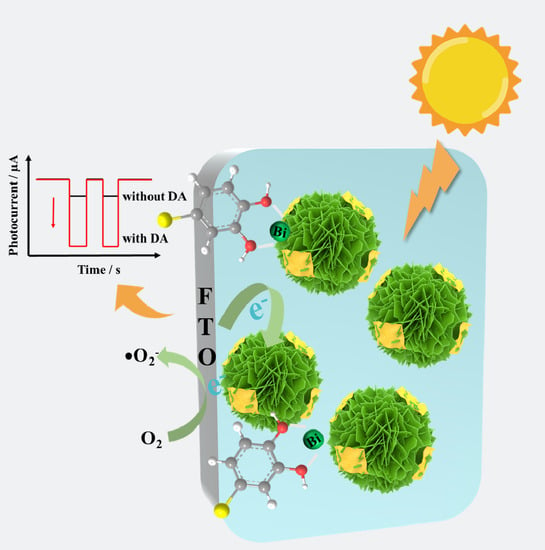
Bi2WO6@g-C3N4 Heterostructure for Cathodic Photoelectrochemical Dopamine Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatuses
2.3. Syntheses of Bi2WO6@g-C3N4 Composites
2.4. Fabrication of Bi2WO6@g-C3N4/FTO PEC Sensor
3. Results and Discussion
3.1. Morphology Characterization of Bi2WO6@g-C3N4 Composites
3.2. Structure and Chemical Compositions of Bi2WO6@g-C3N4 Composites
3.3. Construction of the PEC Sensor
3.4. PEC Sensor for DA Detection
3.5. Selectivity and Stability of the PEC Sensor
3.6. Real Samples Analysis
3.7. PEC Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berke, J.D. What does dopamine mean? Nat. Neurosci. 2018, 21, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, M.; Gao, P.; Gao, W.; Bian, Z.; Jia, N. Photoelectrochemical sensing of dopamine using gold-TiO2 nanocomposites and visible-light illumination. Microchim. Acta 2019, 186, 326. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gomez, F.A.; Miao, Y.; Cui, P.; Lee, W. A colorimetric assay system for dopamine using microfluidic paper-based analytical devices. Talanta 2019, 194, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Segura-Aguilar, J.; Paris, I.; Munoz, P.; Ferrari, E.; Zecca, L.; Zucca, F.A. Protective and toxic roles of dopamine in Parkinson’s disease. J. Neurochem. 2014, 129, 898–915. [Google Scholar] [CrossRef]
- Faraone, S.V. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav. Rev. 2018, 87, 255–270. [Google Scholar] [CrossRef]
- Guo, Z.; Seol, M.L.; Kim, M.S.; Ahn, J.H.; Choi, Y.K.; Liu, J.H.; Huang, X.J. Sensitive and selective electrochemical detection of dopamine using an electrode modified with carboxylated carbonaceous spheres. Analyst 2013, 138, 2683–2690. [Google Scholar] [CrossRef]
- Zhuang, X.M.; Gao, X.Q.; Tian, C.Y.; Cui, D.L.; Luan, F.; Wang, Z.G.; Xiong, Y.; Chen, L.X. Synthesis of europium(iii)-doped copper nanoclusters for electrochemiluminescence bioanalysis. Chem. Commun. 2020, 56, 5755–5758. [Google Scholar] [CrossRef]
- Peng, J.; Han, C.L.; Ling, J.; Liu, C.J.; Ding, Z.T.; Cao, Q.E. Selective fluorescence quenching of papain-Au nanoclusters by self-polymerization of dopamine. Luminescence 2018, 33, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, M.; Aoyama, C.; Nomura, H.; Toyoda, T.; Matsuki, N.; Funatsu, T. Simultaneous determination of dopamine and 3,4-dihydroxyphenylacetic acid in mouse striatum using mixed-mode reversed-phase and cation-exchange high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2010, 51, 712–715. [Google Scholar] [CrossRef]
- Shin, J.W.; Kim, K.J.; Yoon, J.; Jo, J.; El-Said, W.A.; Choi, J.W. Silver nanoparticle modified electrode covered by graphene oxide for the enhanced electrochemical detection of dopamine. Sensors 2017, 17, 2771. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Huang, Y.; Chen, C.; Xiao, A.; Guo, T.; Guan, B.O. Improved detection sensitivity of gamma-aminobutyric acid based on graphene oxide interface on an optical microfiber. Phys. Chem. Chem. Phys. 2018, 20, 14117–14123. [Google Scholar] [CrossRef] [PubMed]
- Kokulnathan, T.; Ahmed, F.; Chen, S.M.; Chen, T.W.; Hasan, P.M.Z.; Bilgrami, A.L.; Darwesh, R. Rational confinement of yttrium vanadate within three-dimensional graphene aerogel: Electrochemical analysis of monoamine neurotransmitter (dopamine). ACS Appl. Mater. Interfaces 2021, 13, 10987–10995. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, Q.; Du, X.; Qian, J.; Mao, H.; Wang, K. Visible light photoelectrochemical sensor for ultrasensitive determination of dopamine based on synergistic effect of graphene quantum dots and TiO2 nanoparticles. Anal. Chim. Acta 2015, 853, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Hun, X.; Wang, S.; Wang, S.; Zhao, J.; Luo, X. A photoelectrochemical sensor for ultrasensitive dopamine detection based on single-layer NanoMoS2 modified gold electrode. Sens. Actuators B Chem. 2017, 249, 83–89. [Google Scholar] [CrossRef]
- Ma, W.; Wang, L.; Zhang, N.; Han, D.; Dong, X.; Niu, L. Biomolecule-free, selective detection of o-diphenol and its derivatives with WS2/TiO2-based photoelectrochemical platform. Anal. Chem. 2015, 87, 4844–4850. [Google Scholar] [CrossRef]
- Wu, Z.; Han, F.; Wang, T.; Guan, L.; Liang, Z.; Han, D.; Niu, L. A recognition-molecule-free photoelectrochemical sensor based on Ti3C2/TiO2 heterostructure for monitoring of dopamine. Biosensors 2023, 13, 526. [Google Scholar] [CrossRef]
- Han, F.J.; Song, Z.Q.; Xu, J.N.; Dai, M.J.; Luo, S.L.; Han, D.X.; Niu, L.; Wang, Z.X. Oxidized titanium carbide MXene-enabled photoelectrochemical sensor for quantifying synergistic interaction of ascorbic acid based antioxidants system. Biosens. Bioelectron. 2021, 177, 112978. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.; Jin, H.; Sun, Y.; Ma, X.; Zhang, E.; Wang, H.; Kong, Z.; Xi, J.; Ji, Z. Constructing two-dimension MoS2 /Bi2WO6 core-shell heterostructure as carriers transfer channel for enhancing photocatalytic activity. Mater. Res. Bull. 2017, 85, 140–146. [Google Scholar] [CrossRef]
- Li, C.; Chen, G.; Sun, J.; Feng, Y.; Liu, J.; Dong, H. Ultrathin nanoflakes constructed erythrocyte-like Bi2WO6 hierarchical architecture via anionic self-regulation strategy for improving photocatalytic activity and gas-sensing property. Appl. Catal. B 2015, 163, 415–423. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, W.Z.; Wang, L.; Sun, S.M. Enhancement of visible-light photocatalysis by coupling with narrow-band-gap semiconductor: A case study on Bi2S3/Bi2WO6. ACS Appl. Mater. Interfaces 2012, 4, 593–597. [Google Scholar] [CrossRef]
- Li, C.; Chen, G.; Sun, J.; Rao, J.; Han, Z.; Hu, Y.; Zhou, Y. A novel mesoporous single-crystal-like Bi2WO6 with enhanced photocatalytic activity for pollutants degradation and oxygen production. ACS Appl. Mater. Interfaces 2015, 7, 25716–25724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, W.; Zhou, L.; Xu, H. Bi2WO6 nano and microstructures: Shape control and associated visible-light-driven photocatalytic activities. Small 2007, 3, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, W.; Chen, Z.; Zhou, L.; Xu, H.; Zhu, W. Fabrication of flower-like Bi2WO6 superstructures as high performance visible-light driven photocatalysts. J. Mater. Chem. 2007, 17, 2526–2532. [Google Scholar] [CrossRef]
- Qi, S.; Zhang, R.; Zhang, Y.; Liu, X.; Xu, H. Preparation and photocatalytic properties of Bi2WO6/g-C3N4. Inorg. Chem. Commun. 2021, 132, 108761. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Nie, Q.; Hu, L.; Cui, Y.; Tan, Z.; Yu, H. One-step synthesis of metallic Bi deposited Bi2WO6 nanoclusters for enhanced photocatalytic performance: An experimental and DFT study. Appl. Surf. Sci. 2021, 559, 149970. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Sun, K.; Wan, J.; Fu, F.; Fan, J. Novel phosphorus-doped Bi2WO6 monolayer with oxygen vacancies for superior photocatalytic water detoxication and nitrogen fixation performance. Chem. Eng. J. 2021, 411, 128629. [Google Scholar] [CrossRef]
- Peng, J.; Zhuge, W.; Liu, Y.; Zhang, C.; Yang, W.; Huang, Y. Photoelectrochemical dopamine sensor Based on Cu-Doped Bi2WO6 micro-Flowers sensitized cobalt tetraaminophthalocyanine functionalized graphene oxide. J. Electrochem. Soc. 2019, 166, B1612–B1619. [Google Scholar] [CrossRef]
- Wang, X.; Rong, X.; Zhang, Y.; Luo, F.; Qiu, B.; Wang, J.; Lin, Z. Homogeneous photoelectrochemical aptasensors for tetracycline based on sulfur-doped g-C3N4/n-GaN heterostructures formed through self-assembly. Anal. Chem. 2022, 94, 3735–3742. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Y.; Yang, L.; Yang, R. Green preparation of in-situ oxidized TiO2/Ti3C2 heterostructure for photocatalytic hydrogen production. Adv. Powder Technol. 2021, 32, 4857–4861. [Google Scholar] [CrossRef]
- Zhang, J.; Shang, M.; Gao, Y.; Yan, J.; Song, W. High-performance VS2 QDs-based type II heterostructured photoanode for ultrasensitive aptasensing of lysozyme. Sens. Actuators B Chem. 2020, 304, 127411. [Google Scholar] [CrossRef]
- Adhikari, S.; Kim, D.-H. Synthesis of Bi2S3/Bi2WO6 hierarchical microstructures for enhanced visible light driven photocatalytic degradation and photoelectrochemical sensing of ofloxacin. Chem. Eng. J. 2018, 354, 692–705. [Google Scholar] [CrossRef]
- Zhang, M. Synthesis of Bi2WO6/TiO2 flake nano-heterostructure photocatalyst and its photocatalytic performance under visible light irradiation. J. Mater. Sci. Mater. Electron. 2020, 31, 20129–20138. [Google Scholar] [CrossRef]
- Zhao, B.; Luo, Y.; Qu, X.; Hu, Q.; Zou, J.; He, Y.; Liu, Z.; Zhang, Y.; Bao, Y.; Wang, W.; et al. Graphite-like carbon nitride nanotube for electrochemiluminescence featuring high efficiency, high stability, and ultrasensitive ion detection capability. J. Phys. Chem. Lett. 2021, 12, 11191–11198. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Q.; Fang, Z.; Li, W.; Zheng, Y.; Ma, J.; Wang, Z.; Zhao, L.; Liu, S.; Shen, Y.; et al. Carbon nitride of five-membered rings with low optical bandgap for photoelectrochemical biosensing. Chem 2021, 7, 2708–2721. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Xu, Z.; Zhu, Y.; Chen, G.; Cui, Z. Synthesis of g-C3N4/NiO p–n heterojunction materials with ball-flower morphology and enhanced photocatalytic performance for the removal of tetracycline and Cr6+. J. Mater. Sci. 2019, 54, 11417–11434. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, Z.; Lin, X.; Han, F.; Liang, Z.; Huang, L.; Dai, M.; Han, D.; Han, L.; Niu, L. A label-free PEC aptasensor platform based on g-C3N4/BiVO4 heterojunction for tetracycline detection in food analysis. Food Chem. 2023, 402, 134258. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Wang, C.; Hu, X.; Liu, Y.; Wang, G. Sensitive detection of glyphosate based on a Cu-BTC MOF/g-C3N4 nanosheet photoelectrochemical sensor. Electrochim. Acta 2019, 317, 341–347. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, S.; Zhu, Y.; Fan, G.; Han, D.; Qu, P.; Zhao, W. Cathodic photoelectrochemical bioanalysis. TrAC Trend. Anal. Chem. 2019, 114, 81–88. [Google Scholar] [CrossRef]
- Weng, S.; Hu, J.; Lu, M.; Ye, X.; Pei, Z.; Huang, M.; Xie, L.; Lin, S.; Liu, P. In situ photogenerated defects on surface-complex BiOCl (0 1 0) with high visible-light photocatalytic activity: A probe to disclose the charge transfer in BiOCl (0 1 0)/surface-complex system. Appl. Catal. B 2015, 163, 205–213. [Google Scholar] [CrossRef]
- Yang, C.; Chai, H.; Xu, P.; Wang, P.; Wang, X.; Shen, T.; Zheng, Q.; Zhang, G. One-step synthesis of a 3D/2D Bi2WO6/g-C3N4 heterojunction for effective photocatalytic degradation of atrazine: Kinetics, degradation mechanisms and ecotoxicity. Sep. Purif. Technol. 2022, 288, 120609. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Zhong, L.; Liu, D.; Cao, X.; Cui, F. Oxygen vacancy-rich 2D/2D BiOCl-g-C3N4 ultrathin heterostructure nanosheets for enhanced visible-light-driven photocatalytic activity in environmental remediation. Appl. Catal. B 2018, 220, 290–302. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Novel Bi2WO6 modified by N-doped graphitic carbon nitride photocatalyst for efficient photocatalytic degradation of phenol under visible light. Appl. Catal. B 2020, 268, 118426. [Google Scholar] [CrossRef]
- Duan, Y.; Li, X.; Lv, K.; Zhao, L.; Liu, Y. Flower-like g-C3N4 assembly from holy nanosheets with nitrogen vacancies for efficient NO abatement. Appl. Surf. Sci. 2019, 492, 166–176. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, H.; Won, M.; Kim, E.; Li, M.; Kim, J.S. Codoping g-C3N4 with boron and graphene quantum dots: Enhancement of charge transfer for ultrasensitive and selective photoelectrochemical detection of dopamine. Biosens. Bioelectron. 2023, 224, 115050. [Google Scholar] [CrossRef]
- Kong, W.; Zhu, D.; Luo, R.; Yu, S.; Ju, H. Framework-promoted charge transfer for highly selective photoelectrochemical biosensing of dopamine. Biosens. Bioelectron. 2022, 211, 114369. [Google Scholar] [CrossRef]
- Peng, J.; Li, X.; Liu, Y.; Zhuge, W.; Zhang, C.; Huang, Y. Photoelectrochemical sensor based on zinc phthalocyanine semiconducting polymer dots for ultrasensitive detection of dopamine. Sens. Actuators B Chem. 2022, 360, 131619. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Cai, G.; Li, M.; Tang, D. In situ formation of (001)TiO2/Ti3C2 heterojunctions for enhanced photoelectrochemical detection of dopamine. Electrochem. Commun. 2021, 125, 106987. [Google Scholar] [CrossRef]
- Yao, W.; Guo, H.; Liu, H.; Li, Q.; Xue, R.; Wu, N.; Li, L.; Wang, M.; Yang, W. Simultaneous electrochemical determination of acetaminophen and dopamine based on metal-organic framework/multiwalled carbon nanotubes-Au@Ag nanocomposites. J. Electrochem. Soc. 2019, 166, B1258–B1267. [Google Scholar] [CrossRef]
- Liu, S.; Shi, F.; Zhao, X.; Chen, L.; Su, X. 3-Aminophenyl boronic acid-functionalized CuInS2 quantum dots as a near-infrared fluorescence probe for the determination of dopamine. Biosens. Bioelectron. 2013, 47, 379–384. [Google Scholar] [CrossRef]
- Hao, Q.; Wang, P.; Ma, X.; Su, M.; Lei, J.; Ju, H. Charge recombination suppression-based photoelectrochemical strategy for detection of dopamine. Electrochem. Commun. 2012, 21, 39–41. [Google Scholar] [CrossRef]
- Moser, J.; Punchihewa, S.; Infelta, P.P.; Graetzel, M. Surface complexation of colloidal semiconductors strongly enhances interfacial electron-transfer rates. Langmuir 1991, 7, 3012–3018. [Google Scholar] [CrossRef]







| Analyte | Added (µM) | Found (µM) | Recovery (%) | RSD (%) (n = 3) |
|---|---|---|---|---|
| Human Blood serum | 0.50 | 0.52 | 104.0 | 3.6 |
| 10 | 10.14 | 101.4 | 1.4 | |
| 25 | 24.62 | 98.5 | 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Su, Y.; Han, F.; Liang, Z.; Han, D.; Qin, D.; Niu, L. Bi2WO6@g-C3N4 Heterostructure for Cathodic Photoelectrochemical Dopamine Sensor. Chemosensors 2023, 11, 404. https://doi.org/10.3390/chemosensors11070404
Wu Z, Su Y, Han F, Liang Z, Han D, Qin D, Niu L. Bi2WO6@g-C3N4 Heterostructure for Cathodic Photoelectrochemical Dopamine Sensor. Chemosensors. 2023; 11(7):404. https://doi.org/10.3390/chemosensors11070404
Chicago/Turabian StyleWu, Zhifang, Ying Su, Fangjie Han, Zhishan Liang, Dongxue Han, Dongdong Qin, and Li Niu. 2023. "Bi2WO6@g-C3N4 Heterostructure for Cathodic Photoelectrochemical Dopamine Sensor" Chemosensors 11, no. 7: 404. https://doi.org/10.3390/chemosensors11070404
APA StyleWu, Z., Su, Y., Han, F., Liang, Z., Han, D., Qin, D., & Niu, L. (2023). Bi2WO6@g-C3N4 Heterostructure for Cathodic Photoelectrochemical Dopamine Sensor. Chemosensors, 11(7), 404. https://doi.org/10.3390/chemosensors11070404