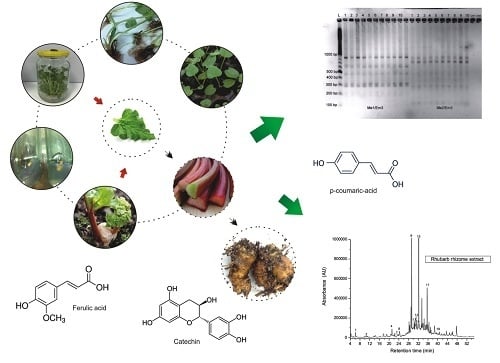
Micropropagation, Genetic Fidelity and Phenolic Compound Production of Rheum rhabarbarum L.
Abstract
:1. Introduction
2. Results
2.1. In Vitro Culture Initiation and Stabilization
2.2. Multiplication Stage
2.3. Rooting and Acclimatization
2.4. SRAP Analysis
2.5. Phenolic Compounds
3. Discussion
4. Materials and Methods
4.1. In Vitro Culture
4.1.1. Initiation and Stabilization
4.1.2. Multiplication Stage
4.1.3. In Vitro Rooting and Acclimatization
4.2. SRAP Analysis
4.3. Chemical Analyses
4.3.1. Chemicals and Standards
4.3.2. Plant Material and Extraction
4.3.3. Analytical HPLC-PDA Determination
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takeoka, G.R.; Dao, L.; Harden, L.; Pantoja, A.; Kuhl, J.C. Antioxidant activity, phenolic and anthocyanin contents of various rhubarb (Rheum spp.) varieties. Int. J. Food Sci. Technol. 2012, 48, 172–178. [Google Scholar] [CrossRef]
- Lepse, L. Adapting technology for local rhubarb (Rheum rhaponticum L.) clones propagation In vitro. Latv. J. Agron. 2005, 8, 324–327. [Google Scholar]
- Duke, J.A. Handbook of Medicinal Herbs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Xiao, P.; He, L.; Wang, L. Ethnopharmacologic study of Chinese rhubarb. J. Ethnopharmacol. 1984, 10, 275–293. [Google Scholar] [CrossRef]
- Aslam, M.; Dayal, R.; Javed, K.; Fahamiya, N.; Mujeeb, M.; Husain, A. Pharmacognostical and phytochemical evaluation of Rheum emodi Wall. Curr. Pharma Res. 2012, 2, 471–479. [Google Scholar]
- Krafczyk, N.; Kötke, M.K.; Lehnert, N.; Glomb, M.A. Phenolic composition of rhubarb. Eur. Food Res. Technol. 2008, 228, 187–196. [Google Scholar] [CrossRef]
- Ye, M.; Hsn, J.; Chen, H.; Zheng, J.; Guo, D. Analysis of phenolic compounds in rhubarbs using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom 2007, 18, 82–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, V.; Guha, G.; Kumar, R.A. Antioxidant and anti-cancer potentials of Rheum emodi rhizome extracts. Evid. Based Complementary Altern. Med. 2011, 697986. [Google Scholar] [CrossRef] [Green Version]
- Zargar, B.A.; Masoodi, M.H.; Ahmed, B.; Ganie, S.A. Phytoconstituents and therapeutic uses of Rheum emodi wall. ex Meissn. Food Chem. 2011, 128, 585–589. [Google Scholar] [CrossRef]
- Komatsu, K.; Nagayama, Y.; Tanaka, K.; Ling, Y.; Cai, S.-Q.; Omote, T.; Meselhy, M.R. Comparative Study of Chemical Constituents of Rhubarb from Different Origins. Chem. Pharm. Bull. 2006, 54, 1491–1499. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Park, J.Y.; Harima, S.; Yoshikawa, M. Antioxidant constituents from Rhubarb: Structural requirements of stilbenes for the activity and structures of two new anthraquinone glucosides. Bioorg. Med. Chem. 2001, 9, 41–50. [Google Scholar] [CrossRef]
- Pham, D.Q.; Ba, D.T.; Dao, N.T.; Choi, G.J.; Vu, T.T.; Kim, J.C.; Giang, T.P.L.; Vu, H.D.; Dang, Q.L. Antimicrobial efficacy of extracts and constituents fractionated from Rheum tanguticum Maxim. Ex Balf. Rhizomes against phytopathogenic fungi and bacteria. Ind. Crops Prod. 2017, 108, 442–450. [Google Scholar] [CrossRef]
- Dūma, M.; Alsina, I.; Dubova, L. Changes of chemical composition of rhubarb during vegetation. Acta Hortic. 2016, 1142, 253–260. [Google Scholar] [CrossRef]
- Medyńska, E.; Smolarz, H.D. Comparative study of phenolic acids from underground parts of Rheum palmatum L., R. rhaponticum L. and R. undulatum L. Acta Soc. Bot. Pol. 2005, 74, 275–279. [Google Scholar]
- Pandey, A.; Belwal, T.; Sekar, K.C.; Bhatt, I.D.; Rawal, R.S. Optimization of ultrasonic-assisted extraction (UAE) of phenolics and antioxidant compounds from rhizomes of Rheum moorcroftianum using response surface methodology (RSM). Ind. Crops Prod. 2018, 119, 218–225. [Google Scholar] [CrossRef]
- Smolarz, H.D.; Medyńska, E.; Matysik, G. Determination of emodin and phenolic acids in the petioles of Rheum undulatum and Rheum rhaponticum. J. Planar Chromatogr. 2005, 18, 318–322. [Google Scholar] [CrossRef]
- Will, F.; Dietrich, H. Processing and chemical composition of rhubarb (Rheum rhabarbarum) juice. LWT Food Sci. Technol. 2013, 50, 673–678. [Google Scholar] [CrossRef]
- Clifford, M.N. Anthocyanins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Arvindekar, A.U.; Laddha, K.S. An efficient microwave-assisted extraction of anthraquinones from Rheum emodi: Optimization using RSM, UV and HPLC analysis and antioxidant studies. Ind. Crops Prod. 2016, 83, 587–595. [Google Scholar] [CrossRef]
- Shen, N.; Cui, Y.; Xu, W.; Zhao, X.; Yang, L. Impact of phosphorus and potassium fertilizers on growth and antraquinone content in Rheum tanguticum Maxim. Ex Balf. Ind. Crops Prod. 2017, 107, 312–319. [Google Scholar] [CrossRef]
- Rajkumar, V.; Guha, G.; Kumar, R.A. Apoptosis induction in MDA-MB-435S, Hep3B and PC-3 cell lines by Rheum emodi rhizome extracts. Asian Pac. J. Cancer Prev. 2011, 12, 1197–1200. [Google Scholar]
- Góraj-Koniarska, J.; Saniewski, M.; Ueda, J.; Miyamoto, K. Effect of methyl jasmonate on gummosis in petioles of culinary rhubarb (Rheum rhabarbarum L.) and the determination of sugar composition of the gum. Acta Physiol. Plant. 2018, 40, 30. [Google Scholar] [CrossRef]
- Dal Toso, R.; Melandri, F. Sustainable sourcing of natural food ingredients by plant cell cultures. Agro Food Ind. Hi Tech. 2011, 22, 30–32. [Google Scholar]
- Lepse, L. Comparison of in vitro and traditional propagation methods of rhubarb (Rheum rhabarbarum) according to morphological features and yield. Acta Hortic. 2009, 812, 265–270. [Google Scholar] [CrossRef]
- Kolewe, M.E.; Gaurav, V.; Roberts, S.C. Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol. Pharm. 2008, 5, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Musa, I.F.; Anu Bakar, N.A.; Karsani, S.A.; Yaacon, J.S. In vitro regeneration and ISSR-Based genetic fidelity analysis of Orthosiphon stamineus Benth. Agronomy 2019, 9, 778. [Google Scholar] [CrossRef] [Green Version]
- Kozak, D.; Salata, A. Effect of cytokinins on in vitro multiplication of rhubarb (Rheum rhaponticum L.) ‘Karpow lipskiego’ shoots and ex vitro acclimatization and growth. Acta Sci. Pol. Hortorum Cultus 2011, 10, 75–87. [Google Scholar]
- Zhao, Y.; Grout, B.W.W.; Crisp, C. Inalvertent selection for unwanted morphological forms during micropropagation ddversely affects field performance of European Rhubarb (Rheum rhaponticum). Acta Hortic. 2003, 616, 301–308. [Google Scholar] [CrossRef]
- Robarts, D.W.H.; Wolfe, A.D. Sequence-related amplified polymorphism (SRAP) markers: A potential resource for studies in plant molecular biology. Appl. Plant. Sci. 2014, 2, 1400017. [Google Scholar] [CrossRef]
- Padma, M.N.; Ravishankar, G.A. In vitro propagation and genetic fidelity study of plant regenerated from inverted hypocotyl explants of eggplant (Solanum melongena L.) cv. Arka Shirish. 3 Biotech 2013, 3, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Li, Q.; Li, H.B.; Ai, J.; Qin, H.Y.; Piao, Z.Y. Plantlet regeneration through somatic embryogenesis in Schisandra chinensis (Turcz) Baill. and analysis of genetic stability of regenerated plants by SRAP markers. Bangladesh J. Bot. 2014, 44, 881–888. [Google Scholar]
- Li, X.; Wang, X.; Luan, C.; Yang, J.; Cheng, S.; Dai, Z.; Mei, P.; Huang, C. Somatic embryogenesis from mature zygotic embryos of Distylium chinense (Fr.) Diels and assessment of genetic fidelity of regenerated plants by SRAP markers. Plant. Growth Regul. 2014, 74, 11–21. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Nonaka, G.; Nishioka, I. Studies on rhubarb (Rhei rhizoma) XV1 simultaneous determination of phenolic constituents by high-performance liquid chromatography. Chem. Pharm. Bull. 1989, 37, 999–1004. [Google Scholar]
- Tripathi, B.; Bhatia, R.; Pandey, A.; Gaur, J.; Chawala, G.; Walia, S.; Choi, E.H.; Attri, P. Potential antioxidant anthraquinones isolated from Rheum emodi showing nematicidal activity against Meloidogyne Incognita. J. Chem. 2014, 2014, 652526. [Google Scholar] [CrossRef] [Green Version]
- Stevanato, R.; Bertelle, M.; Fabris, S. Photoprotective characteristics of natural antioxidant polyphenols. Regul. Toxicol. Pharmacol. 2014, 69, 71–77. [Google Scholar] [CrossRef]
- Benayad, Z.; Martinez-Villaluenga, C.; Frias, J.; Gomez-Cordoves, C.; Es-Safi, N.E. Phenolic composition, antioxidant and anti-inflammatory activities of extracts from Moroccan Opuntia ficus-indica flowers obtained by different extraction methods. Ind. Crops Prod. 2014, 1, 412–420. [Google Scholar] [CrossRef] [Green Version]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef]
- Braicu, C.; Pilecki, V.; Bălăcescu, O.; Irimie, A.; Berindean Neagoe, I. The relationship between biological activities and structure of flavan-3-ols. Int. J. Mol. Sci. 2011, 12, 9342–9353. [Google Scholar] [CrossRef] [Green Version]
- Harborne, J.B. Nature, distribution and function of plant flavonoids. Prog. Clin. Biol. Res. 1986, 213, 15–24. [Google Scholar] [PubMed]
- La Casa, C.; Villegas, I.; Alarcón de la Lastra, C.; Motilva, V.; Martín Calero, M.J. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J. Ethnopharmacol. 2000, 71, 45–53. [Google Scholar] [CrossRef]
- Javed, H.; Khan, M.M.; Ahmad, A.; Vaibhav, K.; Ahmad, M.E.; Khan, A.; Ashafaq, M.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience 2012, 17, 340–352. [Google Scholar] [CrossRef] [PubMed]
- El-Ashry, A.A.; Gabr, A.M.; Arafa, N.M.; El-Bahr, M.K. Rutin accumulation in gardenia calli cultures as a response to phenyl alanine and salicylic acid. Bull. Natl. Res. Cent. 2019, 43, 141. [Google Scholar] [CrossRef] [Green Version]
- Bulgakov, V.P.; Inyushkina, Y.V.; Fedoreyev, S.A. Rosmarinic acid and its derivatives: Biotechnology and applications. Crit. Rev. Biotechnol. 2012, 32, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Le Claire, E.; Schwaiger, S.; Banaigs, B.; Stuppner, H.; Gafner, F. Distribution of a new rosmarinic acid derivative in Eryngium alpinum L. and other Apiaceae. J. Agric. Food Chem. 2005, 53, 4367–4372. [Google Scholar] [CrossRef]
- Ngo, Y.L.; Lau, C.H.; Chua, L.S. Review on rosmarinic acid extraction, fractionation and its anti-diabetic potential. Food Chem. Toxicol. 2018, 121, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, R.; Hatayama, K.; Takahashi, T.; Hayashi, T.; Sato, Y.; Sato, D.; Ohta, K.; Nakano, H.; Seki, C.; Endo, Y.; et al. Structure-activity relations of rosmarinic acid derivatives for the amyloid β aggregation inhibition and antioxidant properties. Eur. J. Med. Chem. 2017, 138, 1066–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, I.M.; Park, M.R.; Chun, J.C.; Yun, S.J. Resveratrol accumulation and resveratrol synthase gene expression in response to abiotic stresses and hormone in peanut plants. Plant. Sci. 2003, 164, 103–109. [Google Scholar] [CrossRef]
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In vitro and in vivo studies about metabolism, bioavailability and biological effects in animal models and humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [Green Version]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Cao, Y.; Fu, Z.D.; Wang, F.; Liu, H.Y.; Han, R. Anti-angiogenic activity of resveratrol, a natural compound from medicinal plants. J. Asian Nat. Prod. Res. 2005, 7, 205–213. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, A. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lodhi, M.A.; Guang-Ning, Z.; Weeden, F.N.F.; Reisch, B.I. A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant. Mol. Biol. Rep. 1994, 12, 6–13. [Google Scholar] [CrossRef]
- Pop, R.; Ardelean, M.; Pamfil, D.; Gaboreanu, I.M. The efficiency of different DNA isolation and purification in ten cultivars of Vitis vinifera. Bul. USAMV CN 2003, 59, 259–261. [Google Scholar]
- Li, G.; Quiros, C.F. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: Its application to mapping and gene tagging in Brassica. Theor. Appl. Genet. 2001, 103, 455–461. [Google Scholar] [CrossRef]
- Azeez, H.; Ibrahim, K.; Pop, R.; Pamfil, D.; Hȃrţa, M.; Bobiş, O. Changes induced by gamma ray irradiation on biomass production and secondary metabolites accumulation in Hypericum triquetrifolium Turra callus cultures. Ind. Crops Prod. 2017, 108, 183–189. [Google Scholar] [CrossRef]
- da Graça Campos, M.; Markham, K.R. Structure Information from HPLC and on-line Measured Absorption Spectra: Flavones, Flavonols and Phenolic Acids; Coimbra University Press: Coimbra, Portugal, 2007. [Google Scholar]



| Types of Hormone Used (4 mg L−1) | Average No. of Shoot Clumps/ Vessel | Average Petiole Length (cm) | Rooted Clumps (%) | Average No. of Roots/ Shoot Clump | Multiplication Rate/Explant |
|---|---|---|---|---|---|
| BA | 24.5 ± 2.5 c | 3.7 ± 0.3 a | 0 | 0.0 ± 0.0 a | 5 ± 0.5 c |
| Kin | 12.5 ± 1.1 a | 8.00 ± 0.3 c | 60 | 14.3 ± 3.7 d | 2.5 ± 0.2 a |
| mT | 21.2 ± 2.3 b | 5.7 ± 0.2 b | 17 | 3.0 ± 1.1 b | 4.2 ± 0.5 b |
| 2-IP | 12.3 ± 1.5 a | 9.6 ± 1.6 d | 31 | 5.8 ± 2.4 c | 2.6 ± 0.5 a,1 |
| Types of Hormone Used (4 mg L−1) | Plant Height (cm) | No. of Leaves/Plant | Root Length (cm) |
|---|---|---|---|
| 2iP | 5.2 ± 0.6 c | 2.2 ± 0.2 a | 11.1 ± 1.3 b |
| Kin | 3.9 ± 0.6 a | 2.1 ± 0.3 a | 10.0 ± 1.5 a |
| only MSm | 4.3 ± 0.6 b | 2.0 ± 0.2 a | 10.1 ± 1.4 a,1 |
| SRAP Primer Combination | Number of Monomorphic Bands | Range of Amplification Products (bp) |
|---|---|---|
| me1/em3 | 4 | 289–753 |
| me2/em3 | 6 | 184–975 |
| me3/em6 | 5 | 203–1215 |
| me4/em2 | 3 | 294–548 |
| me6/em8 | 3 | 220–645 |
| me8/em2 | 5 | 287–756 |
| me5/em2 | 3 | 356–793 |
| me6/em1 | 4 | 278–654 |
| Compound | In vitro | Field | |||||
|---|---|---|---|---|---|---|---|
| Type of Extract | Rhizome | Stalk | Leaf | Rhizome | Stalk | Leaf | |
| 1 | Gallic acid | 86.3 b | 33.9 a | 28.3 a | 124.0 b,* | 32.6 a | 148.5 b,* |
| 2 | Protocatechuic acid | 4.6 a | 2.3 a | - a | 57.7 c,* | 33.7 b,* | 21.5 a,* |
| 3 | p-OH-benzoic acid | 14.5 b | 19.7 c,* | - a | - a | 12.4 b | 12.2 b,* |
| 4 | Catechin | 807.3 b | - a | - a | 1463.3 b,* | - a | - a |
| 5 | Vanillic acid | 25.3 c,* | 15.3 b,* | - a | - | - | - |
| 6 | Clorogenic acid | - a | 7.7 b | - a | - a | 6.1 b | - a |
| 7 | Caffeic acid | 23.9 b | - a | 41.1 c,* | 58.2 c,* | - a | 33.3 b |
| 8 | Syringic acid | 18.3 b | 16.2 b | - a | 71.9 b,* | 24.5 a,* | 82.7 b,* |
| 9 | p-cumaric acid | 624.1 c | 44.2 a | 80.4 b | 1733.8 b,* | 426.7 a,* | 246.0 a,* |
| 10 | Vitexin | - a | - a | 93.6 b | - a | - a | 515.6 b,* |
| 11 | Rutin | - a | - a | 330.0 b | - a | - a | 672.0 b,* |
| 12 | Ferulic acid | 69.2 c | 57.1 b | - a | 2690.3 c,* | 71.4 b,* | - a |
| 13 | Isoquercitrin | 239.5 c,* | 147.2 a | 174.2 b | - a | 218.0 b,* | 572.0 c,* |
| 14 | RA derivate1 | 260.9 c | 55.9 b | - a | 1318.6 c,* | 158.9 b,* | 54.7 a,* |
| 15 | RA derivate2 | 17281.3 c,* | 179.0 b | - a | 6697.8 c | 1072.4 b,* | 506.7 a,* |
| 16 | Rosmarinic acid (RA) | 192.3 b | 179.0 b,* | - a | 1506.5 c,* | 118.6 b | 65.8 a,* |
| 17 | Resveratrol | 371.7 b,* | - a | - a | 229.4 b | - a | - a |
| 18 | Quercitrin | - a | - a | 54.9 b | - a | - a | 206.5 b,* |
| 19 | Apigenin | 11.6 b | - a | 56.9 c | 78.7 a,* | 122.0 b,* | 114.3 b,* |
| 20 | Galangin | - a | 3.5 b,* | 6.3 c | 3.5 a,* | - a | 245.3 b,*,1 |
| No. | Forward Primer | Sequences 5′-3′ | Reverse Primer | Sequences 3′-5′ |
|---|---|---|---|---|
| 1 | Me1 | TGA GTC CAA ACC GGA TA | Em6 | GAC TGC GTA CGA ATT GCA |
| 2 | Me2 | TGA GTC CAA ACC GGA GC | Em1 | GAC TGC GTA CGA ATT AAT |
| 3 | Me2 | TGA GTC CAA ACC GGA GC | Em6 | GAC TGC GTA CGA ATT GCA |
| 4 | Me3 | TGA GTC CAA ACC GGA AT | Em3 | GAC TGC GTA CGA ATT GAC |
| 5 | Me4 | TGA GTC CAA ACC GGA CC | Em2 | GAC TGC GTA CGA ATT TGC |
| 6 | Me5 | TGA GTC CAA ACC GGA AG | Em2 | GAC TGC GTA CGA ATT TGC |
| 7 | Me5 | TGA GTC CAA ACC GGA AG | Em6 | GAC TGC GTA CGA ATT GCA |
| 8 | Me6 | TGA GTC CAA ACC GGA CA | Em1 | GAC TGC GTA CGA ATT AAT |
| 9 | Me6 | TGA GTC CAA ACC GGA CT | Em8 | GAC TGC GTA CGA ATT CAC |
| 10 | Me8 | TGA GTC CAA ACC GGA CT | Em2 | GAC TGC GTA CGA ATT TGC |
| 11 | Me8 | TGA GTC CAA ACC GGA CT | Em3 | GAC TGC GTA CGA ATT GAC |
| 12 | Me8 | TGA GTC CAA ACC GGA CT | Em6 | GAC TGC GTA CGA ATT GCA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clapa, D.; Borsai, O.; Hârța, M.; Bonta, V.; Szabo, K.; Coman, V.; Bobiș, O. Micropropagation, Genetic Fidelity and Phenolic Compound Production of Rheum rhabarbarum L. Plants 2020, 9, 656. https://doi.org/10.3390/plants9050656
Clapa D, Borsai O, Hârța M, Bonta V, Szabo K, Coman V, Bobiș O. Micropropagation, Genetic Fidelity and Phenolic Compound Production of Rheum rhabarbarum L. Plants. 2020; 9(5):656. https://doi.org/10.3390/plants9050656
Chicago/Turabian StyleClapa, Doina, Orsolya Borsai, Monica Hârța, Victoriţa Bonta, Katalin Szabo, Vasile Coman, and Otilia Bobiș. 2020. "Micropropagation, Genetic Fidelity and Phenolic Compound Production of Rheum rhabarbarum L." Plants 9, no. 5: 656. https://doi.org/10.3390/plants9050656
APA StyleClapa, D., Borsai, O., Hârța, M., Bonta, V., Szabo, K., Coman, V., & Bobiș, O. (2020). Micropropagation, Genetic Fidelity and Phenolic Compound Production of Rheum rhabarbarum L. Plants, 9(5), 656. https://doi.org/10.3390/plants9050656