Glucose Oxidase Captured into Electropolymerized p-Coumaric Acid towards the Development of a Glucose Biosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biosensor Membrane Fabrication
2.2. Biosensor Electro-Analytical Evaluation and Characterization
3. Results and Discussion
3.1. Biosensing Membrane Electropolymerization
3.2. Glucose Biosensor Evaluation
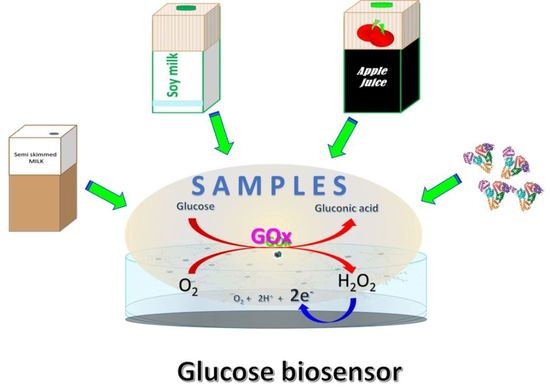
3.3. Glucose Biosensors Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, H.; Ashrafizadeh, M.; Zarrabi, A.; et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother. 2022, 146, 112563. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, N.C.J.; Mbanya, C.J.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projection for 2045. Diabetes Res. Clin. Pract. 2021, 183, 109119. [Google Scholar] [CrossRef]
- Zou, Y.; Chu, Z.; Guo, J.; Liu, S.; Ma, X.; Guo, J. Minimally invasive electrochemical continuous glucose monitoring sensors: Recent progress and perspective. Biosens. Bioelectron. 2023, 225, 115103. [Google Scholar] [CrossRef]
- Cosnier, S. Biosensors based on electropolymerized films: New trends. Anal. Bioanal. Chem. 2003, 337, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Gonzalez, R.; Horton, A.; Li, T. Immobilization of Enzymes by Polymeric Materials. Catalysts 2021, 11, 1211. [Google Scholar] [CrossRef]
- Asgher, M.; Shahid, M.; Kamal, S.; Iqbal, M.H.N. Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. J. Mol. Catal. B Enzym. 2014, 101, 56–66. [Google Scholar] [CrossRef]
- Martinkova, P.; Kostelnik, A.; Valek, T.; Pohanka, M. Main streams in the construction of biosensors and their applications. Int. J. Electrochem. Sci. 2017, 12, 7386–7403. [Google Scholar] [CrossRef]
- Spychalska, K.; Zając, D.; Baluta, S.; Halicka, K.; Cabaj, J. Functional Polymers Structures for (Bio)Sensing Application—A Review. Polymers 2020, 12, 1154. [Google Scholar] [CrossRef]
- Çetin, Z.M.; Guven, N.; Apetrei, R.-M.; Camurlu, P. Highly sensitive detection of glucose via glucose oxidase immobilization onto conducting polymer-coated composite polyacrylonotrile nanofibers. Enzyme Microb. 2023, 164, 110178. [Google Scholar] [CrossRef]
- Teles, R.R.F.; Fonseca, P.L. Applications of polymers for biomolecule immobilization in electrochemical biosensors. Mater. Sci. Eng. C 2008, 28, 1530–1543. [Google Scholar] [CrossRef]
- Nambiar, S.; Yeow, T.W.J. Conductive polymer-based sensor for biomedical applications. Biosens. Bioelectron. 2011, 26, 1825–1832. [Google Scholar] [CrossRef]
- Lange, U.; Roznyatovskaya, V.N.; Mirsky, M.V. Conducting polymers in chemical sensors and arrays. Anal. Chim. Acta 2008, 614, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Somasundrum, M.; Aoki, K. The steady-state current at microcylinder electrodes modified by enzymes immobilized in conducting or non-conducting material. J. Electroanal. Chem. 2002, 530, 40–46. [Google Scholar] [CrossRef]
- Yuqing, M.; Jianrong, C.; Xiaohua, W. Using electropolymerized non-conducting polymers to develop enzyme amperometric biosensors. Trends Biotechnol. 2004, 22, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, J. Polymers of phenylenediamines. Prog. Polym. Sci. 2015, 41, 1–31. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Hocevar, B.S.; Ogoreve, B. One-step electropolymeric co-immobilization of glucose oxidase end heparin for amperometric biosensing of glucose. Analyst 2000, 125, 1431–1434. [Google Scholar] [CrossRef]
- Malitesta, C.; Palmisano, F.; Torsi, L.; Zambonin, G.P. Glucose Fast-Response Amperometric Sensor Based on Glucose Oxidase Immobilized in an Electropolymerized Poly(o-phenylendiamine) Film. Anal. Chem. 1990, 62, 2735–2740. [Google Scholar] [CrossRef]
- Calia, G.; Monti, P.; Marceddu, S.; Dettori, A.M.; Fabbri, D.; Jaoua, S.; O’Neill, D.R.; Delogu, G.; Migheli, Q. Electropolymerized phenol derivates as permselective polymers for biosensor applications. Analyst 2015, 140, 3607–3615. [Google Scholar] [CrossRef]
- Guerriri, A.; De Benedetto, E.G.; Palmisano, F.; Zambonin, G.P. Electrosynthesized non-conductive polymers as permselective membranes in amperometric enzyme electrodes: A glucose biosensor based on a co-crosslinked glucose oxidase/overoxidized polypyrrole bilayer. Biosens. Bioelectron. 1998, 13, 103–112. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Cui, Y. Transdermal amperometric biosensors for continuous glucose monitoring in diabetes. Talanta 2023, 253, 124033. [Google Scholar] [CrossRef]
- Windmiller, R.J.; Valdés-Ramírez, G.; Zhuo, N.; Zhuo, M.; Miller, R.P.; Jin, C.; Brozik, M.S.; Polsky, R.; Katz, E.; Narayan, R.; et al. Bicomponent Microneedle Array Biosensor for Minimally-Invasive Glutamate Monitoring. Electroanalysis 2011, 23, 2302–2309. [Google Scholar] [CrossRef]
- Maghraby, R.Y.; El-Shabasy, M.R.; Ibrahim, H.A.; Azzazy, E.-S.M.H. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Guss, E.; Yakupova, E. Electrochemical Sensors Based on Electropolymerized Natural Phenolic Antioxidants and Their Analytical Application. Sensors 2021, 21, 8385. [Google Scholar] [CrossRef]
- Lima, M.T.; Soares, I.P.; do Nascimento, A.L.; Franco, L.D.; Pereira, C.A.; Ferreira, F.L. A novel electrochemical sensor for simultaneous determination of cadmium and lead using graphite electrodes modified with poly(p-coumaric acid). Microchem. J. 2021, 168, 106306. [Google Scholar] [CrossRef]
- Santiago-Luna, D.F.; Luis, G.L.; Valdés-Ramírez, G. Electrochemical Detection of Pb2+ and Cd2+ Ions in Aqueous Samples Employing Modified Carbon Paste electrode (β-cyclodextrin and p-coumaric Acid). ECS Trans. 2023, 110, 309. [Google Scholar] [CrossRef]
- Valdés-Ramírez, G.; Galicia, L. Biosensing membrane base on ferulic acid and glucose oxidase for an amperometric glucose biosensor. Molecules 2021, 26, 3757. [Google Scholar] [CrossRef]
- Boz, H. p-Coumaric acid in cereals: Presence, antioxidant and antimicrobial effects. Int. J. Food Sci. Technol. 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Boo, C.Y. p-Coumaric Acid as AnActive Ingredient in Cosmetics: A Review Focusing on its Antimelanogenic effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.P.; Victorelli, D.F.; Fonseca-Santos, B.; Chorilli, M. A Review of Analytical Methods for p-Coumaric Acid in Plant-Based Products, Beverages, and Biological Matrices. Crit. Rev. Anal. Chem. 2018, 49, 21–31. [Google Scholar] [CrossRef]
- Tümay, O.S.; Sanko, V.; Şenocak, A.; Orroji, Y.; Demirbas, E.; Yoon, Y.; Khataee, A. Direct and selective determination of p-coumaric acid in food samples via layered Nb4AlC3-MAX phase. Food Chem. 2023, 403, 134130. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, P.; Novak, I.; Seruga, M.; Oliveira-Brett, A.M. Electroanalytical Oxidation of p-Coumaric Acid. Anal. Lett. 2007, 40, 3309–3321. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Kozlova, E.; Budnikov, H. Selective electrochemical sensor based on the electropolymerized p-coumaric acid for the direct determination of L-cysteine. Electrochim. Acta 2018, 270, 369–377. [Google Scholar] [CrossRef]
- Biegler, T.; Rand, D.A.J.; Woods, R.J. Limiting oxygen coverage on platinized platinum; Relevance to determination of real platinum area by hydrogen adsorption. J. Electroanal. Chem. Interfacial Electrochem. 1971, 29, 269–277. [Google Scholar] [CrossRef]
- Doña, R.J.M.; Herrera, M.J.A.; Pérez, P.J. Determination of the Real Surface Area of Pt Electrodes by Hydrogen Adsorption Using Cyclic Voltammetry. J. Chem. Ed. 2000, 77, 1195. [Google Scholar] [CrossRef]
- Fogh-Andersen, N.; Altura, B.; Altura, B.T.; Siggaard-Andersen, O. Composition of Interstitial Fluid. Clin. Chem. 1995, 41, 1522–1525. [Google Scholar] [CrossRef]
- Benvidi, A.; Dadras, A.; Abbasi, S.; Tezerjani, M.D.; Rezaeinasab, M.; Tabaraki, R.; Namazian, M. Experimental and computational study of the pKa of coumaric acid derivates. J. Chin. Chem. Soc. 2019, 66, 589–593. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Xiong, H.; Yang, Z. The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes.modified. Open Chem. 2021, 19, 961–973. [Google Scholar] [CrossRef]
- Updique, J.S.; Shults, C.M.; Gilligan, J.B.; Rhodes, K.R. A subcutaneous glucose sensor with improved longevity, dynamic range, and stability of calibration. Diabetes Care. 2000, 23, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Wu, X.; Shi, C.; Dai, Y.; Zhang, J.; Liu, W.; Wu, C.; Zhang, Y.; Huang, X.; Zeng, W. Flexible enzymatic biosensor based on graphene sponge for glucose detection in human sweat. Surf 2023, 36, 102525. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Sundramoorthy, A.K.; Arya, S. Synthesis and characterization of wearable cupros oxide /conductive fabric enable non-enzymatic electrochemical sensing of glucose. Ionics 2023, 29, 2501–2513. [Google Scholar] [CrossRef]
- Gao, N.; Cai, Z.; Chang, G.; He, Y. Non-invasive andwearable glucose biosensor based on gel electrolyte for detection of human sweat. J. Mater. Sci. 2023, 58, 890–901. [Google Scholar] [CrossRef]
- Bi, R.; Ma, X.; Miao, K.; Ma, P.; Wang, Q. Enzymatic biosensor based on dendritic gold nanostructure and enzyme precipitation coating for glucose sensing and detection. Enzyme Microb. Technol. 2023, 162, 110132. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Baycan, F. Fabricating a new immobilization matrix based on a conjugated polymer and application as a glucose biosensor. J. Appl. Polym. Sci. 2023, 140, 53268. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Yuan, M.; Yu, J.; Wang, Z.; Chen, X. One-step laser synthesis platinum nanostructures 3D porous graphene:A flexible dual-functional electrochemical biosensor for glucose and pH detection in humanperspiration. Talanta 2023, 257, 124362. [Google Scholar] [CrossRef]
- Promsuwan, K.; Soleh, A.; Samoson, K.; Sisahas, K.; Wangchuk, S.; Saichanapan, J.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Novel biosensor platform for glucose monitoring via smarthphone based on battery-less NFC potentiostat. Talanta 2023, 256, 124266. [Google Scholar] [CrossRef]









| Sample | Total Sugar g/100 mL Concentration (Label) * | Total Sugar g/100 mL Concentration Utilizing This Glucose Biosensor |
|---|---|---|
| Flavored soy milk | 4 | 3.92 ± 0.06 |
| Semi-skimmed flavored milk | 7.1 | 7.26 ± 0.33 |
| Fruit juice | 10.5 | 10.41 ± 0.30 |
| Sensor Structure | Application | Sensitivity | LOD | Linear Range | Applied Potential | Reference |
|---|---|---|---|---|---|---|
| GOx/CTs/GS/PB (GCGP) | sweat | 1.79 nA/mMcm2 | 2.45 μM | 8.17–100 μM | 0.075 V vs. Ag/AgCl | [40] |
| Cu2O—coated CF | 3739 mA/mMcm2 | 97.08 μM | 0–500 μM | 0.5 V vs. Ag/AgCl | [41] | |
| GOx—gel—rGO—Au/SPGE | sweat | 27.4 μA/mMcm2 53.7 μA/mMcm2 | 1.25 μM | 1.25–850 μM 0.85–7.72 mM | −0.3 V vs. Ag/AgCl | [42] |
| GOxEPC-DenAu/CC (FcOH) | serum | 72.45 μA/mMcm2 | 6.7 μM | 0.02–31.7 mM | 0.28 V vs. Ag/AgCl | [43] |
| GPE/PThBN/AuNPs/GOx | 0.1326 μA/mMcm2 | 0.034 mM | 2.97 μM–2.087 mM | −0.5 V vs. Ag/AgCl | [44] | |
| GOx/Pt-HEC/LSG | sweat | 69.64 μA/mMcm2 | 0.23 μM | 5–3000 μM | 0.60 V vs. Ag/AgCl | [45] |
| GOx-AuNPs-PEDOT:PSS/PB-G | 0.15 μM | 0.5–500 μM | 0.0 V vs. Ag/AgCl | [46] | ||
| Pt/poly(p-CA-GOx) | Proteins and commercial beverages | 54.06 mA/mMcm2 33.86 mA/mMcm2 | 0.66 mM | 1.5 mM 5–30 mM | 0.60 V vs. Ag/AgCl | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés-Ramírez, G.; Galicia, L. Glucose Oxidase Captured into Electropolymerized p-Coumaric Acid towards the Development of a Glucose Biosensor. Chemosensors 2023, 11, 345. https://doi.org/10.3390/chemosensors11060345
Valdés-Ramírez G, Galicia L. Glucose Oxidase Captured into Electropolymerized p-Coumaric Acid towards the Development of a Glucose Biosensor. Chemosensors. 2023; 11(6):345. https://doi.org/10.3390/chemosensors11060345
Chicago/Turabian StyleValdés-Ramírez, Gabriela, and Laura Galicia. 2023. "Glucose Oxidase Captured into Electropolymerized p-Coumaric Acid towards the Development of a Glucose Biosensor" Chemosensors 11, no. 6: 345. https://doi.org/10.3390/chemosensors11060345
APA StyleValdés-Ramírez, G., & Galicia, L. (2023). Glucose Oxidase Captured into Electropolymerized p-Coumaric Acid towards the Development of a Glucose Biosensor. Chemosensors, 11(6), 345. https://doi.org/10.3390/chemosensors11060345