Enhanced Visible Light Absorption and Photophysical Features of Novel Isomeric Magnesium Phthalocyaninates with Cyanophenoxy Substitution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Materials
2.3. Methods
2.3.1. Fluorescence Quantum Yield (Φx) and Lifetime (τf) Determination
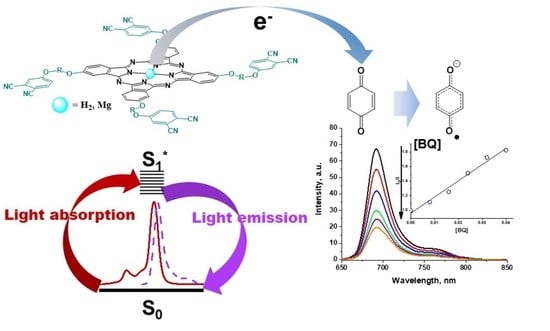
2.3.2. Fluorescence Quenching Studies
2.3.3. Singlet Oxygen Quantum Yield (ΦΔ) Determination
2.4. Synthesis of Substituted Phthalodinitriles
2.4.1. 4,4′-[1,3-Phenylenebis(oxy)]diphthalonitrile (2)
2.4.2. 4,4′-[1,4-Phenylenebis(oxy)]diphthalonitrile (3)
2.5. Synthesis of Magnesium Tetrakisdicyanophenoxyphenoxyphthalocyaninates
2.5.1. Mg(II) Tetrakis-4-[3-(3,4-dicyanophenoxy)phenoxy]phthalocyaninate (4)
2.5.2. Mg(II) Tetrakis-4-[4-(3,4-dicyanophenoxy)phenoxy]phthalocyaninate (5)
2.6. Synthesis of Metal-Free Tetrakis-4-[3,4-dicyanophenoxyphenoxyphthalocyanines
2.6.1. Tetrakis-4-[3-(3,4-dicyanophenoxy)phenoxy]phthalocyanine (6)
2.6.2. Tetrakis-4-[4-(3,4-dicyanophenoxy)phenoxy]phthalocyanine (7)
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Spectroscopic Properties of Phthalocyaninates
3.3. Aggregation Studies
3.4. Photophysical Studies
3.4.1. Fluorescence Quantum Yields and Lifetimes
3.4.2. Fluorescence Quenching
3.4.3. Singlet Oxygen Quantum Yield (ΦΔ) Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrett, P.A.; Dent, C.E.; Linstead, R.P. Phthalocyanines. Part VII. Phthalocyanine as a Co-ordinating Group. A General Investigation of the Metallic Derivatives. J. Chem. Soc. 1934, 1936, 1719–1736. [Google Scholar] [CrossRef]
- Kuzmina, E.A.; Dubinina, T.V.; Vasilevsky, P.N.; Saveliev, M.S.; Gerasimenko, A.Y.; Borisova, N.E.; Tomilova, L.G. Novel octabromo-substituted lanthanide(III) phthalocyanines—Prospective compounds for nonlinear optics. Dye. Pigment. 2021, 185, 108871. [Google Scholar] [CrossRef]
- Mutlu, F.; Pişkin, M.; Canpolat, E.; Öztürk, Ö.F. The new zinc(II) phthalocyanine directly conjugated with 4-butylmorpholine units: Synthesis, characterization, thermal, spectroscopic and photophysical properties. J. Mol. Struct. 2020, 1201, 127169. [Google Scholar] [CrossRef]
- De Almeida, R.H.D.; Monroy-Guzmán, F.; Juárez, C.R.A.; Rocha, J.M.; Bustos, E.B. Electrochemical detector based on a modified graphite electrode with phthalocyanine for the elemental analysis of actinides. Chemosphere 2021, 276, 130114. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, G.Y. Measurement of singlet oxygen generation of 9(Hydroxymethyl)anthracene substituted silicon phthalocyanine by sono-photochemical and photochemical studies. J. Mol. Struct. 2021, 1226, 30–32. [Google Scholar] [CrossRef]
- Atmaca, G.Y. Investigation of the differences between sono-photochemical and photochemical studies for singlet oxygen generation of indium phthalocyanine. Inorg. Chim. Acta 2021, 515, 120052. [Google Scholar] [CrossRef]
- Atmaca, G.Y. Investigation of singlet oxygen efficiency of di-axially substituted silicon phthalocyanine with sono-photochemical and photochemical studies. Polyhedron 2021, 193, 114894. [Google Scholar] [CrossRef]
- Orman, E.B.; Sağlam, M.B.; Özkaya, A.R. Novel peripherally substituted metal-free, zinc (II), and cobalt (II) phthalocyanines with 1,1′-thiobis(2-napthol) and additional tetraphthalonitrile groups: Synthesis, aggregation behavior, electrochemical redox and electrocatalytic oxygen reducing prope. Synth. Met. 2020, 263, 116351. [Google Scholar] [CrossRef]
- Silva, N.; Calderón, S.; Páez, M.A.; Oyarzún, M.P.; Koper, M.T.M.; Zagal, J.H. Probing the Fen +/Fe(n − 1)+ redox potential of Fe phthalocyanines and Fe porphyrins as a reactivity descriptor in the electrochemical oxidation of cysteamine. J. Electroanal. Chem. 2018, 819, 502–510. [Google Scholar] [CrossRef]
- Demirbaş, Ü.; Ömeroğlu, İ.; Akçay, H.T.; Durmuş, M.; Kantekin, H. Synthesis, characterization, photophysical and photochemical properties of peripherally tetra benzodioxane substituted metal-free phthalocyanine and its zinc(II) and magnesium(II) derivatives. J. Mol. Struct. 2021, 1223, 128992. [Google Scholar] [CrossRef]
- Yalazan, H.; Köç, M.; Fandaklı, S.; Nas, A.; Durmuş, M.; Kantekin, H. Synthesis, characterization, and photochemical properties of novel peripherally and non-peripherally tetra substituted zinc(II) and magnesium(II) phthalocyanines containing 4-(1,5-diphenyl-4,5-dihydro-1H-pyrazol-3-yl)phenol units. Polyhedron 2019, 170, 576–583. [Google Scholar] [CrossRef]
- Yalazan, H.; Tekintas, K.; Serdaroğlu, V.; Saka, E.T.; Kahriman, N.; Kantekin, H. Design, syntheses, spectroscopic, aggregation properties of novel peripheral octa-substituted zinc(II), magnesium(II) and lead(II) phthalocyanines and investigation of their photocatalytic properties on the photooxidation of 4-nitrophenol. Inorg. Chem. Commun. 2020, 118, 107998. [Google Scholar] [CrossRef]
- Farajzadeh, N.; Kösoğlu, G.; Erdem, M.; Eryürek, G.; Koçak, M.B. Nonlinear optical properties of peripheral symmetrically and non-symmetrically 4-(trifluoromethoxy)phenoxy substituted zinc phthalocyanines. Synth. Met. 2020, 266, 116440. [Google Scholar] [CrossRef]
- Demirbaş, Ü.; Akyüz, D.; Akçay, H.T.; Koca, A.; Menteşe, E.; Kantekin, H. Novel 1,2,4-triazole substituted metallo-phthalocyanines: Synthesis, characterization and investigation of electrochemical and spectroelectrochemical properties. J. Mol. Struct. 2018, 1173, 205–212. [Google Scholar] [CrossRef]
- Biyiklioglu, Z.; Sofuoğlu, A. Peripherally and non-peripherally electropolymerizable (2-{2-[4-(1H-pyrrol-1-yl)phenoxy]ethoxy}ethoxy) group substituted cobalt(II), manganese(III) phthalocyanines: Synthesis and electrochemistry. J. Mol. Struct. 2020, 1212, 128144. [Google Scholar] [CrossRef]
- Akyüz, D.; Demirbaş, Ü.; Akçay, H.T. Synthesis, characterization and electrochemistry of 1-phenoxypropan-2-yloxy substituted phthalocyanines. J. Organomet. Chem. 2020, 923, 121455. [Google Scholar] [CrossRef]
- Vashurin, A.; Maizlish, V.; Kuzmin, I.; Znoyko, S.; Morozova, A.; Razumov, M.; Koifman, O. Symmetrical and difunctional substituted cobalt phthalocyanines with benzoic acids fragments: Synthesis and catalytic activity. J. Porphyr. Phthalocyanines 2017, 21, 37–47. [Google Scholar] [CrossRef]
- Husain, A.; Ganesan, A.; Sebastian, M.; Makhseed, S. Large ultrafast nonlinear optical response and excellent optical limiting behaviour in pyrene-conjugated zinc(II) phthalocyanines at a near-infrared wavelength. Dye. Pigment. 2021, 184, 108787. [Google Scholar] [CrossRef]
- Lobo, C.S.; Tomé, V.A.; Schaberle, F.A.; Calvete, M.J.F.; Pereira, M.M.; Serpa, C.; Arnaut, L.G. Biocompatible ring-deformed indium phthalocyanine label for near-infrared photoacoustic imaging. Inorg. Chim. Acta 2021, 514, 119993. [Google Scholar] [CrossRef]
- Markad, G.B.; Padma, N.; Chadha, R.; Gupta, K.C.; Rajarajan, A.K.; Deb, P.; Kapoor, S. Mutual influence on aggregation and magnetic properties of graphene oxide and copper phthalocyanine through non-covalent, charge transfer interaction. Appl. Surf. Sci. 2020, 505, 144624. [Google Scholar] [CrossRef]
- Berezin, D.B.; Makarov, V.V.; Znoyko, S.A.; Mayzlish, V.E.; Kustov, A.V. Aggregation of water soluble octaanionic phthalocyanines and their photoinactivation antimicrobial effect in vitro. Mendeleev Commun. 2020, 30, 621–623. [Google Scholar] [CrossRef]
- Gök, Y.; Gök, H.Z. Effect of substituent patterns on the aggregation and photophysical properties of novel C2-symmetric diol-based peripherally and non-peripherally zinc phthalocyanines. J. Mol. Struct. 2020, 1206, 127717. [Google Scholar] [CrossRef]
- Vashurin, A.; Erzunov, D.; Kazaryan, K.; Tonkova, S.; Tikhomirova, T.; Filippova, A.; Koifman, O. Synthesis, catalytic, spectroscopic, fluorescent and coordination properties of dicyanophenoxy-substituted phthalocyaninates of d-metals. Dye. Pigment. 2020, 174, 108018. [Google Scholar] [CrossRef]
- Erzunov, D.A.; Vashurin, A.S.; Koifman, O.I. Synthesis and spectral properties of isomers of cobalt tetrakis(dicyanophenoxy)phthalocyaninate. Russ. Chem. Bull. 2018, 67, 2250–2252. [Google Scholar] [CrossRef]
- Erzunov, D.; Sarvin, I.; Belikova, A.; Vashurin, A. Synthesis and Spectroscopic and Luminescent Properties of Er, Yb and Lu Complexes with Cyano-Substituted Phthalocyanine Ligands. Molecules 2022, 27, 4050. [Google Scholar] [CrossRef] [PubMed]
- Gouterman, M. The Porphyrins Vol III: Physical Chemistry; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Fery-Forgues, S.; Lavabre, D. Are fluorescence quantum yields so tricky to measure? A demonstration using familiar stationery products. J. Chem. Educ. 1999, 76, 1260–1264. [Google Scholar] [CrossRef]
- De Boni, L.; Piovesan, E.; Gaffo, L.; Mendonça, C.R. Resonant nonlinear absorption in Zn-phthalocyanines. J. Phys. Chem. A 2008, 112, 6803–6807. [Google Scholar] [CrossRef]
- Wöhrle, D. Polymere aus Nitrilen. Die Makromol. Chem. 1972, 160, 35–101. [Google Scholar] [CrossRef]
- Nemykin, V.N.; Kobayashi, N.; Mytsyk, V.M.; Volkov, S.V. The solid phase, room-temperature synthesis of metal-free and metallophthalocyanines, particularly of 2,3,9,10,16,17,23,24-octacyanophthalocyanines. Chem. Lett. 2000, 29, 546–547. [Google Scholar] [CrossRef]
- Rose, J. Advanced Physico-Chemical Experiments; J. Wiley: London, UK, 1964. [Google Scholar]
- Gandini, S.C.M.; Yushmanov, V.E.; Borissevitch, I.E.; Tabak, M. Intaraction of the tetra(4-sulfonatophenyl)porphyrin with ionic surfactants: Aggregation and location of micelles. Langmuir 1999, 15, 6233–6243. [Google Scholar] [CrossRef]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The exciton model in molecular spectroscopy. Pure Appl. Chem. 2009, 11, 371–392. [Google Scholar] [CrossRef] [Green Version]
- Gmrk, G.; Karaolan, G.K.; Erdomu, A.; Gl, A.; Avcata, U. A novel phthalocyanine conjugated with four salicylideneimino complexes: Photophysics and fluorescence quenching studies. Dye. Pigment. 2012, 95, 280–289. [Google Scholar] [CrossRef]
- Kaestner, L.; Cesson, M.; Kassab, K.; Christensen, T.; Edminsen, P.D.; Cook, M.J.; Chambrier, I.; Cesson, G.J.M. Zinc octa-n-alkyl phthalocyanines in photodynamic therapy: Photophysical properties, accumulation and apoptosis in cell cultures, studies in erythrocytes and topical application to Balb/c mice skin. Photochem. Photobiol. Sci. 2003, 2, 660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Guo, W. Imidazole Functionalized Magnesium Phthalocyanine Photosensitizer: Modified Photophysics, Singlet Oxygen Generation and Photooxidation Mechanism. J. Phys. Chem. 2012, 116, 7651–7657. [Google Scholar] [CrossRef]










| Sample | CHCl3 | Acetone | DMF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| λmaxabs, nm (lgε) | λmaxem, nm | Stokes Shift, nm | λmaxabs, nm (lgε) | λmaxem, nm | Stokes Shift, nm | λmaxabs, nm (lgε) | λmaxem, nm | Stokes Shift, nm | |
| 4 | 680 (4.59) | 689 | 9 | 674 (4.81) | 684 | 10 | 677 (4.77) | 687 | 10 |
| 5 | 681 (4.34) | 693 | 12 | 674 (4.43) | 683 | 9 | 678 (4.55) | 689 | 11 |
| 6 | 664, 700 (3.95) | 707 | 7 | 661, 694 (3.96) | 702 | 8 | 667, 699 (3.97) | 702 | 3 |
| 7 | 665, 701 (3.91) | 707 | 6 | 662, 695 (3.95) | 703 | 8 | 677, 700 (3.97) | 703 | 3 |
| Sample | CHCl3 | Acetone | DMF | THF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Φx * | τf (ns) * | KSV | Φx | τF | KSV | Φx | τF | KSV | ΦΔ | |
| 4 | 0.34 | 5.62 | 78.36 | 0.18 | 5.85 | 61.99 | 0.21 | 5.49 | 32.15 | 0.11 |
| 5 | 0.32 | 5.66 | 62.15 | 0.14 | 5.50 | 93.71 | 0.19 | 5.38 | 28.44 | 0.04 |
| 6 | 0.15 | 6.02 | 29.84 | 0.14 | 6.01 | 73.65 | 0.09 | 4.98 | 31.32 | - |
| 7 | 0.13 | 6.03 | 22.24 | 0.13 | 5.93 | 39.40 | 0.06 | 5.25 | 23.92 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erzunov, D.; Tonkova, S.; Belikova, A.; Vashurin, A. Enhanced Visible Light Absorption and Photophysical Features of Novel Isomeric Magnesium Phthalocyaninates with Cyanophenoxy Substitution. Chemosensors 2022, 10, 503. https://doi.org/10.3390/chemosensors10120503
Erzunov D, Tonkova S, Belikova A, Vashurin A. Enhanced Visible Light Absorption and Photophysical Features of Novel Isomeric Magnesium Phthalocyaninates with Cyanophenoxy Substitution. Chemosensors. 2022; 10(12):503. https://doi.org/10.3390/chemosensors10120503
Chicago/Turabian StyleErzunov, Dmitry, Svetlana Tonkova, Anastasia Belikova, and Arthur Vashurin. 2022. "Enhanced Visible Light Absorption and Photophysical Features of Novel Isomeric Magnesium Phthalocyaninates with Cyanophenoxy Substitution" Chemosensors 10, no. 12: 503. https://doi.org/10.3390/chemosensors10120503
APA StyleErzunov, D., Tonkova, S., Belikova, A., & Vashurin, A. (2022). Enhanced Visible Light Absorption and Photophysical Features of Novel Isomeric Magnesium Phthalocyaninates with Cyanophenoxy Substitution. Chemosensors, 10(12), 503. https://doi.org/10.3390/chemosensors10120503