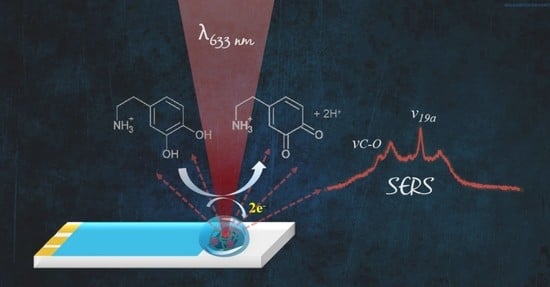
Nickel Tetrasulfonated Phthalocyanine Decorated with AuNP as a Double Sensorial Platform: SERS and Electrochemical
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sensing Platform: Fabrication and Characterization
2.3. Surface Characterization and Spectroelectrochemical Analysis
3. Results and Discussion
3.1. Modified SPCE: Fabrications Steps and Surface Characterization
3.2. Cyclic Voltammetry and Electrochemical Impedance Spectroscopy
3.3. Spectroelectrochemistry of SPCE/p-NiTsPc/AuNP towards Dopamine Oxidation
3.4. Determination of Dopamine at the SPCE/p-NiTsPc/AuNP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhai, Y.; Zhu, Z.; Zhou, S.; Zhu, C.; Dong, S. Recent advances in spectroelectrochemistry. Nanoscale 2018, 10, 3089–3111. [Google Scholar] [CrossRef]
- Kaim, W.; Fiedler, J. Spectroelectrochemistry: The best of two worlds. Chem. Soc. Rev. 2009, 38, 3373–3382. [Google Scholar] [CrossRef]
- Dos Santos, M.F.; Katic, V.; Dos Santos, P.L.; Pires, B.M.; Formiga, A.L.B.; Bonacin, J.A. 3D-Printed low-cost spectroelectrochemical cell for in situ raman measurements. Anal. Chem. 2019, 91, 10386–10389. [Google Scholar] [CrossRef]
- Maringa, A.; Nyokong, T. The influence of gold nanoparticles on the electroactivity of nickel tetrasulfonated phthalocyanine. J. Porphyr. Phthalocyanines 2014, 18, 642–651. [Google Scholar] [CrossRef]
- Mbokou Foukmeniok, S.; Bako, R.F.Y.; Ilboudo, O.; Karanga, Y.; Njanja, E.; Pontie, M.; Tapsoba, I.; Tonle Kenfack, I.; Djouaka, R. Sensitive Carbon Fiber Microelectrode for the Quantification of Diuron in Quality Control of a Commercialized Formulation. Int. J. Anal. Chem. 2022, 2022, 9p. [Google Scholar] [CrossRef]
- Musa, I.; Raffin, G.; Hangouet, M.; Martin, M.; Alcacer, A.; Zine, N.; Bellagambi, F.; Jaffrezic-Renault, N.; Errachid, A. Development of a Chitosan/Nickel Phthalocyanine Composite based Conductometric Micro-sensor for Methanol Detection. Electroanalysis 2022, 34, 1–11. [Google Scholar] [CrossRef]
- Pereira-Rodrigues, N.; Albin, V.; Koudelka-Hep, M.; Auger, V.; Pailleret, A.; Bedioui, F. Nickel tetrasulfonated phthalocyanine based platinum microelectrode array for nitric oxide oxidation. Electrochem. Commun. 2002, 4, 922–927. [Google Scholar] [CrossRef]
- Xu, H.; Yang, F. The interplay of dopamine metabolism abnormalities and mitochondrial defects in the pathogenesis of schizophrenia. Transl. Psychiatry 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Birtwistle, J.; Baldwin, D. Role of dopamine in schizophrenia and Parkinson’s disease. Br. J. Nurs. 1998, 7, 832–841. [Google Scholar] [CrossRef]
- Olmo, F.; Garoz-Ruiz, J.; Colina, A.; Heras, A. Derivative UV/Vis spectroelectrochemistry in a thin-layer regime: Deconvolution and simultaneous quantification of ascorbic acid, dopamine and uric acid. Anal. Bioanal. Chem. 2020, 412, 6329–6339. [Google Scholar] [CrossRef]
- González-Diéguez, N.; Colina, A.; López-Palacios, J.; Heras, A. Spectroelectrochemistry at screen-printed electrodes: Determination of dopamine. Anal. Chem. 2012, 84, 9146–9153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Sun, W.; Cao, S.H.; Jiang, W.L.; Peng, H.; Cai, S.H.; Chen, Z. NMR spectroelectrochemistry in studies of dopamine oxidation. Electrochemistry 2020, 88, 200–204. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Baokang, J.I.N.; Xiangqin, L.I.N. In-situ FTIR spectroelectrochemical study of dopamine at a glassy carbon electrode in a neutral solution. Anal. Sci. 2002, 18, 931–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibañez, D.; Santidrian, A.; Heras, A.; Kalbáč, M.; Colina, A. Study of adenine and guanine oxidation mechanism by surface-enhanced Raman spectroelectrochemistry. J. Phys. Chem. C 2015, 119, 8191–8198. [Google Scholar] [CrossRef] [Green Version]
- de Lucena, N.C.; Miyazaki, C.M.; Shimizu, F.M.; Constantino, C.J.L.; Ferreira, M. Layer-by-layer composite film of nickel phthalocyanine and montmorillonite clay for synergistic effect on electrochemical detection of dopamine. Appl. Surf. Sci. 2018, 436, 957–966. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.S.; Rubira, R.J.G.; Silva, J.N.; Aléssio, P. Role of Iron Phthalocyanine Coordination in Catecholamines Detection. Surfaces 2021, 4, 323–335. [Google Scholar] [CrossRef]
- Martin, C.S.; Gouveia-Caridade, C.; Crespilho, F.N.; Constantino, C.J.L.; Brett, C.M.A. Iron Phthalocyanine Electrodeposited Films: Characterization and Influence on Dopamine Oxidation. J. Phys. Chem. C 2016, 120, 15698–15706. [Google Scholar] [CrossRef]
- Yusoff, N.; Pandikumar, A.; Ramaraj, R.; Lim, H.N.; Huang, N.M. Gold nanoparticle based optical and electrochemical sensing of dopamine. Microchim. Acta 2015, 182, 2091–2114. [Google Scholar] [CrossRef]
- Pérez-Morales, M.; Muñoz, E.; Martín-Romero, M.T.; Camacho, L. Anodic electrodeposition of NiTSPP from aqueous basic media. Langmuir 2005, 21, 5468–5474. [Google Scholar] [CrossRef]
- Li, J.; Xie, H.; Chen, L. A sensitive hydrazine electrochemical sensor based on electrodeposition of gold nanoparticles on choline film modified glassy carbon electrode. Sensors Actuators B Chem. 2011, 153, 239–245. [Google Scholar] [CrossRef]
- Obirai, J.; Bedioui, F.; Nyokong, T. Electro-oxidation of phenol and its derivatives on poly-Ni(OH)TPhPyPc modified vitreous carbon electrodes. J. Electroanal. Chem. 2005, 576, 323–332. [Google Scholar] [CrossRef]
- Rosłonek, G.; Taraszewska, J. Electrocatalytic oxidation of alcohols on glassy carbon electrodes electrochemically modified with nickel tetraazamacrocyclic complexes: Mechanism of f. J. Electroanal. Chem. 1992, 325, 285–300. [Google Scholar] [CrossRef]
- Trévin, S.; Bedioui, F.; Devynck, J. New electropolymerized nickel porphyrin films. Application to the detection of nitric oxide in aqueous solution. J. Electroanal. Chem. 1996, 408, 261–265. [Google Scholar] [CrossRef]
- Trevin, S.; Fethi, B.; Villegas, M.G.G.; Bied-Charreton, C. Electropolymerized nickel macrocyclic complex-based films: Design and electrocatalytic application. J. Mater. Chem. 1997, 7, 923–928. [Google Scholar] [CrossRef]
- Elias, J.; Gizowska, M.; Brodard, P.; Widmer, R.; Dehazan, Y.; Graule, T.; Michler, J.; Philippe, L. Electrodeposition of gold thin films with controlled morphologies and their applications in electrocatalysis and SERS. Nanotechnology 2012, 23. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, J.; Di, J.; Tu, Y. Electrodeposition of large size gold nanoparticles on indium tin oxide glass and application as refractive index sensor. Electrochem. Commun. 2009, 11, 1034–1037. [Google Scholar] [CrossRef]
- Resmi, P.E.; Raveendran, J.; Suneesh, P.V.; Ramanchandran, T.; Nair, B.G.; Satheesh Babu, T.G. Screen-printed carbon electrode for the electrochemical detection of conjugated bilirubin. Mater. Lett. 2021, 304, 130574. [Google Scholar] [CrossRef]
- Mazzaracchio, V.; Tomei, M.R.; Cacciotti, I.; Chiodoni, A.; Novara, C.; Castellino, M.; Scordo, G.; Amine, A.; Moscone, D.; Arduini, F. Inside the different types of carbon black as nanomodifiers for screen-printed electrodes. Electrochim. Acta 2019, 317, 673–683. [Google Scholar] [CrossRef]
- Furini, L.N.; Martin, C.S.; Camacho, S.A.; Rubira, R.J.G.; Fernandes, J.D.; Silva, E.A.; Gomes, T.C.; Stunges, G.M.; Constantino, C.J.L.; Alessio, P. Electrochemical properties of nickel phthalocyanine: The effect of thin film morphology tuned by deposition techniques. Thin Solid Films 2020, 699, 137897. [Google Scholar] [CrossRef]
- Maximino, M.D.; Martin, C.S.; Paulovich, F.V.; Alessio, P. Layer-by-Layer Thin Film of Iron Phthalocyanine as a Simple and Fast Sensor for Polyphenol Determination in Tea Samples. J. Food Sci. 2016, 81, C2344–C2351. [Google Scholar] [CrossRef]
- Zucolotto, V.; Ferreira, M.; Cordeiro; Ḿarcia, R.; Constantino, C.J.L.; Balogh, D.T.; Zanatta, A.R.; Moreira, W.C.; Oliveira, O.N. Unusual interactions binding iron tetrasulfonated phthalocyanine and poly(allylamine hydrochloride) in layer-by-layer films. J. Phys. Chem. B 2003, 107, 3733–3737. [Google Scholar] [CrossRef]
- Aroca, R.; Martin, F. Trace analysis of tetrasulphonated copper phthalocyanine by surface enhanced Raman spectroscopy. J. Raman Spectrosc. 1986, 17, 243–247. [Google Scholar] [CrossRef]
- Wing, R.M.; Callahan, K.P. Characterization of metal-oxygen bridge systems. Inorg. Chem. 1969, 8, 871–874. [Google Scholar] [CrossRef]
- Fox, B.G.; Shanklin, J.; Ai, J.; Loehr, T.M.; Sanders-Loehr, J. Resonance Raman Evidence for an Fe-O-Fe Center in Stearoyl-ACP Desaturase. Primary Sequence Identity with Other Diiron-Oxo Proteins. Biochemistry 1994, 33, 12776–12786. [Google Scholar] [CrossRef] [PubMed]
- Basova, T.V.; Kiselev, V.G.; Latteyer, F.; Peisert, H.; Chassé, T. Molecular organization in the thin films of gallium(III) phthalocyanine chloride and its μ-(oxo)dimer: Optical spectroscopy and XPS study. Appl. Surf. Sci. 2014, 322, 242–248. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2000; ISBN 978-0-471-04372-0. [Google Scholar]
- Ognjanović, M.; Stanković, D.M.; Jović, M.; Krstić, M.P.; Lesch, A.; Girault, H.H.; Antić, B. Inkjet-Printed Carbon Nanotube Electrodes Modified with Dimercaptosuccinic Acid-Capped Fe3O4 Nanoparticles on Reduced Graphene Oxide Nanosheets for Single-Drop Determination of Trifluoperazine. ACS Appl. Nano Mater. 2020, 3, 4654–4662. [Google Scholar] [CrossRef]
- Petković, B.B.; Ognjanović, M.; Krstić, M.; Stanković, V.; Babincev, L.; Pergal, M.; Stanković, D.M. Boron-doped diamond electrode as efficient sensing platform for simultaneous quantification of mefenamic acid and indomethacin. Diam. Relat. Mater. 2020, 105, 107785. [Google Scholar] [CrossRef]
- Lee, J.; Murugappan, K.; Arrigan, D.W.M.; Silvester, D.S. Oxygen reduction voltammetry on platinum macrodisk and screen-printed electrodes in ionic liquids: Reaction of the electrogenerated superoxide species with compounds used in the paste of Pt screen-printed electrodes? Electrochim. Acta 2013, 101, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Agboola, B.O.; Ozoemena, K.I.; Nyokong, T. Electrochemical properties of benzylmercapto and dodecylmercapto tetra substituted nickel phthalocyanine complexes: Electrocatalytic oxidation of nitrite. Electrochim. Acta 2006, 51, 6470–6478. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Brett, A.M.O. Electrochemistry: Principles, Methods, and Applications; Oxford University Press: Oxford, UK, 1993; ISBN 0198553889. [Google Scholar]
- Kavazoi, H.S.; Martin, C.S.; Alessio, P. Comparative study of tetrasulfonated phthalocyanine modified screen-printed electrodes in paraquat. Synth. Met. 2022, 284, 116988. [Google Scholar] [CrossRef]
- Boonkaew, S.; Chaiyo, S.; Jampasa, S.; Rengpipat, S.; Siangproh, W.; Chailapakul, O. An origami paper-based electrochemical immunoassay for the C-reactive protein using a screen-printed carbon electrode modified with graphene and gold nanoparticles. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef]
- Khater, M.; de la Escosura-Muñiz, A.; Quesada-González, D.; Merkoçi, A. Electrochemical detection of plant virus using gold nanoparticle-modified electrodes. Anal. Chim. Acta 2019, 1046, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Chaiyo, S.; Mehmeti, E.; Siangproh, W.; Hoang, T.L.; Nguyen, H.P.; Chailapakul, O.; Kalcher, K. Non-enzymatic electrochemical detection of glucose with a disposable paper-based sensor using a cobalt phthalocyanine–ionic liquid–graphene composite. Biosens. Bioelectron. 2018, 102, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.R.; Noori, M.T.; Ghangrekar, M.M. Carbon supported nickel-phthalocyanine/MnOx as novel cathode catalyst for microbial fuel cell application. Int. J. Hydrogen Energy 2017, 42, 23085–23094. [Google Scholar] [CrossRef]
- Georgescu State, R.; Stefanov, C.; van Staden, J.K.F.; van Staden, R.I.S. Application of a Tetraamino Cobalt(II) Phthalocyanine Modified Screen Printed Carbon Electrode for the Sensitive Electrochemical Determination of L-Dopa in Pharmaceutical and Biological Samples. Electroanalysis 2021, 33, 1778–1788. [Google Scholar] [CrossRef]
- Cheng, H.; Qiu, H.; Zhu, Z.; Li, M.; Shi, Z. Investigation of the electrochemical behavior of dopamine at electrodes modified with ferrocene-filled double-walled carbon nanotubes. Electrochim. Acta 2012, 63, 83–88. [Google Scholar] [CrossRef]
- Shi, Z.; Wu, X.; Zou, Z.; Yu, L.; Hu, F.; Li, Y.; Guo, C.; Li, C.M. Screen-printed analytical strip constructed with bacteria-templated porous N-doped carbon nanorods/Au nanoparticles for sensitive electrochemical detection of dopamine molecules. Biosens. Bioelectron. 2021, 186, 113303. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Wilson, D.; Gonçalves, D.; Oliveira, O.N. Low-cost screen-printed electrodes based on electrochemically reduced graphene oxide-carbon black nanocomposites for dopamine, epinephrine and paracetamol detection. J. Colloid Interface Sci. 2018, 515, 101–108. [Google Scholar] [CrossRef]
- Jha, O.; Yadav, T.K.; Yadav, R.A. Structural and vibrational study of a neurotransmitter molecule: Dopamine [4-(2-aminoethyl) benzene-1,2-diol]. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 473–484. [Google Scholar] [CrossRef]
- Figueiredo, M.L.B.; Martin, C.S.; Furini, L.N.; Rubira, R.J.G.; Batagin-Neto, A.; Alessio, P.; Constantino, C.J.L. Surface-enhanced Raman scattering for dopamine in Ag colloid: Adsorption mechanism and detection in the presence of interfering species. Appl. Surf. Sci. 2020, 522, 146466. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, J.; Jia, Z.; Huo, D.; Liu, Q.; Zhong, D.; Hu, Y.; Yang, M.; Bian, M.; Hou, C. In-situ growth of gold nanoparticles on a 3D-network consisting of a MoS2/rGO nanocomposite for simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Microchim. Acta 2019, 186, 92. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shi, W.; Bao, N.; Yu, C.; Gu, H. Electrochemically reduced graphene oxide and gold nanoparticles on an indium tin oxide electrode for voltammetric sensing of dopamine. Microchim. Acta 2019, 186, 310. [Google Scholar] [CrossRef] [PubMed]
- Harsini, M.; Widyaningrum, B.A.; Fitriany, E.; Paramita, D.R.A.; Farida, A.N.; Baktir, A.; Kurniawan, F.; Sakti, S.C.W. Electrochemical synthesis of polymelamine/gold nanoparticle modified carbon paste electrode as voltammetric sensor of dopamine. Chin. J. Anal. Chem. 2022, 50, 100052. [Google Scholar] [CrossRef]
- Ben Ali Hassine, C.; Kahri, H.; Barhoumi, H. Enhancing Dopamine Detection Using Glassy Carbon Electrode Modified with Graphene Oxide, Nickel and Gold Nanoparticles. J. Electrochem. Soc. 2020, 167, 027516. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Sridevi, V. In Situ Electrodeposited Gold Nanoparticles on Polyaniline-Modified Electrode Surface for the Detection of Dopamine in Presence of Ascorbic Acid and Uric Acid. Electrocatalysis 2021, 12, 415–435. [Google Scholar] [CrossRef]
- Uppachai, P.; Srijaranai, S.; Poosittisak, S.; Md Isa, I.; Mukdasai, S. Supramolecular Electrochemical Sensor for Dopamine Detection Based on Self-Assembled Mixed Surfactants on Gold Nanoparticles Deposited Graphene Oxide. Molecules 2020, 25, 2528. [Google Scholar] [CrossRef]
- Sipuka, D.S.; Arotiba, O.A.; Sebokolodi, T.I.; Tsekeli, T.R.; Nkosi, D. Gold-dendrimer Nanocomposite Based Electrochemical Sensor for Dopamine. Electroanalysis 2023, 35. [Google Scholar] [CrossRef]
- Luo, Q.; Su, Y.; Zhang, H. Sensitive dopamine sensor based on electrodeposited gold nanoparticles and electro-modulated MoS2 nanoflakes. J. Iran. Chem. Soc. 2023, 20, 731–738. [Google Scholar] [CrossRef]
- Pari, M.; Reddy, K.R.V.; Fasiulla, K.B.C. Amperometric determination of dopamine based on an interface platform comprising tetra-substituted Zn2+ phthalocyanine film layer with embedment of reduced graphene oxide. Sensors Actuators A Phys. 2020, 316, 112377. [Google Scholar] [CrossRef]
- Luhana, C.; Mashazi, P. Simultaneous Detection of Dopamine and Paracetamol on Electroreduced Graphene Oxide–Cobalt Phthalocyanine Polymer Nanocomposite Electrode. Electrocatalysis 2023, 14, 406–417. [Google Scholar] [CrossRef]
- Luhana, C.; Moyo, I.; Tshenkeng, K.; Mashazi, P. In-sera selectivity detection of catecholamine neurotransmitters using covalent composite of cobalt phthalocyanine and aminated graphene quantum dots. Microchem. J. 2022, 180, 107605. [Google Scholar] [CrossRef]
- Mounesh; Malathesh, P.; Praveen Kumara, N.Y.; Jilani, B.S.; Mruthyunjayachari, C.D.; Venugopala Reddy, K.R. Synthesis and characterization of tetra-ganciclovir cobalt (II) phthalocyanine for electroanalytical applications of AA/DA/UA. Heliyon 2019, 5, e01946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazloum-Ardakani, M.; Beitollahi, H.; Ganjipour, B.; Naeimi, H.; Nejati, M. Electrochemical and catalytic investigations of dopamine and uric acid by modified carbon nanotube paste electrode. Bioelectrochemistry 2009, 75, 1–8. [Google Scholar] [CrossRef]
- Wang, H.-S.; Li, T.-H.; Jia, W.-L.; Xu, H.-Y. Highly selective and sensitive determination of dopamine using a Nafion/carbon nanotubes coated poly(3-methylthiophene) modified electrode. Biosens. Bioelectron. 2006, 22, 664–669. [Google Scholar] [CrossRef]








| Electrode Modifier | Linear Range (μmol/L) | Limit of Detection (μmol/L) | Ref. |
|---|---|---|---|
| 3D-MoS2/rGO/Au | 0.3–198.3 | 0.11 | [53] |
| ErGO-AuNP/ITO | 0.02–200 | 0.015 | [54] |
| AuNPs/polymelanine/CPE | 0.1–11 | 0.067 | [55] |
| GCE/EG-Ni-Au(NPs) | 0.2–100 | 0.10 | [56] |
| GCE/PANI/AuNPs | 20–100 | 16.0 | [57] |
| TBABr/SDS⋅AuNPs/GO/GCE | 0.02–1.0 | 0.01 | [58] |
| GCE-PPI-AuNPs | 0.1–125 | 0.16 | [59] |
| AuNPs/1T-MoS2/GCE | 0.2–80 | 0.16 | [60] |
| GCE/rGO−Zn(II)TPEBiPc | 0.05–0.8 | 0.0016 | [61] |
| GCE-ERGO/polyCoTAPc | 2.0–100 | 0.095 | [62] |
| Au-IPA-AmGQD-CoTCPhOPc | 1.0–50 | 0.20 | [63] |
| CoTGPc/GCE | 2–12 | 1.0 | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, C.S.; Kavazoi, H.S.; Miyazaki, C.M.; Alessio, P.; Constantino, C.J.L. Nickel Tetrasulfonated Phthalocyanine Decorated with AuNP as a Double Sensorial Platform: SERS and Electrochemical. Chemosensors 2023, 11, 372. https://doi.org/10.3390/chemosensors11070372
Martin CS, Kavazoi HS, Miyazaki CM, Alessio P, Constantino CJL. Nickel Tetrasulfonated Phthalocyanine Decorated with AuNP as a Double Sensorial Platform: SERS and Electrochemical. Chemosensors. 2023; 11(7):372. https://doi.org/10.3390/chemosensors11070372
Chicago/Turabian StyleMartin, Cibely Silva, Henry Seitiro Kavazoi, Celina Massumi Miyazaki, Priscila Alessio, and Carlos José Leopoldo Constantino. 2023. "Nickel Tetrasulfonated Phthalocyanine Decorated with AuNP as a Double Sensorial Platform: SERS and Electrochemical" Chemosensors 11, no. 7: 372. https://doi.org/10.3390/chemosensors11070372
APA StyleMartin, C. S., Kavazoi, H. S., Miyazaki, C. M., Alessio, P., & Constantino, C. J. L. (2023). Nickel Tetrasulfonated Phthalocyanine Decorated with AuNP as a Double Sensorial Platform: SERS and Electrochemical. Chemosensors, 11(7), 372. https://doi.org/10.3390/chemosensors11070372