] [CrossRef]
- Pasin, F.; Bedoya, L.C.; Bernabé-Orts, J.M.; Gallo, A.; Simón-Mateo, C.; Orzaez, D.; García, J.A. Multiple T-DNA Delivery to Plants Using Novel Mini Binary Vectors with Compatible Replication Origins. ACS Synth. Biol. 2017, 6, 1962–1968. [Google Scholar] [CrossRef]
- Deshpande, A.; Dhadi, S.R.; Hager, E.J.; Ramakrishna, W. Anticancer activity of rice callus suspension culture. Phytother. Res. 2012, 26, 1075–1081. [Google Scholar] [CrossRef]
- Mittal, J.; Sharma, M.M. Enhanced production of berberine in In vitro regenerated cell of Tinospora cordifolia and its analysis through LCMS QToF. 3 Biotech 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rady, M.R.; Aboul-Enein, A.M.; Ibrahim, M.M. Active compounds and biological activity of in vitro cultures of some Echinacea purpurea varieties. Bull. Nat. Res. Cent. 2018, 42, 1–12. [Google Scholar] [CrossRef]
- Rahpeyma, S.A.; Moieni, A.; Jalali Javaran, M. Paclitaxel production is enhanced in suspension-cultured hazel (Corylus avellana L.) cells by using a combination of sugar, precursor, and elicitor. Eng. Life Sci. 2015, 15, 234–242. [Google Scholar] [CrossRef]
- Suprun, A.R.; Ogneva, Z.V.; Dubrovina, A.S.; Kiselev, K.V. Effect of spruce PjSTS1a, PjSTS2, or PjSTS3 gene overexpression on stilbene biosynthesis in callus cultures of Vitis amurensis Rupr. Biotechnol. Appl. Biochem. 2019, 1–7. [Google Scholar] [CrossRef]
- Zheng, J.; Zhuang, Y.; Mao, H.Z.; Jang, I.C. Overexpression of SrDXS1 and SrKAH enhances steviol glycosides content in transgenic Stevia plants. BMC Plant Biol. 2019, 19, 1–16. [Google Scholar] [CrossRef]
- Hidalgo, D.; Martínez-Márquez, A.; Cusidó, R.; Bru-Martínez, R.; Palazón, J.; Corchete, P. Silybum marianum cell cultures stably transformed with Vitis vinifera stilbene synthase accumulate t-resveratrol in the extracellular medium after elicitation with methyl jasmonate or methylated β-cyclodextrins. Eng. Life Sci. 2017, 17, 686–694. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Duan, W.; He, H.; Su, P.; Zhang, M.; Song, G.; Fu, C.; Yu, L. Enhancing taxane biosynthesis in cell suspension culture of Taxus chinensis by overexpressing the neutral/alkaline invertase gene. Process Biochem. 2015, 50, 651–660. [Google Scholar] [CrossRef]
- Mehrotra, S.; Srivastava, V.; Rahman, L.U.; Kukreja, A.K. Hairy root biotechnology—Indicative timeline to understand missing links and future outlook. Protoplasma 2015, 252, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Nigutová, K.; Kusari, S.; Sezgin, S.; Petijová, L.; Henzelyová, J.; Bálintová, M.; Spiteller, M.; Čellárová, E. Chemometric evaluation of hypericin and related phytochemicals in 17 in vitro cultured Hypericum species, hairy root cultures and hairy root-derived transgenic plants. J. Pharm. Pharmacol. 2019, 71, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottaki, Z.; Rezayian, M.; Niknam, V.; Ebrahimzadeh, H.; Mirmasoumi, M. Using hairy roots for production of secondary metabolites in Artemisia. Plant Biotechnol. Rep. 2019, 13, 263–271. [Google Scholar] [CrossRef]
- Matvieieva, N.; Drobot, K.; Duplij, V.; Ratushniak, Y.; Shakhovsky, A.; Kyrpa-Nesmiian, T.; Mickevičius, S.; Brindza, J. Flavonoid content and antioxidant activity of Artemisia vulgaris L. “hairy” roots. Prep. Biochem. Biotechnol. 2019, 49, 82–87. [Google Scholar] [CrossRef]
- Guo, Z.; Tan, H.; Lv, Z.; Ji, Q.; Huang, Y.; Liu, J.; Chen, D.; Diao, Y.; Si, J.; Zhang, L. Targeted expression of Vitreoscilla hemoglobin improves the production of tropane alkaloids in Hyoscyamus niger hairy roots. Sci. Rep. 2018, 8, 1–12. [Google Scholar]
- Yin, Y.; Zhang, X.; Gao, Z.; Hu, T.; Yang, L.; Zhang, Z.; Li, W.; Liu, Y. Over-expressing root-specific β-amyrin synthase gene increases glycyrrhizic acid content in hairy roots of glycyrrhiza uralensis. Chin. Herb. Med. 2019, 11, 192–199. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; Mcphail, A.T. Plant Antitumor Agents.VI.The Isolation and Structure of Taxol, a Novel Antileukemic and Antitumor Agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef]
- Stage, T.B.; Bergmann, T.K.; Kroetz, D.L. Clinical Pharmacokinetics of Paclitaxel Monotherapy: An Updated Literature Review. Clin. Pharmacokinet. 2018, 57, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Fukaya, K.; Tanaka, Y.; Sato, A.C.; Kodama, K.; Yamazaki, H.; Ishimoto, T.; Nozaki, Y.; Iwaki, Y.M.; Yuki, Y.; Umei, K.; et al. Synthesis of paclitaxel. 1. synthesis of the abc ring of paclitaxel by SmI2-mediated cyclization. Org. Lett. 2015, 17, 2570–2573. [Google Scholar] [CrossRef]
- Ho, C.; Chang, S.; Lung, J. The Strategies to Increase Taxol Production by Using Taxus mairei Cells Transformed with TS and DBAT Genes. Int. J. 2005, 3, 179–185. [Google Scholar]
- Sykłowska-Baranek, K.; Pilarek, M.; Bonfill, M.; Kafel, K.; Pietrosiuk, A. Perfluorodecalin-supported system enhances taxane production in hairy root cultures of Taxus x media var. Hicksii carrying a taxadiene synthase transgene. Plant Cell Tissue Organ Cult. 2015, 120, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.M.; Kim, H.S.; Jeon, J.H.; Kim, S.H.; Moon, B.K.; Song, J.Y.; Shim, S.H.; Baek, K.H. Metabolic engineering of Nicotiana benthamiana for the increased production of taxadiene. Plant Cell Rep. 2014, 33, 895–904. [Google Scholar] [CrossRef]
- Sykłowska-Baranek, K.; Grech-Baran, M.; Naliwajski, M.R.; Bonfill, M.; Pietrosiuk, A. Paclitaxel production and PAL activity in hairy root cultures of Taxus x media var. Hicksii carrying a taxadiene synthase transgene elicited with nitric oxide and methyl jasmonate. Acta Physiol. Plant. 2015, 37, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Martino, E.; Della Volpe, S.; Terribile, E.; Benetti, E.; Sakaj, M.; Centamore, A.; Sala, A.; Collina, S. The long story of camptothecin: From traditional medicine to drugs. Bioorg. Med. Chem. Lett. 2017, 27, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Sakato, K.; Tanaka, H.; Mukai, N.; Misawa, M. Isolation and Identification of Camptothecin from Cells of Camptotheca acuminata Suspension Cultures. Agric. Biol. Chem. 1974, 38, 217–218. [Google Scholar] [CrossRef]
- Ni, X.; Wen, S.; Wang, X.; Wang, W.; Xu, H.; Kai, G. Enhancement of camptothecin production in Camptotheca acuminata hairy roots by overexpressing ORCA3 gene. J. Appl. Pharm. Sci. 2011, 1, 85–88. [Google Scholar]
- Yan Pi Effects of over-expression of allene oxide cyclase on camptothecin production by cell cultures of Camptotheca acuminata. Afr. J. Biotechnol. 2012, 11, 6535–6541.
- Cui, L.; Ni, X.; Ji, Q.; Teng, X.; Yang, Y.; Wu, C.; Zekria, D.; Zhang, D.; Kai, G. Co-overexpression of geraniol-10-hydroxylase and strictosidine synthase improves anti-cancer drug camptothecin accumulation in Ophiorrhiza pumila. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetterauer, B.; Wildi, E.; Wink, M. Production of the anticancer compound camptothecin in root and hairy root cultures of Ophiorrhiza mungos L. In Biotechnological Approaches for Medicinal and Aromatic Plants—Conservation, Genetic Improvement and Utilization; Nitish, K., Ed.; Springer: Singapore, 2018; pp. S303–S341. [Google Scholar]
- Wang, C.; Wu, C.; Wang, Y.; Xie, C.; Shi, M.; Nile, S.; Zhou, Z.; Kai, G. Transcription factor OpWRKY3 is involved in the development and biosynthesis of camptothecin and its precursors in Ophiorrhiza pumila hairy roots. Int. J. Mol. Sci. 2019, 20, 3996. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Camino, Á.; Umerez, M.; Martin-Guerrero, I.; García de Andoin, N.; Santos, B.; Sastre, A.; Echebarria-Barona, A.; Astigarraga, I.; Navajas, A.; Garcia-Orad, A. Mir-pharmacogenetics of Vincristine and peripheral neurotoxicity in childhood B-cell acute lymphoblastic leukemia. Pharm. J. 2018, 18, 704–712. [Google Scholar] [CrossRef]
- Setty, B.A.; Stanek, J.R.; Mascarenhas, L.; Miller, A.; Bagatell, R.; Okcu, F.; Nicholls, L.; Lysecki, D.; Gupta, A.A. VIncristine, irinotecan, and temozolomide in children and adolescents with relapsed rhabdomyosarcoma. Pediatr. Blood Cancer 2018, 65, e26728. [Google Scholar] [CrossRef]
- Büyükkapu Bay, S.; Kebudi, R.; Görgün, O.; Zülfikar, B.; Darendeliler, E.; Çakır, F.B. Vincristine, irinotecan, and temozolomide treatment for refractory/relapsed pediatric solid tumors: A single center experience. J. Oncol. Pharm. Pract. 2019, 25, 1343–1348. [Google Scholar] [CrossRef]
- Schönning, A.; Karlén, J.; Frisk, T.; Heyman, M.; Svahn, J.E.; Øra, I.; Kawan, L.; Holmqvist, B.M.; Björklund, C.; Harila-Saari, A.; et al. Venous thrombosis in children and adolescents with Hodgkin lymphoma in Sweden. Thromb. Res. 2017, 152, 64–68. [Google Scholar] [CrossRef]
- Batra, S.; Wistinghausen, B. Pediatric Cancer: Solid Tumors and Lymphoma. In Mount Sinai Expert Guides; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 543–555. [Google Scholar]
- Schiller, G.J.; Damon, L.E.; Coutre, S.E.; Hsu, P.; Bhat, G.; Douer, D. High-dose vincristine sulfate liposome injection, for advanced, relapsed, or refractory philadelphia chromosome-negative acute lymphoblastic leukemia in an adolescent and young adult subgroup of a phase 2 clinical trial. J. Adolesc. Young Adult Oncol. 2018, 7, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, K.; Nishikawa, J.; Moriwaki, J.; Sato, T. Effects of over-expression of strictosidine synthase and tryptophan decarboxylase on alkaloid production by cell cultures of Catharanthus roseus. J. Gen. Plant Pathol. 2013, 79, 414–419. [Google Scholar]
- Sazegari, S.; Niazi, A.; Shahriari-Ahmadi, F.; Moshtaghi, N.; Ghasemi, Y. CrMYC1 transcription factor overexpression promotes the production of low abundance terpenoid indole alkaloids in Catharanthus roseus. Plant OMICS 2018, 11, 30–36. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, C.; Xiong, Z.; Wang, H.; Fu, X.; Shen, Q.; Peng, B.; Ma, Y.; Sun, X.; Tang, K. CrERF5, an AP2/ERF transcription factor, positively regulates the biosynthesis of bisindole alkaloids and their precursors in Catharanthus roseus. Front. Plant Sci. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Lelario, F.; Scrano, L.; de Franchi, S.; Bonomo, M.G.; Salzano, G.; Milan, S.; Milella, L.; Bufo, S.A. Identification and antimicrobial activity of most representative secondary metabolites from different plant species. Chem. Biol. Technol. Agric. 2018, 5, 1–12. [Google Scholar] [CrossRef]
- Radulovic, N.S.; Blagojevic, P.D.; Stojanovic-Radic, Z.Z.; Stojanovic, N.M. Antimicrobial Plant Metabolites: Structural Diversity and Mechanism of Action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Çetinkaya, R.A.; Yenilmez, E.; Petrone, P.; Yılmaz, S.; Bektöre, B.; Şimsek, B.; Kula Atik, T.; Özyurt, M.; Ünlü, A. Platelet-rich plasma as an additional therapeutic option for infected wounds with multi-drug resistant bacteria: In vitro antibacterial activity study. Eur. J. Trauma Emerg. Surg. 2019, 45, 555–565. [Google Scholar] [CrossRef]
- Mahmoud, M.; Askora, A.; Barakat, A.B.; Rabie, O.E.F.; Hassan, S.E. Isolation and characterization of polyvalent bacteriophages infecting multi drug resistant Salmonella serovars isolated from broilers in Egypt. Int. J. Food Microbiol. 2018, 266, 8–13. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Seong, E.S.; Yu, C.Y.; Kim, S.H.; Chung, I.M. Evaluation of phenolic compounds and antimicrobial activities in transgenic Codonopsis lanceolata plants via overexpression of the γ-tocopherol methyltransferase (γ-tmt) gene. S. Afr. J. Bot. 2017, 109, 25–33. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Yu, C.Y.; Chung, I.M. Assessment of the phenolic profile, antimicrobial activity and oxidative stability of transgenic Perilla frutescens L.overexpressing tocopherol methyltransferase (γ-tmt) gene. Plant Physiol. Biochem. 2017, 118, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Kowalczyk, T.; Rijo, P.; Białas, A.J.; Wielanek, M.; Wysokińska, H.; Garcia, C.; Toma, M.; Śliwiński, T.; Skała, E. Over-Expression of AtPAP1 Transcriptional Factor Enhances Phenolic Acid Production in Transgenic Roots of Leonurus sibiricus L. and Their Biological Activities. Mol. Biotechnol. 2018, 60, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Kowalczyk, T.; Santangelo, S.; Białas, A.J.; Toma, M.; Wieczfinska, J.; Śliwiński, T.; Skała, E. The Extract of Leonurus sibiricus Transgenic Roots with AtPAP1 Transcriptional Factor Induces Apoptosis via DNA Damage and Down Regulation of Selected Epigenetic Factors in Human Cancer Cells. Neurochem. Res. 2018, 43, 1363–1370. [Google Scholar] [CrossRef] [Green Version]
- Sitarek, P.; Kowalczyk, T.; Picot, L.; Michalska-Hejduk, D.; Bijak, M.; Białas, A.J.; Wielanek, M.; Śliwiński, T.; Skała, E. Growth of Leonurus sibiricus L. roots with over-expression of AtPAP1 transcriptional factor in closed bioreactor, production of bioactive phenolic compounds and evaluation of their biological activity. Ind. Crops Prod. 2018, 122, 732–739. [Google Scholar] [CrossRef]
- Sitarek, P.; Synowiec, E.; Kowalczyk, T.; Śliwiński, T.; Skała, E. An in vitro estimation of the cytotoxicity and genotoxicity of root extract from Leonurus sibiricus L. overexpressing AtPAP1 against different cancer cell lines. Molecules 2018, 23, 2049. [Google Scholar] [CrossRef] [Green Version]
- Zalesińska, M.D.; Drąg, M.; Poręba, M.; Borska, S.; Kulbacka, J. Anticancer properties of ester derivatives of betulin in human metastatic melanoma cells (Me-45). Cancer Cell Int. 2017, 17, 1–7. [Google Scholar]
- Suzuki, H.; Fukushima, E.O.; Shimizu, Y.; Seki, H.; Fujisawa, Y.; Ishimoto, M.; Osakabe, K.; Osakabe, Y.; Muranaka, T. Lotus japonicus Triterpenoid Profile and Characterization of the CYP716A51 and LjCYP93E1 Genes Involved in Their Biosynthesis in Planta. Plant Cell Physiol. 2019, 60, 2496–2509. [Google Scholar] [CrossRef]
- Xia, K.; Liu, X.; Zhang, Q.; Qiang, W.; Guo, J.; Lan, X.; Chen, M.; Liao, Z. Promoting scopolamine biosynthesis in transgenic Atropa belladonna plants with pmt and h6h overexpression under field conditions. Plant Physiol. Biochem. 2016, 106, 46–53. [Google Scholar] [CrossRef]
- Sohrabi, S.M.; Ismaili, A.; Nazarian-Firouzabadi, F. Simultaneous over-expression and silencing of some benzylisoquinoline alkaloid biosynthetic genes in opium poppy. Ind. Crops Prod. 2018, 123, 581–590. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.M.; Meng, X.F.; Lin, J.S.; Li, Y.J.; Hou, B.K. Ectopic expression of glycosyltransferase UGT76E11 increases flavonoid accumulation and enhances abiotic stress tolerance in Arabidopsis. Plant Biol. 2018, 20, 10–19. [Google Scholar] [CrossRef]
- Lee, W.J.; Kim, J.; Lee, D.; Hong, S.W.; Lee, H. Arabidopsis UDP-glycosyltransferase 78D1-overexpressing plants accumulate higher levels of kaempferol 3-O-β-d-glucopyranoside than wild-type plants. Appl. Biol. Chem. 2017, 60, 647–652. [Google Scholar] [CrossRef]
- Wang, F.; Kong, W.; Wong, G.; Fu, L.; Peng, R.; Li, Z.; Yao, Q. AtMYB12 regulates flavonoids accumulation and abiotic stress tolerance in transgenic Arabidopsis thaliana. Mol. Gen. Genom. 2016, 291, 1545–1559. [Google Scholar] [CrossRef]
- Wang, F.; Ren, G.; Li, F.; Qi, S.; Xu, Y.; Wang, B.; Yang, Y.; Ye, Y.; Zhou, Q.; Chen, X. A chalcone synthase gene AeCHS from Abelmoschus esculentus regulates flavonoid accumulation and abiotic stress tolerance in transgenic Arabidopsis. Acta Physiol. Plant. 2018, 40, 1–13. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Kong, W.; Peng, R.; Liu, Q.; Yao, Q. The Antirrhinum AmDEL gene enhances flavonoids accumulation and salt and drought tolerance in transgenic Arabidopsis. Planta 2016, 244, 59–73. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, Y.H.; Kim, S.H.; Jeong, Y.J.; Kim, C.Y.; Lee, J.S.; Bae, J.Y.; Ahn, M.J.; Jeong, J.C.; Lee, H.S.; et al. Overexpression of the IbMYB1 gene in an orange-fleshed sweet potato cultivar produces a dual-pigmented transgenic sweet potato with improved antioxidant activity. Physiol. Plant. 2015, 153, 525–537. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, S.H.; Park, S.; Lee, H.U.; Lee, J.S.; Park, W.S.; Ahn, M.J.; Kim, Y.H.; Jeong, J.C.; Lee, H.S.; et al. Enhanced accumulation of carotenoids in sweetpotato plants overexpressing IbOr-Ins gene in purple-fleshed sweetpotato cultivar. Plant Physiol. Biochem. 2015, 86, 82–90. [Google Scholar] [CrossRef]
- Mierziak, J.; Wojtasik, W.; Kostyn, K.; Czuj, T.; Szopa, J.; Kulma, A. Crossbreeding of transgenic flax plants overproducing flavonoids and glucosyltransferase results in progeny with improved antifungal and antioxidative properties. Mol. Breed. 2014, 34, 1917–1932. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Khaldun, A.B.M.; Chen, J.; Zhang, C.; Lv, H.; Yuan, L.; Wang, Y. A R2R3-MYB transcription factor regulates the flavonol biosynthetic pathway in a traditional Chinese medicinal plant, Epimedium sagittatum. Front. Plant Sci. 2016, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.J.; An, C.H.; Woo, S.G.; Park, J.H.; Lee, K.W.; Lee, S.H.; Rim, Y.; Jeong, H.J.; Ryu, Y.B.; Kim, C.Y. Enhanced production of resveratrol derivatives in tobacco plants by improving the metabolic flux of intermediates in the phenylpropanoid pathway. Plant Mol. Biol. 2016, 92, 117–129. [Google Scholar] [CrossRef]
- Shi, J.; Li, W.; Gao, Y.; Wang, B.; Li, Y.; Song, Z. Enhanced rutin accumulation in tobacco leaves by overexpressing the NtFLS2 gene. Biosci. Biotechnol. Biochem. 2017, 81, 1721–1725. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Qi, L.; Yang, J.; Wu, C.; Liu, Y.; Huang, L. A Scutellaria baicalensis R2R3-MYB gene, SbMYB8, regulates flavonoid biosynthesis and improves drought stress tolerance in transgenic tobacco. Plant Cell Tissue Organ Cult. 2015, 120, 961–972. [Google Scholar] [CrossRef]
- Gwak, Y.S.; Han, J.Y.; Adhikari, P.B.; Ahn, C.H.; Choi, Y.E. Heterologous production of a ginsenoside saponin (compound K) and its precursors in transgenic tobacco impairs the vegetative and reproductive growth. Planta 2017, 245, 1105–1119. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhang, J.; Yu, B.; Wang, J.; Wang, D. The MYB transcription factor StMYBA1 from potato requires light to activate anthocyanin biosynthesis in transgenic tobacco. J. Plant Biol. 2017, 60, 93–101. [Google Scholar] [CrossRef]
- Sun, W.; Meng, X.; Liang, L.; Li, Y.; Zhou, T.; Cai, X.; Wang, L.; Gao, X. Overexpression of a Freesia hybrida flavonoid 3-O-glycosyltransferase gene, Fh3GT1, enhances transcription of key anthocyanin genes and accumulation of anthocyanin and flavonol in transgenic petunia (Petunia hybrida). Vitro Cell. Dev. Biol. Plant 2017, 53, 478–488. [Google Scholar] [CrossRef]
- Ai, T.N.; Naing, A.H.; Yun, B.W.; Lim, S.H.; Kim, C.K. Overexpression of rsmyb1 enhances anthocyanin accumulation and heavy metal stress tolerance in transgenic petunia. Front. Plant Sci. 2018, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, J.; Pei, T.; Bai, Z.; Han, R.; Liang, Z. Overexpression of SmANS enhances anthocyanin accumulation and alters phenolic acids content in Salvia miltiorrhiza and Salvia miltiorrhiza bge f. Alba plantlets. Int. J. Mol. Sci. 2019, 20, 2225. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sun, G.; Zhong, Z.; Ji, L.; Zhang, Y.; Zhou, J.; Zheng, X.; Deng, K. Overexpression of AtEDT1 promotes root elongation and affects medicinal secondary metabolite biosynthesis in roots of transgenic Salvia miltiorrhiza. Protoplasma 2017, 254, 1617–1625. [Google Scholar] [CrossRef]
- Yang, N.; Zhou, W.; Su, J.; Wang, X.; Li, L.; Wang, L.; Cao, X.; Wang, Z. Overexpression of smMYC2 increases the production of phenolic acids in Salvia miltiorrhiza. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Niu, J.; Li, B.; Huang, Y.; Han, L.; Liu, Y.; Zhou, W.; Hu, S.; Li, L.; Wang, D.; et al. Molecular characterization and overexpression of SmJMT increases the production of phenolic acids in Salvia miltiorrhiza. Int. J. Mol. Sci. 2018, 19, 3788. [Google Scholar] [CrossRef] [Green Version]
- Lim, W.; Li, J. Co-expression of onion chalcone isomerase in Del/Ros1-expressing tomato enhances anthocyanin and flavonol production. Plant Cell Tissue Organ Cult. 2017, 128, 113–124. [Google Scholar] [CrossRef]
- Jian, W.; Cao, H.; Yuan, S.; Liu, Y.; Lu, J.; Lu, W.; Li, N.; Wang, J.; Zou, J.; Tang, N.; et al. SlMYB75, an MYB-type transcription factor, promotes anthocyanin accumulation and enhances volatile aroma production in tomato fruits. Hortic. Res. 2019, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, P. Standardisation of an Agroinfiltration Protocol for Eggplant Fruits and Proving its Usefulness by Over-expressing the SmHQT Gene. Preprints 2019. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Sun, S.; Luo, S.; Zhang, J.; Xiao, X.; Zhang, L.; Wang, F.; Liu, S. Arabidopsis AtPAP1 transcription factor induces anthocyanin production in transgenic Taraxacum brevicorniculatum. Plant Cell Rep. 2014, 33, 669–680. [Google Scholar] [CrossRef]
- Nomani, M.; Sadat Noori, S.A.; Tohidfar, M.; Ramshini, H. Overexpression of TPS2 gene to increase thymol content using Agrobacterium tumefaciens-mediated transformation in Trachyspermum ammi (Qom ecotype). Ind. Crops Prod. 2019, 130, 63–70. [Google Scholar] [CrossRef]
- Pandey, V.; Srivastava, R.; Akhtar, N.; Mishra, J.; Mishra, P.; Verma, P.C. Expression of Withania somnifera Steroidal Glucosyltransferase gene Enhances Withanolide Content in Hairy Roots. Plant Mol. Biol. Rep. 2016, 34, 681–689. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, W.; Zhang, Q.; Xiang, L.; Liu, X.; Chen, M.; Lin, Z.; Wang, Q.; Liao, Z. Overexpression of artemisinic aldehyde Δ11 (13) reductase gene-enhanced artemisinin and its relative metabolite biosynthesis in transgenic Artemisia annua L. Biotechnol. Appl. Biochem. 2015, 62, 17–23. [Google Scholar] [CrossRef]
- Inthima, P.; Nakano, M.; Otani, M.; Niki, T.; Nishijima, T.; Koshioka, M.; Supaibulwatana, K. Overexpression of the gibberellin 20-oxidase gene from Torenia fournieri resulted in modified trichome formation and terpenoid metabolities of Artemisia annua. Plant Cell Tissue Organ Cult. 2017, 129, 223–236. [Google Scholar] [CrossRef]
- Fan, G.; Nie, T.; Huang, Y.; Zhan, Y. GSNOR deficiency enhances betulin production in Betula platyphylla. Trees Struct. Funct. 2018, 32, 847–853. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Zhu, K.; Yang, W.; Ye, J.; Chai, L.; Xu, Q.; Deng, X. The citrus transcription factor CsMADS6 modulates carotenoid metabolism by directly regulating carotenogenic genes. Plant Physiol. 2018, 176, 2657–2676. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Poudereux, I.; Muñoz-Bertomeu, J.; Navarro, A.; Arrillaga, I.; Segura, J. Enhanced levels of S-linalool by metabolic engineering of the terpenoid pathway in spike lavender leaves. Metab. Eng. 2014, 23, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Reddy, V.A.; Panicker, D.; Mao, H.Z.; Kumar, N.; Rajan, C.; Venkatesh, P.N.; Chua, N.H.; Sarojam, R. Metabolic engineering of terpene biosynthesis in plants using a trichome-specific transcription factor MsYABBY5 from spearmint (Mentha spicata). Plant Biotechnol. J. 2016, 14, 1619–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.M.; Park, D.J.; Lee, D.G.; Song, H.J.; Kang, S.M.; Min, J.Y.; Moon, B.C.; Lee, C.K.; Jeon, K.S.; Shivappakarigar, C.; et al. Over expression of IPP isomerase and limonene synthase enzymes in Mentha spicata and their influence on the terpenoid metabolism. Rom. Biotechnol. Lett. 2015, 20, 10358–10368. [Google Scholar]
- Hamachi, A.; Nisihara, M.; Saito, S.; Rim, H.; Takahashi, H.; Islam, M.; Uemura, T.; Ohnishi, T.; Ozawa, R.; Maffei, M.E.; et al. Overexpression of geraniol synthase induces heat stress susceptibility in Nicotiana tabacum. Planta 2019, 249, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Kim, Y.J.; Sukweenadhi, J.; Zhang, D.; Yang, D.C. PgLOX6 encoding a lipoxygenase contributes to jasmonic acid biosynthesis and ginsenoside production in Panax ginseng. J. Exp. Bot. 2016, 67, 6007–6019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadaun, J.S.; Sangwan, N.S.; Narnoliya, L.K.; Singh, N.; Bansal, S.; Mishra, B.; Sangwan, R.S. Over-expression of DXS gene enhances terpenoidal secondary metabolite accumulation in rose-scented geranium and Withania somnifera: Active involvement of plastid isoprenogenic pathway in their biosynthesis. Physiol. Plant. 2017, 159, 381–400. [Google Scholar] [CrossRef]
- Wei, T.; Gao, Y.; Deng, K.; Zhang, L.; Yang, M.; Liu, X.; Qi, C.; Wang, C.; Song, W.; Zhang, Y.; et al. Enhancement of tanshinone production in Salvia miltiorrhiza hairy root cultures by metabolic engineering. Plant Methods 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Deng, C.; Hao, X.; Shi, M.; Fu, R.; Wang, Y.; Zhang, Y.; Zhou, W.; Feng, Y.; Makunga, N.P.; Kai, G. Tanshinone production could be increased by the expression of SmWRKY2 in Salvia miltiorrhiza hairy roots. Plant Sci. 2019, 284, 1–8. [Google Scholar] [CrossRef]
- Vaccaro, M.; Malafronte, N.; Alfieri, M.; de Tommasi, N.; Leone, A. Enhanced biosynthesis of bioactive abietane diterpenes by overexpressing AtDXS or AtDXR genes in Salvia sclarea hairy roots. Plant Cell Tissue Organ Cult. 2014, 119, 65–77. [Google Scholar] [CrossRef]
- Tian, N.; Liu, F.; Wang, P.; Yan, X.; Gao, H.; Zeng, X.; Wu, G. Overexpression of BraLTP2, a lipid transfer protein of Brassica napus, results in increased trichome density and altered concentration of secondary metabolites. Int. J. Mol. Sci. 2018, 19, 1733. [Google Scholar] [CrossRef] [Green Version]
- Chahel, A.A.; Zeng, S.; Yousaf, Z.; Liao, Y.; Yang, Z.; Wei, X.; Ying, W. Plant-specific transcription factor LrTCP4 enhances secondary metabolite biosynthesis in Lycium ruthenicum hairy roots. Plant Cell Tissue Organ Cult. 2019, 136, 323–337. [Google Scholar] [CrossRef]
- Yamazaki, M.; Rai, A.; Yoshimoto, N.; Saito, K. Perspective: Functional genomics towards new biotechnology in medicinal plants. Plant Biotechnol. Rep. 2018, 12, 69–75. [Google Scholar] [CrossRef]

| Plant Species | Vector/Genetic Construct | Plant Material | Extraction Solvent | Class of Compounds | Effect | References |
|---|---|---|---|---|---|---|
| Atropa belladonna L. | pXI vector containing NtPMT and HnH6H | Whole plant | methanol and acetate acetate (methanol:50mM ammonium acetate = 58:42) | Alkaloids | Enhanced biosynthesis of scopolamine | [106] |
| Papaver somniferum L. | pTRV2-BBE, pTRV2-COM, pTRV2-BBECOM | Leaves | Methanol | Alkaloids | Changes in the different alkaloids content | [107] |
| Arabidopsis thaliana (L.) Heynh. | pBI121 vector containing UGT76E11 | Seedlings | Methanol | Polyphenols | increased content of flavonoid glycosides (kaempferol 3-O-[6″-O-(rhamnosyl) glucoside] 7-O-rhamnoside kaempferol 3-O-glucoside 7- O-rhamnoside, kaempferol 3-O-rhamnoside 7-O-rhamnoside quercetin 3-O-rhamnoside 7-O-rhamnoside and quercetin 3-O-glucoside 7-O-rhamnoside) | [108] |
| Arabidopsis thaliana (L.) Heynh. | 35Spro: AtUGT78D1 | Seedlings | Methanol | Polyphenols | Increased accumulation of flavonoids | [109] |
| Arabidopsis thaliana (L.) Heynh. | pCAMBIA1301-AtMYB12 | Seedlings | HCl-methanol | Polyphenols | Increased content of flavonoids | [110] |
| Arabidopsis thaliana (L.) Heynh. | pCAMBIA1301- AeCHS | Seedlings | HCl-methanol | Polyphenols | Increased level of flavonoids | [111] |
| Arabidopsis thaliana (L.) Heynh. | pCAMBIA1301-AmDEL | Seedlings | HCl-methanol | Polyphenols | Increased level of flavonoids | [112] |
| Ipomoea batatas (L. Poir.) | pCam-SPO-IbMYB1a | Storage root | Methanol | Polyphenols | Increased anthocyanin content | [113] |
| Ipomoea batatas (L. Poir.) | pGWB11 vector containing IbOr | Storage root | HCl-methanol | Polyphenols | Enhanced accumulation of zeaxanthin and β-carotene | [114] |
| Leonurus sibiricus L. | pCAMBIA1305.1-AtPAP1 | Hairy roots | Methanol-water | Polyphenols | Higher phenolic acid content. In addition, tested extracts with higher amounts of phenolic acids showed better antimicrobial and cytotoxic effect. | [102] |
| Linum usitatissimum L. | pBinAR | Whole plants | HCl-methanol | Polyphenols | Increased level of flavonoids | [115] |
| Nicotiana benthamiana Domin | pMV-EsMYBF1 | Flowers | HCl-methanol | Polyphenols | Increased production of flavonol content | [116] |
| Nicotiana tabacum L. | pGR-STS and pGR-ROST | Whole plant | 80% metanol | Polyphenols | Increased production of resveratrol derivatives (piceid, piceid methyl ether, resveratrol methyl ether O-hexoside, and 5-methyl resveratrol-3,4-O-β-d-diglucopyranoside) | [117] |
| Nicotiana tabacum L. | pK2GW7 vector containing NtFLS2 | Leaves | Methanol-water-chloroform (5:2:2) | Polyphenols | Increased accumulation of rutin | [118] |
| Nicotiana tabacum L. | pCambia1305 containing SbMYB8 | Leaves | Ethyl alcohol | Polyphenols | higher caffeoylquinic acid contents | [119] |
| Nicotiana tabacum L. | pZIP-Bar containing PgDDS, CYP716A47 and UGT71A28 | Leaves | 100% methanol | Polyphenols | Enhanced production of ginsenoside saponin | [120] |
| Nicotiana tabacum L. | pSAK277 vector containing 35S:StMYBA1-1 construct | Leaves | HCl-methanol | Polyphenols | Enhanced anthocyanin accumulation | [121] |
| Petunia x hybrida hort. ex E.Vilm | pBI-121 containing Fh3GT1 | Blooming flowers | HCl-methanol | Polyphenols | Increased production of cyaniding, peonidin derivatives, kaempferol derivatives and quercetin derivatives | [122] |
| Petunia x hybrida hort. ex E.Vilm | pB7WG2D vector containing RsMYB1 | Leaves | HCl-methanol | Polyphenols | Enhanced accumulation of flavonoids | [123] |
| Salvia miltiorrhiza Bunge | pCAMBIA2300 vector containing SmANS | Plantlets | HCl-methanol | Polyphenols | Increased anthocyanin accumulation, flavonols and proanthocyanidins biosynthesis | [124] |
| Salvia miltiorrhiza Bunge | pCB2006-EDT1 | Roots | 80% methanol | Polyphenols | Increased accumulation of salvianolic acids | [125] |
| Salvia miltiorrhiza Bunge | pEarleyGate201–SmMYC2 | Roots | 75% methanol | Polyphenols | Enhanced production of hydrophilic phenolic acids | [126] |
| Salvia miltiorrhiza Bunge | pEarleyGate202-SmJMT | Roots | Methanol-acetone (7:3) | Polyphenols | Increased production of salvianolic and rosmarinic acids | [127] |
| Solanum lycopersicum L. | pE1775-CHI pDEL.ROS | Flesh and peel | HCl-methanol | Polyphenols | Enhanced anthocyanins and flavonols | [128] |
| Solanum lycopersicum L. | K303 vector containing SlMYB75 | Fruits | 80% methanol | Polyphenols | Increased accumulation of anthocyanin, phenolics and flavonoids | [129] |
| Solanum melongena L. | pBIN19+SmHQT pBIN19+p19 | Fruits | Methanol-water (80:20) | Polyphenols | Increased level of phenolic compounds | [130] |
| Taraxacum brevicorniculatum Korol. | pBI-AtPAP1 | Leaves | Methanol and formic acid | Polyphenols | Increased production of anthocyanins, phenolic acids and flavonoids | [131] |
| Trachyspermum ammi L. Sprague | pBI121-TP | Leaves | 80% ethanol | Polyphenols | Increased production of thymol | [132] |
| Withania somnifera L. | pYL436 vector containing Ws-Sgtl4 | Hairy roots | Methanol | Steroids | Increased withanolide and withanolide-A contents | [133] |
| Artemisia annua L. | pCAMBIA1305–DBR2 | Leaves | Methanol | Terpenoids | Increased level of artemisinin | [134] |
| Artemisia annua L. | pIG-TfGA20ox2 | Leaves | Methanol | Terpenoids | Increased production of artemisinin, sesquiterpenes. Eucalyptol, borneol, α-caryophyllene, β-guaiene, δ-cadinene and β-cubebene and isomultiflorenone were detected only in transgenic extract | [135] |
| Betula platyphylla | pSGRNAi-GSNOR | Cell suspension or plantlet stems | Ethanol | Triterpenoids | Increased betulin content | [136] |
| Citrus grandis L. | pK2- CsMADS6 | Calli and fruit | - | Terpenoids | increased carotenoid contents | [137] |
| Lavandula latifolia Medik. | pBILIS | Leaves | Hexane | Triterpenoids | Increased production of terpenes (S-linalool) | [138] |
| Mentha spicata L. | pK7WG2D- MsYABBY5 | Leaves | Ethyl acetate | Triterpenoids | Increased production of terpenes by gene silencing | [139] |
| Mentha spicata L. | pB1121 vector containing IPP | Whole plants | - | Terpenoids | Increased production of terpenoids | [140] |
| Nicotiana tabacum L. | pSKAN35SGES | Leaves | Methanol | Triterpenoids | Increased production of terpenes | [141] |
| Panax ginseng CA Meyer | pCAMBIA1390 vector containing PgLOX6 | Roots | 80% methanol | Terpenoids | Increased production of ginsenosides | [142] |
| Pelargonium graveolens L’Her and Withania somnifera (L.) Dunal | pBI121 vector containing GrDXS | Whole plants | - | Terpenoids | Increased production of essential oil and withanolides | [143] |
| Salvia miltiorrhiza Bunge | pBI121 vector containing SmMDS | Hairy roots | 80% methanol | Terpenoids | increased accumulation of tanshinones (dihydrotanshinone I, cryptotanshinone, tanshinone I andtanshinone IIA) | [144] |
| Salvia miltiorrhiza Bunge | pCAMBIA2300sm-SmWRKY2 | Hairy roots | Methanol/di- chloromethane (3:1) | Terpenoids | Increased accumulation of tanshinones | [145] |
| Salvia sclarea L. | PKYLX71:35S vector containing DXS or DXR | Hairy roots | Acetone | Terpenoids | Enhanced biosynthesis of abietane diterpenes | [146] |
| Brassica rapa L. | pBI121S vector containing BraLTP2 | Leaves | Methanol-water | Different metabolites | Upregulation of 43 different secondary metabolites. | [147] |
| Lycium ruthenicum Murr. | pCAMBIA1307-TCP4-OE | Hairy roots | Methanol | Different metabolites | higher relative abundances of different secondary metabolites | [148] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, T.; Wieczfinska, J.; Skała, E.; Śliwiński, T.; Sitarek, P. Transgenesis as a Tool for the Efficient Production of Selected Secondary Metabolites from Plant in Vitro Cultures. Plants 2020, 9, 132. https://doi.org/10.3390/plants9020132
Kowalczyk T, Wieczfinska J, Skała E, Śliwiński T, Sitarek P. Transgenesis as a Tool for the Efficient Production of Selected Secondary Metabolites from Plant in Vitro Cultures. Plants. 2020; 9(2):132. https://doi.org/10.3390/plants9020132
Chicago/Turabian StyleKowalczyk, Tomasz, Joanna Wieczfinska, Ewa Skała, Tomasz Śliwiński, and Przemysław Sitarek. 2020. "Transgenesis as a Tool for the Efficient Production of Selected Secondary Metabolites from Plant in Vitro Cultures" Plants 9, no. 2: 132. https://doi.org/10.3390/plants9020132
APA StyleKowalczyk, T., Wieczfinska, J., Skała, E., Śliwiński, T., & Sitarek, P. (2020). Transgenesis as a Tool for the Efficient Production of Selected Secondary Metabolites from Plant in Vitro Cultures. Plants, 9(2), 132. https://doi.org/10.3390/plants9020132