Non-Enzymatic Electrochemical Sensing of Glucose with a Carbon Black/Polyaniline/Silver Nanoparticle Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Characterization and Methods
2.2.1. Characterization Techniques
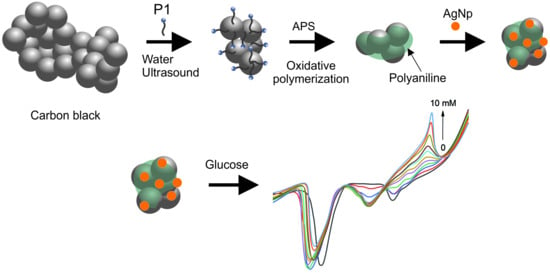
2.2.2. Core–Shell Composite Synthesis
2.2.3. Synthesis of Silver Nanoparticles
2.2.4. Electrode Modification and Electrochemical Analysis
3. Results
3.1. Morphology
3.2. Functional Groups
3.3. Electrochemical Characterization of Core–Shell Composites
3.4. Characterization of the Electrocatalytic System and Modified Electrode
3.4.1. Morfología de AgNP y CB-PANI.1-1/AgNP
3.4.2. Analysis of Functional Groups of Silver Nanoparticles and CB-PANI.1-1/AgNP Composite
3.4.3. Analysis of the Electroactive Area of the Modified Electrode
3.5. Glucose Detection Assay
3.6. Interference Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obreja, V.V.N. On the Performance of Supercapacitors with Electrodes Based on Carbon Nanotubes and Carbon Activated Material—A Review. Phys. E Low-Dimens. Syst. Nanostructures 2008, 40, 2596–2605. [Google Scholar] [CrossRef]
- Yogeswaran, U.; Chen, S. Recent Trends in the Application of Carbon Nanotubes–Polymer Composite Modified Electrodes for Biosensors: A Review. Anal. Lett. 2008, 41, 210–243. [Google Scholar] [CrossRef]
- Lufrano, F.; Staiti, P. Mesoporous Carbon Materials as Electrodes for Electrochemical Supercapacitors. Int. J. Electrochem. Sci. 2010, 5, 903–916. [Google Scholar] [CrossRef]
- Han, Z.; Li, H.; Xiao, J.; Song, H.; Li, B.; Cai, S.; Chen, Y.; Ma, Y.; Feng, X. Ultralow-Cost, Highly Sensitive, and Flexible Pressure Sensors Based on Carbon Black and Airlaid Paper for Wearable Electronics. ACS Appl. Mater. Interfaces 2019, 11, 33370–33379. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Cinti, S.; Mazzaracchio, V.; Scognamiglio, V.; Amine, A.; Moscone, D. Carbon Black as an Outstanding and Affordable Nanomaterial for Electrochemical (Bio)Sensor Design. Biosens. Bioelectron. 2020, 156, 112033. [Google Scholar] [CrossRef]
- Khodabakhshi, S.; Fulvio, P.F.; Andreoli, E. Carbon Black Reborn: Structure and Chemistry for Renewable Energy Harnessing. Carbon N. Y. 2020, 162, 604–649. [Google Scholar] [CrossRef]
- Silva, T.A.; Moraes, F.C.; Janegitz, B.C.; Fatibello-Filho, O.; Ganta, D. Electrochemical Biosensors Based on Nanostructured Carbon Black: A Review. J. Nanomater. 2017, 2017, 4571614. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Silva, T.A.; Vicentini, F.C.; Fatibello-Filho, O. Effect of Carbon Black Functionalization on the Analytical Performance of a Tyrosinase Biosensor Based on Glassy Carbon Electrode Modified with Dihexadecylphosphate Film. Enzym. Microb. Technol. 2018, 116, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, M.; Lepore, M.; Portaccio, M.; Di Tuoro, D.; Arduini, F.; Moscone, D.; Mita, D.G. Laccase Biosensor Based on Screen-Printed Electrode Modified Withthionine-Carbon Black Nanocomposite, for Bisphenol A Detection. Electrochimica Acta 2013, 109, 340–347. [Google Scholar] [CrossRef]
- Vicentini, F.C.; Raymundo-Pereira, P.A.; Janegitz, B.C.; Machado, S.A.S.; Fatibello-Filho, O. Nanostructured Carbon Black for Simultaneous Sensing in Biological Fluids. Sens. Actuators B Chem. 2016, 227, 610–618. [Google Scholar] [CrossRef]
- Lo, T.W.B.; Aldous, L.; Compton, R.G. The Use of Nano-Carbon as an Alternative to Multi-Walled Carbon Nanotubes in Modified Electrodes for Adsorptive Stripping Voltammetry. Sens. Actuators B Chem. 2012, 162, 361–368. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, Y.; Lv, Z.; Li, M.; Ma, S.; Cui, Z.; Kong, Q. Carbon Nanotubes, Conductive Carbon Black and Graphite Powder Based Paste Electrodes. Int. J. Electrochem. Sci. 2011, 6, 6063–6073. [Google Scholar] [CrossRef]
- Luo, K.; Guo, X.; Shi, N.; Sun, C. Synthesis and Characterization of Core-Shell Nanocomposites of Polyaniline and Carbon Black. Synth. Met. 2005, 151, 293–296. [Google Scholar] [CrossRef]
- Sotzing, G.A.; Phend, J.N.; Grubbs, R.H.; Lewis, N.S. Highly Sensitive Detection and Discrimination of Biogenic Amines Utilizing Arrays of Polyaniline/Carbon Black Composite Vapor Detectors. Chem. Mater. 2000, 12, 593–595. [Google Scholar] [CrossRef]
- Reiner-Rozman, C.; Pichler, B.; Madi, V.; Weißenböck, P.; Hegedüs, T.; Aspermair, P.; Bintinger, J. Optimization of Printed Polyaniline Composites for Gas Sensing Applications. Sensors 2022, 22, 5379. [Google Scholar] [CrossRef]
- Teng, Z.; Zhang, Z.; Li, X. Preparation of Pt Catalysts Supported on Polyaniline Modified Carbon Black and Electrocatalytic Methanol Oxidation. Synth. Met. 2023, 293, 117256. [Google Scholar] [CrossRef]
- Taguchi, M.; Ptitsyn, A.; McLamore, E.S.; Claussen, J.C. Nanomaterial-Mediated Biosensors for Monitoring Glucose. J. Diabetes Sci. Technol. 2014, 8, 403–411. [Google Scholar] [CrossRef]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A Critical Review of Glucose Biosensors Based on Carbon Nanomaterials: Carbon Nanotubes and Graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef]
- Dong, Q.; Ryu, H.; Lei, Y. Metal Oxide Based Non-Enzymatic Electrochemical Sensors for Glucose Detection. Electrochim. Acta 2021, 370, 137744. [Google Scholar] [CrossRef]
- Lakard, B. Electrochemical Biosensors Based on Conducting Polymers: A Review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Armando Zaragoza-Contreras, E.; Stockton-Leal, M.; Hernández-Escobar, C.A.; Hoshina, Y.; Guzmán-Lozano, J.F.; Kobayashi, T. Synthesis of Core-Shell Composites Using an Inverse Surfmer. J. Colloid Interface Sci. 2012, 377, 231–236. [Google Scholar] [CrossRef]
- Vega-Rios, A.; Hernández-Escobar, C.A.; Zaragoza-Contreras, E.A.; Kobayashi, T. Electrical and Electrochemical Properties of Polystyrene/Polyaniline Core-Shell Materials Prepared with the Use of a Reactive Surfactant as the Polyaniline Shell Precursor. Synth. Met. 2013, 167, 64–71. [Google Scholar] [CrossRef]
- Silva-Holguín, P.N.; Reyes-López, S.Y. Synthesis of Hydroxyapatite-Ag Composite as Antimicrobial Agent. Dose-Response 2020, 18, 1559325820951342. [Google Scholar] [CrossRef]
- Kato, Y.; Sugino, T. Effect of the Polyaniline/Carbon Black Additive on the Dispersion State of Carbon Nanotubes and Polymer Actuator Performance. Sens. Actuators A Phys. 2023, 355, 114302. [Google Scholar] [CrossRef]
- Stejskal, J.; Hajná, M.; Kašpárková, V.; Humpolíček, P.; Zhigunov, A.; Trchová, M. Purification of a Conducting Polymer, Polyaniline, for Biomedical Applications. Synth. Met. 2014, 195, 286–293. [Google Scholar] [CrossRef]
- Sugatri, R.I.; Wirasadewa, Y.C.; Saputro, K.E.; Muslih, E.Y.; Ikono, R.; Nasir, M. Recycled Carbon Black from Waste of Tire Industry: Thermal Study. Microsyst. Technol. 2017, 24, 749–755. [Google Scholar] [CrossRef]
- Su, C.; Wang, G.; Huang, F.; Sun, Y. Effect of Carbon Black Modified with Polyaniline on Resistivity Behavior of Polyethylene/Carbon Black Composites. J. Macromol. Sci. Part B 2008, 47, 65–75. [Google Scholar] [CrossRef]
- Jow, J.J.; Hsieh, L.Y.; Cho, H.P.; Chen, H.R.; Kuo, C.W. Determination of Surface Area of Carbon-Black by Simple Cyclic-Voltammetry Measurements in Aqueous H2SO4. J. Ind. Eng. Chem. 2013, 19, 1730–1734. [Google Scholar] [CrossRef]
- Yamada, H.; Yoshii, K.; Asahi, M.; Chiku, M.; Kitazumi, Y. Cyclic Voltammetry Part 1: Fundamentals. Electrochemistry 2022, 90, 102005. [Google Scholar] [CrossRef]
- Arduini, F.; Di Nardo, F.; Amine, A.; Micheli, L.; Palleschi, G.; Moscone, D. Carbon Black-Modified Screen-Printed Electrodes as Electroanalytical Tools. Electroanalysis 2012, 24, 743–751. [Google Scholar] [CrossRef]
- Kausar, A. Electromagnetic Interference Shielding of Polyaniline/Poloxalene/Carbon Black Composite. Int. J. Mater. Chem. 2016, 6, 6–11. [Google Scholar]
- Ahani, M.; Khatibzadeh, M. Size Optimisation of Silver Nanoparticles Synthesised by Gallic Acid Using the Response Surface Methodology. Micro Nano Lett. 2020, 15, 403–408. [Google Scholar] [CrossRef]
- Yoosaf, K.; Ipe, B.I.; Suresh, C.H.; Thomas, K.G. In Situ Synthesis of Metal Nanoparticles and Selective Naked-Eye Detection of Lead Ions from Aqueous Media. J. Phys. Chem. C 2007, 111, 12839–12847. [Google Scholar] [CrossRef]
- Paulkumar, K.; Gnanajobitha, G.; Vanaja, M.; Pavunraj, M.; Annadurai, G. Green Synthesis of Silver Nanoparticle and Silver Based Chitosan Bionanocomposite Using Stem Extract of Saccharum Officinarum and Assessment of Its Antibacterial Activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 035019. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.A.; Sundararaju, S.; Ramaraj, R.; Maniam, G.P.; Govindan, N. Synthesis of Silver Nanoparticles Using Marine Macroalgae Padina Sp. and Its Antibacterial Activity towards Pathogenic Bacteria. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 3. [Google Scholar] [CrossRef]
- Gupta, K.; Jana, P.C.; Meikap, A.K. Optical and Electrical Transport Properties of Polyaniline-Silver Nanocomposite. Synth. Met. 2010, 160, 1566–1573. [Google Scholar] [CrossRef]
- Domínguez-Aragón, A.; Conejo-Dávila, A.S.; Zaragoza-Contreras, E.A.; Dominguez, R.B. Pretreated Screen-Printed Carbon Electrode and Cu Nanoparticles for Creatinine Detection in Artificial Saliva. Chemosensors 2023, 11, 102. [Google Scholar] [CrossRef]
- Pasta, M.; La Mantia, F.; Cui, Y. Mechanism of Glucose Electrochemical Oxidation on Gold Surface. Electrochimica Acta 2010, 55, 5561–5568. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Kang, B.C.; Ha, T.J. Non-Enzymatic Electrochemical Glucose Sensors Based on Polyaniline/Reduced-Graphene-Oxide Nanocomposites Functionalized with Silver Nanoparticles. J. Mater. Chem. C 2020, 8, 5112–5123. [Google Scholar] [CrossRef]
- Quan, H.; Park, S.U.; Park, J. Electrochemical Oxidation of Glucose on Silver Nanoparticle-Modified Composite Electrodes. Electrochim. Acta 2010, 55, 2232–2237. [Google Scholar] [CrossRef]
- Poletti Papi, M.A.; Caetano, F.R.; Bergamini, M.F.; Marcolino-Junior, L.H. Facile Synthesis of a Silver Nanoparticles/Polypyrrole Nanocomposite for Non-Enzymatic Glucose Determination. Mater. Sci. Eng. C 2017, 75, 88–94. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Salim, H.; Hassan, I.U.; Awan, T.; Arshad, F.; Pedram, M.Z.; Ahmed, W.; Qurashi, A. Recent Advances in Non-Enzymatic Glucose Sensors Based on Metal and Metal Oxide Nanostructures for Diabetes Management—A Review. Front. Chem. 2021, 9, 748957. [Google Scholar] [CrossRef]
- Joshi, A.C.; Markad, G.B.; Haram, S.K. Rudimentary Simple Method for the Decoration of Graphene Oxide with Silver Nanoparticles: Their Application for the Amperometric Detection of Glucose in the Human Blood Samples. Electrochim. Acta 2015, 161, 108–114. [Google Scholar] [CrossRef]
- Vassilyev, Y.B.; Khazova, O.A.; Nikolaeva, N.N. Kinetics and Mechanism of Glucose Electrooxidation on Different Electrode-Catalysts Part I. Adsorption and Oxidation on Platinum; Elsevier Sequoia S.A.: Amsterdam, The Netherlands, 1985; Volume 196. [Google Scholar]
- Abd El Rehim, S.S.; Hassan, H.H.; Ibrahim, M.A.M.; Amin, M.A. Electrochemical Behaviour of a Silver Electrode in NaOH Solutions. Monatshefte Fuer Chem. Chem. Mon. 1998, 129, 1103–1117. [Google Scholar] [CrossRef]
- Pasta, M.; Ruffo, R.; Falletta, E.; Mari, C.M.; Pina, C. Della Alkaline Glucose Oxidation on Nanostructured Gold Electrodes. Gold Bull 2010, 43, 57–64. [Google Scholar] [CrossRef]
- Aparicio-Martínez, E.P.; Vega-Rios, A.; Osuna, V.; Dominguez, R.B. Salivary Glucose Detection with Laser Induced Graphene/AgNPs Non-Enzymatic Sensor. Biosensors 2023, 13, 207. [Google Scholar] [CrossRef]
- Ravichandran, R.; Martinez, J.G.; Jager, E.W.H.; Phopase, J.; Turner, A.P.F. Type i Collagen-Derived Injectable Conductive Hydrogel Scaffolds as Glucose Sensors. ACS Appl. Mater. Interfaces 2018, 10, 16244–16249. [Google Scholar] [CrossRef]
- Comba, F.N.; Romero, M.R.; Garay, F.S.; Baruzzi, A.M. Mucin and Carbon Nanotube-Based Biosensor for Detection of Glucose in Human Plasma. Anal. Biochem. 2018, 550, 34–40. [Google Scholar] [CrossRef]
- Asif, M.H.; Razaq, A.; Akbar, N.; Danielsson, B.; Sultana, I. Facile Synthesis of Multisegment Au/Ni/Au Nanowire for High Performance Electrochemical Glucose Sensor. Mater. Res. Express 2019, 6, 095028. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Electrochemical Deposition of Silver/Silver Oxide on Reduced Graphene Oxide for Glucose Sensing. J. Solid State Electrochem. 2015, 19, 2255–2263. [Google Scholar] [CrossRef]









| Composite | CB g (mmol) | P1 g (mmol) | APS g (mmol) |
|---|---|---|---|
| CB-PANI.1-1 | 0.2 (0.5563) | 0.2 (0.5563) | 0.1586 (0.6958) |
| CB-PANI.1-2 | 0.1 (0.5563) | 0.2 (0.5563) | 0.1586 (0.6958) |
| CB-PANI.1-4 | 0.05 (0.5563) | 0.2 (0.5563) | 0.1586 (0.6958) |
| Detection System | LOD (µM) | Sensitivity (μA mM−1 cm2) | LROD (mM) | Reference |
|---|---|---|---|---|
| GOx/MWCNTs/Pt | 50 | 288.86 | 0–5 | [48] |
| GOx/CNTMuci/Pt | 3 | 15 | 0.002–0.003 | [49] |
| GOx/Au/Ni/Au/NW | ---- | 123.3 mV/decade | 0.5–10 | [50] |
| Ag/Ag2O/rGO | 0.06 | 32 | 0.2–8 | [51] |
| LIG/AgNPs | 412 | 24.1 | 0–10 | [47] |
| CB-PANI.1-1/AgNP | 520 | 41 | 1–10 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñón-Balderrama, C.I.; Hernández-Escobar, C.A.; Reyes-López, S.Y.; Conejo-Dávila, A.S.; Estrada-Monje, A.; Zaragoza-Contreras, E.A. Non-Enzymatic Electrochemical Sensing of Glucose with a Carbon Black/Polyaniline/Silver Nanoparticle Composite. Chemosensors 2024, 12, 26. https://doi.org/10.3390/chemosensors12020026
Piñón-Balderrama CI, Hernández-Escobar CA, Reyes-López SY, Conejo-Dávila AS, Estrada-Monje A, Zaragoza-Contreras EA. Non-Enzymatic Electrochemical Sensing of Glucose with a Carbon Black/Polyaniline/Silver Nanoparticle Composite. Chemosensors. 2024; 12(2):26. https://doi.org/10.3390/chemosensors12020026
Chicago/Turabian StylePiñón-Balderrama, Claudia Ivone, Claudia Alejandra Hernández-Escobar, Simón Yobanni Reyes-López, Alain Salvador Conejo-Dávila, Anayansi Estrada-Monje, and Erasto Armando Zaragoza-Contreras. 2024. "Non-Enzymatic Electrochemical Sensing of Glucose with a Carbon Black/Polyaniline/Silver Nanoparticle Composite" Chemosensors 12, no. 2: 26. https://doi.org/10.3390/chemosensors12020026
APA StylePiñón-Balderrama, C. I., Hernández-Escobar, C. A., Reyes-López, S. Y., Conejo-Dávila, A. S., Estrada-Monje, A., & Zaragoza-Contreras, E. A. (2024). Non-Enzymatic Electrochemical Sensing of Glucose with a Carbon Black/Polyaniline/Silver Nanoparticle Composite. Chemosensors, 12(2), 26. https://doi.org/10.3390/chemosensors12020026