Quenching of the Photoluminescence of Gold Nanoclusters Synthesized by Pulsed Laser Ablation in Water upon Interaction with Toxic Metal Species in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Gold Nanomaterial by Pulsed Laser Ablation (PLA) in Water
2.3. Separation of the Plasmonic Gold Nanoparticles from the AuNCs
2.4. Dialysis of the AuNCs Colloidal Dispersion
2.5. Optical Spectroscopies
2.6. Analysis by TEM
2.7. Quenching of the Photoluminescence upon Interaction with Heavy Metal Ions: Linear Range, Sensitivity and Limit of Detection
3. Results and Discussion
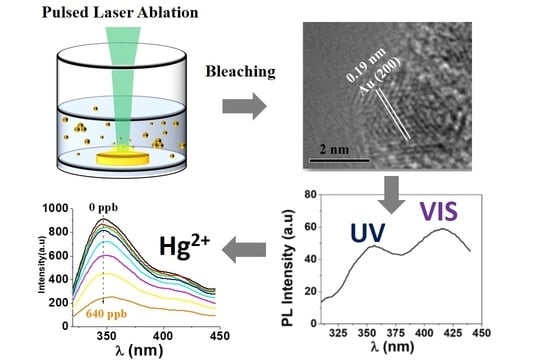
3.1. Bleaching of the Fresh Colloidal Dispersion of Gold Nanomaterial
3.2. Dimensions and Extinction Properties of the Gold Nanomaterial before after the Bleaching
3.3. Characterization of the AuNCs by HRTEM and Photoluminescence Spectroscopy
3.4. Quenching of the Photoluminescence of the AuNCs upon Interaction with the HMI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Sheng, H.; Astruc, D.; Zhu, M. Atomically Precise Noble Metal Nanoclusters as Efficient Catalysts: A Bridge between Structure and Properties. Chem. Rev. 2020, 120, 526–622. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef]
- Chang, H.; Karan, N.S.; Shin, K.; Bootharaju, M.S.; Nah, S.; Chae, S.I.; Baek, W.; Lee, S.; Kim, J.; Son, Y.J.; et al. Highly Fluorescent Gold Cluster Assembly. J. Am. Chem. Soc. 2021, 143, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef]
- Chen, Y.; Montana, D.M.; Wei, H.; Cordero, J.M.; Schneider, M.; Le Guével, X.; Chen, O.; Bruns, O.T.; Bawendi, M.G. Shortwave Infrared in Vivo Imaging with Gold Nanoclusters. Nano Lett. 2017, 17, 6330–6334. [Google Scholar] [CrossRef]
- Qu, X.; Li, Y.; Li, L.; Wang, Y.; Liang, J.; Liang, J. Fluorescent Gold Nanoclusters: Synthesis and Recent Biological Application. J. Nanomater. 2015, 2015, 1–23. [Google Scholar] [CrossRef]
- Cui, M.; Zhao, Y.; Song, Q. Synthesis, optical properties and applications of ultra-small luminescent gold nanoclusters. TrAC Trends Anal. Chem. 2014, 57, 73–82. [Google Scholar] [CrossRef]
- Deng, H.-H.; Shi, X.-Q.; Wang, F.-F.; Peng, H.-P.; Liu, A.-L.; Xia, X.-H.; Chen, W. Fabrication of Water-Soluble, Green-Emitting Gold Nanoclusters with a 65% Photoluminescence Quantum Yield via Host–Guest Recognition. Chem. Mater. 2017, 29, 1362–1369. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Huang, K.-Y.; He, S.-B.; Peng, H.-P.; Xia, X.-H.; Chen, W.; Deng, H.-H. Single gold nanocluster probe-based fluorescent sensor array for heavy metal ion discrimination. J. Hazard. Mater. 2021, 405, 124259. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Z. Colorimetric detection of Hg2+ by Au nanoparticles formed by H2O2 reduction of HAuCl4 using Au nanoclusters as the catalyst. Sensors Actuators B Chem. 2017, 241, 1063–1068. [Google Scholar] [CrossRef]
- Chen, K.-J.; Hsu, I.-H.; Sun, Y.-C. Determination of methylmercury and inorganic mercury by coupling short-column ion chromatographic separation, on-line photocatalyst-assisted vapor generation, and inductively coupled plasma mass spectrometry. J. Chromatogr. A 2009, 1216, 8933–8938. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Criado, L.; Queipo-Abad, S.; Rodríguez-Cea, A.; Rodríguez-González, P.; Alonso, J.I.G. Comparison of GC-ICP-MS, GC-EI-MS and GC-EI-MS/MS for the determination of methylmercury, ethylmercury and inorganic mercury in biological samples by triple spike species-specific isotope dilution mass spectrometry. J. Anal. At. Spectrom. 2022, 37, 1462–1470. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Highly selective and ultrasensitive detection of Hg2+ based on fluorescence quenching of Au nanoclusters by Hg2+–Au+ interactions. Chem. Commun. 2010, 46, 961–963. [Google Scholar] [CrossRef]
- Kawasaki, H.; Yoshimura, K.; Hamaguchi, K.; Arakawa, R. Trypsin-Stabilized Fluorescent Gold Nanocluster for Sensitive and Selective Hg2+ Detection. Anal. Sci. 2011, 27, 591–596. [Google Scholar] [CrossRef]
- Hu, D.; Sheng, Z.; Gong, P.; Zhang, P.; Cai, L. Highly selective fluorescent sensors for Hg2+ based on bovine serum albumin-capped gold nanoclusters. Anal. 2010, 135, 1411–1416. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Tseng, W.-L. Ultrasensitive Sensing of Hg2+ and CH3Hg+ Based on the Fluorescence Quenching of Lysozyme Type VI-Stabilized Gold Nanoclusters. Anal. Chem. 2010, 82, 9194–9200. [Google Scholar] [CrossRef]
- Liu, X.; Du, D.; Mourou, G. Laser ablation and micromachining with ultrashort laser pulses. IEEE J. Quantum Electron. 1997, 33, 1706–1716. [Google Scholar] [CrossRef]
- Wang, L.; Yin, K.; Deng, Q.; Huang, Q.; He, J.; Duan, J. Wetting Ridge-Guided Directional Water Self-Transport. Adv. Sci. 2022, 9, 2204891. [Google Scholar] [CrossRef]
- Tahir, T.; Pandoli, O.G.; Zaman, Q.; Concas, G.C.; Gisbert, M.J.d.S.; Cremona, M.; Freire, F.L.; Carvalho, I.C.S.; Bevilaqua, P.H.C.; de Sá, D.S.; et al. Thermoelastic pulsed laser ablation of silver thin films with organic metal–SiO2 adhesion layer in water: Application to the sustainable regeneration of glass microfluidic reactors for silver nanoparticles. J. Phys. Commun. 2022, 6, 055005. [Google Scholar] [CrossRef]
- Siano, S.; Agresti, J.; Cacciari, I.; Ciofini, D.; Mascalchi, M.; Osticioli, I.; Mencaglia, A.A. Laser cleaning in conservation of stone, metal, and painted artifacts: State of the art and new insights on the use of the Nd:YAG lasers. Appl. Phys. A 2012, 106, 419–446. [Google Scholar] [CrossRef]
- Cheung, J.; Horwitz, J. Pulsed Laser Deposition History and Laser-Target Interactions. MRS Bull. 1992, 17, 30–36. [Google Scholar] [CrossRef]
- Amendola, V.; Amans, D.; Ishikawa, Y.; Koshizaki, N.; Scirè, S.; Compagnini, G.; Reichenberger, S.; Barcikowski, S. Room-Temperature Laser Synthesis in Liquid of Oxide, Metal-Oxide Core-Shells, and Doped Oxide Nanoparticles. Chem.–A Eur. J. 2020, 26, 9206–9242. [Google Scholar] [CrossRef] [PubMed]
- Kalus, M.-R.; Lanyumba, R.; Lorenzo-Parodi, N.; Jochmann, M.A.; Kerpen, K.; Hagemann, U.; Schmidt, T.C.; Barcikowski, S.; Gökce, B. Determining the role of redox-active materials during laser-induced water decomposition. Phys. Chem. Chem. Phys. 2019, 21, 18636–18651. [Google Scholar] [CrossRef] [PubMed]
- Simakin, A.V.; Astashev, M.E.; Baimler, I.V.; Uvarov, O.V.; Voronov, V.; Vedunova, M.V.; Sevost’Yanov, M.A.; Belosludtsev, K.N.; Gudkov, S.V. The Effect of Gold Nanoparticle Concentration and Laser Fluence on the Laser-Induced Water Decomposition. J. Phys. Chem. B 2019, 123, 1869–1880. [Google Scholar] [CrossRef]
- Del Rosso, T.; A Rey, N.; Rosado, T.; Landi, S.; Larrude, D.G.; Romani, E.C.; Junior, F.L.F.; Quinteiro, S.M.; Cremona, M.; Aucelio, R.Q.; et al. Synthesis of oxocarbon-encapsulated gold nanoparticles with blue-shifted localized surface plasmon resonance by pulsed laser ablation in water with CO2 absorbers. Nanotechnology 2016, 27, 255602. [Google Scholar] [CrossRef]
- Sylvestre, J.-P.; Poulin, S.; Kabashin, A.V.; Sacher, E.; Meunier, A.M.; Luong, J.H.T. Surface Chemistry of Gold Nanoparticles Produced by Laser Ablation in Aqueous Media. J. Phys. Chem. B 2004, 108, 16864–16869. [Google Scholar] [CrossRef]
- Del Rosso, T.; Louro, S.; Deepak, F.; Romani, E.; Zaman, Q.; Tahir; Pandoli, O.; Cremona, M.; Junior, F.F.; De Beule, P.; et al. Biocompatible Au@Carbynoid/Pluronic-F127 nanocomposites synthesized by pulsed laser ablation assisted CO2 recycling. Appl. Surf. Sci. 2018, 441, 347–355. [Google Scholar] [CrossRef]
- Ziefuss, A.R.; Steenbock, T.; Benner, D.; Plech, A.; Göttlicher, J.; Teubner, M.; Grimm-Lebsanft, B.; Rehbock, C.; Comby-Zerbino, C.; Antoine, R.; et al. Photoluminescence of Fully Inorganic Colloidal Gold Nanocluster and Their Manipulation Using Surface Charge Effects. Adv. Mater. 2021, 33, e2101549. [Google Scholar] [CrossRef]
- Zaman, Q.; Souza, J.; Pandoli, O.; Costa, K.Q.; Dmitriev, V.; Fulvio, D.; Cremona, M.; Aucelio, R.Q.; Fontes, G.; Del Rosso, T. Two-color surface plasmon resonance nanosizer for gold nanoparticles. Opt. Express 2019, 27, 3200–3216. [Google Scholar] [CrossRef]
- Palazzo, G.; Valenza, G.; Dell’Aglio, M.; De Giacomo, A. On the stability of gold nanoparticles synthesized by laser ablation in liquids. J. Colloid Interface Sci. 2017, 489, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Shi, Y.; Hua, Q.; Tong, B. Phosphorescent chemosensor for Hg2+ based on an iridium(iii) complex coordinated with 4-phenylquinazoline and carbazole dithiocarbamate. RSC Adv. 2015, 5, 74924–74931. [Google Scholar] [CrossRef]
- Mocak, J.; Bond, A.M.; Mitchell, S.; Scollary, G. A statistical overview of standard (IUPAC and ACS) and new procedures for determining the limits of detection and quantification: Application to voltammetric and stripping techniques (Technical Report). Pure Appl. Chem. 1997, 69, 297–328. [Google Scholar] [CrossRef]
- Jin, R. Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2010, 2, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Ziefuß, A.R.; Reichenberger, S.; Rehbock, C.; Chakraborty, I.; Gharib, M.; Parak, W.J.; Barcikowski, S. Laser Fragmentation of Colloidal Gold Nanoparticles with High-Intensity Nanosecond Pulses is Driven by a Single-Step Fragmentation Mechanism with a Defined Educt Particle-Size Threshold. J. Phys. Chem. C 2018, 122, 22125–22136. [Google Scholar] [CrossRef]
- Giorgetti, E.; Giusti, A.; Giammanco, F.; Laza, S.; Del Rosso, T.; Dellepiane, G. Photodegradation of PAMAM G5-stabilized aqueous suspensions of gold nanoparticles. Appl. Surf. Sci. 2007, 254, 1140–1144. [Google Scholar] [CrossRef]
- Giorgetti, E.; Cicchi, S.; Muniz-Miranda, M.; Margheri, G.; Del Rosso, T.; Giusti, A.; Rindi, A.; Ghini, G.; Sottini, S.; Marcelli, A.; et al. Förster resonance energy transfer (FRET) with a donor–acceptor system adsorbed on silver or gold nanoisland films. Phys. Chem. Chem. Phys. 2009, 11, 9798–9803. [Google Scholar] [CrossRef]
- Villa, A.M.; Doglia, S.M.; De Gioia, L.; Bertini, L.; Natalello, A. Anomalous Intrinsic Fluorescence of HCl and NaOH Aqueous Solutions. J. Phys. Chem. Lett. 2019, 10, 7230–7236. [Google Scholar] [CrossRef]
- Reyes-Gasga, J.; Tehuacanero-Nuñez, S.; Montejano-Carrizales, J.M.; Gao, X.; Jose-Yacaman, M. Analysis of the contrast in icosahedral gold nanoparticles. Top. Catal. 2007, 46, 23–30. [Google Scholar] [CrossRef]
- Braga, M.S.; Jaimes, R.F.V.V.; Borysow, W.; Gomes, O.F.; Salcedo, W.J. Portable Multispectral Colorimeter for Metallic Ion Detection and Classification. Sensors 2017, 17, 1730. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-L.; Leng, Y.-M.; Zhang, Y.-J.; Li, T.-H.; Shen, Z.-Y.; Wu, A.-G. A new simple and reliable Hg2+ detection system based on anti-aggregation of unmodified gold nanoparticles in the presence of O-phenylenediamine. Sensors Actuators B Chem. 2014, 200, 140–146. [Google Scholar] [CrossRef]
- Kim, M.; Taylor, T.J.; Gabbaï, F.P. Hg(II)···Pd(II) Metallophilic Interactions. J. Am. Chem. Soc. 2008, 130, 6332–6333. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Yang, T.; Wang, X.-Y.; Chen, M.-L.; Yu, Y.-L.; Wang, J.-H. Mercury Speciation with Fluorescent Gold Nanocluster as a Probe. Anal. Chem. 2018, 90, 6945–6951. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, Y.; Zhang, C.; Shi, L.; Zhang, G.; Shuang, S.; Dong, C.; Ma, H. Water-soluble gold nanoclusters-based fluorescence probe for highly selective and sensitive detection of Hg2+. Sensors Actuators B Chem. 2016, 224, 458–464. [Google Scholar] [CrossRef]
- Wu, X.-J.; Kong, F.; Zhao, C.-Q.; Ding, S.-N. Ratiometric fluorescent nanosensors for ultra-sensitive detection of mercury ions based on AuNCs/MOFs. Analyst 2019, 144, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Pyykkö, P. Strong Closed-Shell Interactions in Inorganic Chemistry. Chem. Rev. 1997, 97, 597–636. [Google Scholar] [CrossRef]
- Echeverría, R.; López-De-Luzuriaga, J.M.; Monge, M.; Olmos, M.E. The gold(i)⋯lead(ii) interaction: A relativistic connection. Chem. Sci. 2015, 6, 2022–2026. [Google Scholar] [CrossRef] [PubMed]
- López-De-Luzuriaga, J.M.; Monge, M.; Olmos, M.E.; Pascual, D.; Lasanta, T. Amalgamating at the molecular level. A study of the strong closed-shell Au(i)⋯Hg(ii) interaction. Chem. Commun. 2011, 47, 6795–6797. [Google Scholar] [CrossRef]
- Pyykkö, P.; Tamm, T. Theory of the d10−d10 Closed-Shell Attraction. 4. X(AuL)nm+ Centered Systems. Organometallics 1998, 17, 4842–4852. [Google Scholar] [CrossRef]
- Kloo, L. On closed-shell interactions between heavy main-group elements. J. Comput. Chem. 2022, 43, 1985–1996. [Google Scholar] [CrossRef]
- Szalay, S.; Barcza, G.; Szilvási, T.; Veis, L.; Legeza, Ö. The correlation theory of the chemical bond. Sci. Rep. 2017, 7, 2237. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Baligar, R.S.; Singh, H.B.; Butcher, R.J. Reaction of a Metallamacrocycle Leading to a Mercury(II)⋅Palladium(II)⋅Mercury(II) Interaction. Angew. Chem. 2009, 121, 2021–2024. [Google Scholar] [CrossRef]
- Echeverría, R.; López-De-Luzuriaga, J.M.; Monge, M.; Moreno, S.; Olmos, M.E. New Insights into the Au(I)·Pb(II) Closed-Shell Interaction: Tuning of the Emissive Properties with the Intermetallic Distance. Inorg. Chem. 2016, 55, 10523–10534. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wen, X.; Toh, Y.-R.; Ma, X.; Tang, J. Fluorescent Metallic Nanoclusters: Electron Dynamics, Structure, and Applications. Part. Part. Syst. Charact. 2015, 32, 142–163. [Google Scholar] [CrossRef]






| Bleaching Method | None | Grounding | Evaporation/Centrifugation | Salt Precipitation |
|---|---|---|---|---|
| Au concentration | 81 ± 7 ppm | 50 ± 12 ppb | 23 ± 5 ppb | 100 ± 10 ppb |
| UV Emission | Visible Emission | |||||||
|---|---|---|---|---|---|---|---|---|
| HMI | Sensitivity 10−3 (ppb−1) | R2 | LOD ppb/nmol/L | Cmax ppb/nM | Sensitivity 10−3 (ppb−1) | R2 | LOD ppb/nmol/L | Cmax ppb/nmol/L |
| Hg2+ | 4.0 | 0.97 | 3/15 | 640/3200 | 3.0 | 0.80 | 4/23 | 20/115 |
| Pb2+ | 2.0 | 0.98 | 6/30 | 20/96 | 1.9 | 0.92 | 7/34 | 20/96 |
| Cd2+ | 1.2 | 0.88 | 10/88 | 20/177 | 0.5 | 0.75 | 11/97 | 20/177 |
| CH3Hg1+ | 1.5 | 0.98 | 8/38 | 40/185 | 2.9 | 0.89 | 23/106 | 20/143 |
| Kind of Gold Nanocluster | Linearity Range | LOD | Ref. |
|---|---|---|---|
| Lys VI-AuNCs | 15–600 nM | 3.0 pM | [17] |
| Au+-stabilized AuNCs | 1–20 nM | 0.5 nM | [14] |
| trypsin-AuNC | 50–600 nM | 50 nM | [15] |
| l-amino acid-AuNCs | 0.5–8.4 μM | 59 nM | [44] |
| folic acid-AuNCs | 0.1–1.0 μM | 28 nM | [43] |
| AuNCs/MIL-68(In)-NH2/Cys | 20 pM–0.2 μM | 6.9 pM | [45] |
| AuNCs by PLA | 50–3200 nM | 15 nM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir; Freire Jr, F.L.; Aucelio, R.Q.; Cremona, M.; Padilha, J.d.S.; Margheri, G.; Zaman, Q.; Concas, G.C.; Gisbert, M.; Ali, S.; et al. Quenching of the Photoluminescence of Gold Nanoclusters Synthesized by Pulsed Laser Ablation in Water upon Interaction with Toxic Metal Species in Aqueous Solution. Chemosensors 2023, 11, 118. https://doi.org/10.3390/chemosensors11020118
Tahir, Freire Jr FL, Aucelio RQ, Cremona M, Padilha JdS, Margheri G, Zaman Q, Concas GC, Gisbert M, Ali S, et al. Quenching of the Photoluminescence of Gold Nanoclusters Synthesized by Pulsed Laser Ablation in Water upon Interaction with Toxic Metal Species in Aqueous Solution. Chemosensors. 2023; 11(2):118. https://doi.org/10.3390/chemosensors11020118
Chicago/Turabian StyleTahir, Fernando Lazaro Freire Jr, Ricardo Q. Aucelio, Marco Cremona, Juliana da S. Padilha, Giancarlo Margheri, Quaid Zaman, Guilherme C. Concas, Mariana Gisbert, Sajjad Ali, and et al. 2023. "Quenching of the Photoluminescence of Gold Nanoclusters Synthesized by Pulsed Laser Ablation in Water upon Interaction with Toxic Metal Species in Aqueous Solution" Chemosensors 11, no. 2: 118. https://doi.org/10.3390/chemosensors11020118
APA StyleTahir, Freire Jr, F. L., Aucelio, R. Q., Cremona, M., Padilha, J. d. S., Margheri, G., Zaman, Q., Concas, G. C., Gisbert, M., Ali, S., Toloza, C. A. T., Licea, Y. E., Saint’Pierre, T. D., Carvalho, R. S., Khan, R., Mariotto, G., Daldosso, N., Perez, G., & Del Rosso, T. (2023). Quenching of the Photoluminescence of Gold Nanoclusters Synthesized by Pulsed Laser Ablation in Water upon Interaction with Toxic Metal Species in Aqueous Solution. Chemosensors, 11(2), 118. https://doi.org/10.3390/chemosensors11020118