Studies on the Interaction of Rose Bengal with the Human Serum Albumin Protein under Spectroscopic and Docking Simulations Aspects in the Characterization of Binding Sites
Abstract
:1. Introduction
2. Materials
2.1. Experimental Studies
2.2. Molecular Docking Studies
3. Methodologies
3.1. Experimental Studies
3.1.1. Determination of the Binding Constant
3.1.2. Determination of the Binding Site in the HSA
3.1.3. Determination of the Proportional Amount between Ligand and Protein
3.2. Molecular Docking Studies
3.2.1. Protein Database
3.2.2. Molecular Redocking
3.2.3. Conformational Structure of Rose Bengal Dye
3.2.4. Molecular Docking
3.3. Studies to Determine the Attractive and Repulsive Interactions of the Interaction between the RB Dye and the HSA Protein
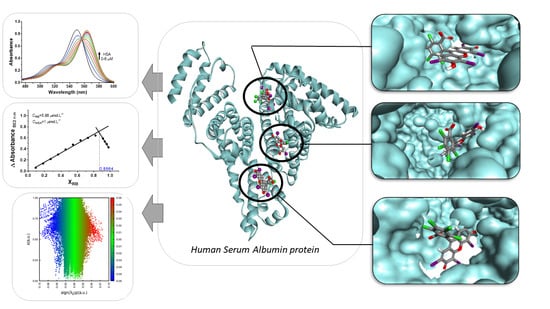
4. Results and Discussions
4.1. Calculation of Ka in the Formation of the RB-HSA Complex
4.2. Determination of the Binding Site
4.3. Ratio between Binding Protein
4.4. Molecular Docking
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamberts, J.J.M.; Neckers, D.C. Rose Bengal and Non-Polar Derivatives: The Birth of Dye Sensitizers for Photooxidation+. Z. Nat. Sect. B J. Chem. 1984, 39, 474–484. [Google Scholar] [CrossRef]
- Zeyada, H.M.; Youssif, M.I.; El-Ghamaz, N.A.; Aboderbala, M.E.O. Spectral, structural, optical and dielectrical studies of UV irradiated Rose Bengal thin films prepared by spin coating technique. Phys. B Condens. Matter 2017, 506, 75–82. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, I.; Liu, L. Photophysics of halogenated fluoresceins: Involvement of both intramolecular electron transfer and heavy atom effect in the deactivation of excited states. Photochem. Photobiol. 2010, 86, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.C.S.; Costa, P.F.A.; Pellosi, D.S.; Calori, I.R.; Vilsinski, B.H.; Estevão, B.M.; Hioka, N.; Caetano, W. Photophysical properties and interaction studies of Rose Bengal derivatives with biomimetic systems based in micellar aqueous solutions. J. Mol. Liq. 2017, 230, 674–685. [Google Scholar] [CrossRef]
- Vanerio, N.; Stijnen, M.; de Mol, B.A.J.M.; Kock, L.M. Biomedical Applications of Photo- and Sono-Activated Rose Bengal: A Review. Photobiomodulation Photomed. Laser Surg. 2019, 37, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Kochevar, I.E.; Lambert, C.R.; Lynch, M.C.; Tedesco, A.C. Comparison of photosensitized plasma membrane damage caused by singlet oxygen and free radicals. Biochim. Biophys. Acta 1996, 1280, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Sola-Llano, R.; Agarrabeitia, A.R.; García-Fresnadillo, D.; López-Arbeloa, I.; Villanueva, A.; Ortiz, M.J.; de la Moya, S.; Martínez-Martínez, V. Exploring BODIPY Derivatives as Singlet Oxygen Photosensitizers for PDT. Photochem. Photobiol. 2020, 96, 458–477. [Google Scholar] [CrossRef]
- Turbay, M.B.E.; Rey, V.; Argañaraz, N.M.; Vieyra, F.E.M.; Aspée, A.; Lissi, E.A.; Borsarelli, C.D. Effect of dye localization and self-interactions on the photosensitized generation of singlet oxygen by rose bengal bound to bovine serum albumin. J. Photochem. Photobiol. B Biol. 2014, 141, 275–282. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Liu, Y.; Hu, D.; Hu, L.; Fei, X.; Zhang, J. Effect of Rapamycin Microspheres in Sjögren Syndrome Dry Eye: Preparation and Outcomes. Ocul. Immunol. Inflamm. 2019, 27, 1357–1364. [Google Scholar] [CrossRef]
- Gundry, R.L.; Fu, Q.; Jelinek, C.A.; Eyk, J.E.V.; Cotter, R.J. Investigation of an albumin-enriched fraction of human serum and its albuminome. Proteom. Clin. Appl. 2007, 1, 73–88. [Google Scholar] [CrossRef]
- Moreira, M.B.; Franciscato, D.S.; Toledo, K.C.F.; de Souza, J.R.B.; Nakatani, H.S.; de Souza, V.R. Investigação da supressão de fluorescência de soro albumina bovina e humana por complexo de rutênio. Quim. Nova 2015, 38, 227–232. [Google Scholar]
- Ascenzi, P.; di Masi, A.; Fanali, G.; Fasano, M. Heme-based catalytic properties of human serum albumin. Cell Death Discov. 2015, 1, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, A.A.; Grüne, T.; Curry, S. Crystallographic Analysis Reveals Common Modes of Binding of Medium and Long-chain Fatty Acids to Human Serum Albumin. J. Mol. Biol. 2000, 303, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural Basis of the Drug-binding Specificity of Human Serum Albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, S.; Almasi, M.; Vaisi-Raygani, A.; Moradi, S.; Ashrafi-Kooshk, M.R.; Shahlaei, M.; Khodarahmi, R. Explorin the interaction between “site-markers, aspirin and esterase-like activity” ternary systems on the human sérum albumin: Direct evidence for modulation of catalytic activity of the protein in different inhibition modes. J. Iran Chem. Soc. 2018, 15, 555–573. [Google Scholar] [CrossRef]
- Manjushree, M.; Revanasiddappa, H.D. Interpretation of the binding interaction between bupropion hydrochloride with human serum albumin: A collective spectroscopic and computational approach Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 209, 264–273. [Google Scholar]
- Cambridge Crystallographic Data Centre Software. GOLD Configuration File User Guide: A Component of the CSD-Discovery Suite; Cambridge Crystallographic Data Centre Software: Cambridge, UK, 2019; pp. 1–80. [Google Scholar]
- de Souza, A.R.; Boza, I.A.F.; Ximenes, V.F.; Yoguim, M.I.; Rodriguez, M.J.D.; Morgon, N.H.; Caracelli, I. Elucidação da quiralidade induzida na molécula dansilglicina na complexação com a proteína albumina do soro humano (HSA). Quím. Nova 2019, 42, 135–142. [Google Scholar] [CrossRef]
- Boto, R.; Peccati, F.; Laplaza, R.; Quan, C.; Carbone, A.; Piquemal, J.P.; Maday, Y.; Contreras-García, J. A new step towards a fast quantification of noncovalent interactions. ChemRxiv. 2020, 6498, 1–31. [Google Scholar]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.P.; Beratan, D.N.; Yang, W. NCIPLOT: A program for plotting non-covalent interaction regions. J. Chem. Theory Comput. 2012, 7, 625–632. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Garcia, J.; Johnson, E.R.; Keinan, S.; Yang, W.; de-la-Roza, A.O.; Quang, C.; Laplaza, R.; Boto, R.A. Nciplot4 Manual. Available online: https://www.lct.jussieu.fr/pagesperso/contrera/NCIPLOT4_MANUAL.pdf (accessed on 2 July 2022).
- Kozuch, S.; Martin, J.M.L. Halogen bonds: Benchmarks and theoretical analysis. J. Chem. Theory Comput. 2013, 9, 1918–1931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Wang, Y.; Xu, M.; Hu, X. UV–Vis spectroscopy combined with chemometric study on the interactions of three dietary flavonoids with copper ions. Food Chem. 2018, 263, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.; Vaishanav, S.K.; Yadav, T.; Sinha, S.; Tiwari, S.; Satnami, M.L.; Ghosh, K.K. Antidepressant drug-protein interactions studied by spectroscopic methods based on fluorescent carbon quantum dots. Heliyon 2019, 5, 1–9. [Google Scholar]
- Alarcón, E.; Edwards, A.M.; Aspee, A.; Moran, F.E.; Borsarelli, C.D.; Lissi, E.A.; Gonzalez-Nilo, D.; Poblete, H.; Scaiano, J.C. Photophysics and photochemistry of dyes bound to human serum albumin are determined by the dye localization. Photochem. Photobiol. Sci. 2010, 9, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Kufareva, I.; Abagyan, R. Methods of protein structure comparison. Methods Mol. Biol. 2012, 857, 231–257. [Google Scholar]
- Si, D.; Moritz, S.A.; Pfab, J.; Hou, J.; Cao, R.; Wang, L.; Wu, T.; Cheng, J. Deep Learning to Predict Protein Backbone Structure from High-Resolution Cryo-EM Density Maps. Sci. Rep. 2020, 10, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dybamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]









| Ka (μM) | Site [14,15,16] | |
|---|---|---|
| HSA | (3.90 ± 0.08) × 105 | |
| +WARFARIN | (2.42 ± 0.95) × 105 | DS1 |
| +IBUPROFEN | (1.35 ± 0.42) × 105 | DS2 or FA6 |
| +DIFLUNISAL | (0.86 ± 0.06) × 105 | DS2 or FA6 |
| +DIAZEPAM | (3.05 ± 0.53) × 105 | DS2 |
| +DIGITOXIN | (2.06 ± 0.59) × 105 | DS3 |
| Site | Radius(Å) | Energy(kcal mol−1) | Interactions | |
|---|---|---|---|---|
| DS1 | 6 | −52.13 | HB. | Lys199 (2.21 Å); Glu292 (1.86 Å) |
| π | His242 (2.79 Å); Ala291 (2.28 Å) | |||
| DS2 | - | - | ||
| DS3 | 5 | −58.79 | HB. | Arg117 (2.11 Å) |
| vdw | Tyr138 (2.48 Å); Arg145 (2.50 Å); Arg186 (3.15 Å); Ser193 (3.01 Å) | |||
| π | Arg186 (3.57 Å); Arg186 (3.48 Å) | |||
| FA6 | 10(flex) * | −67.55 | H.B. | Arg209 (1.48 Å); Lys351 (1.58 Å); Asp324 (1.97 Å) |
| vdw | Arg209 (2.40 Å) | |||
| π | Lys315 (2.78 Å); Lys351 (2.41 Å) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoguim, M.I.; Grandini, G.S.; Bertozo, L.d.C.; Caracelli, I.; Ximenes, V.F.; de Souza, A.R. Studies on the Interaction of Rose Bengal with the Human Serum Albumin Protein under Spectroscopic and Docking Simulations Aspects in the Characterization of Binding Sites. Chemosensors 2022, 10, 440. https://doi.org/10.3390/chemosensors10110440
Yoguim MI, Grandini GS, Bertozo LdC, Caracelli I, Ximenes VF, de Souza AR. Studies on the Interaction of Rose Bengal with the Human Serum Albumin Protein under Spectroscopic and Docking Simulations Aspects in the Characterization of Binding Sites. Chemosensors. 2022; 10(11):440. https://doi.org/10.3390/chemosensors10110440
Chicago/Turabian StyleYoguim, Maurício I., Giulia S. Grandini, Luiza de C. Bertozo, Ignez Caracelli, Valdecir F. Ximenes, and Aguinaldo R. de Souza. 2022. "Studies on the Interaction of Rose Bengal with the Human Serum Albumin Protein under Spectroscopic and Docking Simulations Aspects in the Characterization of Binding Sites" Chemosensors 10, no. 11: 440. https://doi.org/10.3390/chemosensors10110440
APA StyleYoguim, M. I., Grandini, G. S., Bertozo, L. d. C., Caracelli, I., Ximenes, V. F., & de Souza, A. R. (2022). Studies on the Interaction of Rose Bengal with the Human Serum Albumin Protein under Spectroscopic and Docking Simulations Aspects in the Characterization of Binding Sites. Chemosensors, 10(11), 440. https://doi.org/10.3390/chemosensors10110440