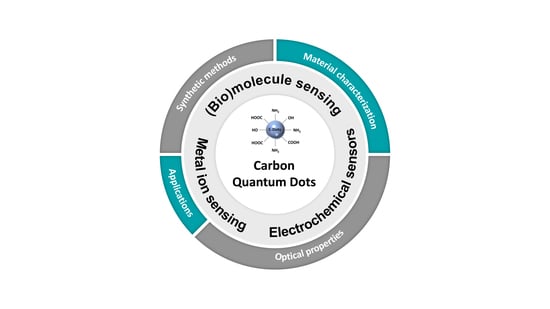
An Overview of the Recent Developments in Carbon Quantum Dots—Promising Nanomaterials for Metal Ion Detection and (Bio)Molecule Sensing
Abstract
:1. Introduction
2. Methods for Preparation of Carbon Quantum Dots
2.1. “Top–Down” Approach
2.2. “Bottom–Up” Approach
2.3. CQDs Preparation from Natural Sources
3. Material Characterization
3.1. Structural and Chemical Properties
3.2. Optical Properties
4. Sensing Mechanisms
5. Photoluminescent Sensing of Metal Ions
5.1. Ferric (Fe3+) Ions Detection
5.2. Mercuric (Hg2+) Ions Detection
5.3. Other Metals Ions Detection
6. Photoluminescent Sensing of Molecules
7. Electrochemical Sensors
8. Conclusions and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and behavior of carbon nanomaterials when interfacing neuronal cells: How far have we come? Carbon 2019, 143, 430–446. [Google Scholar] [CrossRef]
- Meng, W.; Bai, X.; Boyang, W.; Zhongyi, L.; Lu, S.; Yang, B. Biomass-Derived Carbon Dots and Their Applications. Energy Environ. Mat. C 2019, 2, 172–192. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, S.; Ramachandran, V.; Raj, R.P.; Tv, S.; Kumar, S. Carbon Quantum Dots: A Potential Candidate for Diagnostic and Therapeutic Application. In Nanobiomaterial Engineering, 1st ed.; Chandra, P., Prakash, R., Eds.; Springer Nature Singapore: Singapore, 2020; pp. 47–90. [Google Scholar] [CrossRef]
- Ghosh, D.; Sarkar, K.; Devi, P.; Kim, K.H.; Kumara, P. Current and future perspectives of carbon and graphene quantum dots: From synthesis to strategy for building optoelectronic and energy devices. Renew. Sust. Energ. Rev. 2021, 135, 110391. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Wu, H.; Xu, H.; Zhang, Y.; Li, Y.; Li, X.; Fan, L. Carbon dots: A booming material for biomedical applications. Mater. Chem. Front. 2020, 4, 821–836. [Google Scholar] [CrossRef]
- Molaei, M.J. Carbon quantum dots and their biomedical and therapeutic applications: A review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Nair, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Natural carbon-based quantum dots and their applications in drug delivery: A review. Biomed. Pharmacother. 2020, 132, 110834. [Google Scholar] [CrossRef]
- Rastogi, A.; Pandey, F.; Parmar, A.; Singh, S.; Hegde, G.; Manohar, R. Effect of carbonaceous oil palm leaf quantum dot dispersion in nematic liquid crystal on zeta potential, optical texture and dielectric properties. J. Nanostruct. Chem. 2021. [Google Scholar] [CrossRef]
- Devi, P.; Rajput, P.; Thakur, A.; Kim, K.; Kumar, P. Recent advances in carbon quantum dot-based sensing of heavy metals in water. TrAC 2019, 114, 171–195. [Google Scholar] [CrossRef]
- Gao, X.; Du, C.; Zhuang, Z.; Chen, W. Carbon quantum dot-based nanoprobes for metal ion detection. J. Mater. Chem. C 2016, 4, 6927–6945. [Google Scholar] [CrossRef]
- Yoo, D.; Park, Y.; Cheon, B.; Park, M. Carbon Dots as an Effective Fluorescent Sensing Platform for Metal Ion Detection. Nanoscale Res. Lett. 2019, 14, 272. [Google Scholar] [CrossRef] [Green Version]
- Qu, D.; Wang, X.; Bao, Y.; Sun, Z. Recent advance of carbon dots in bio-related applications. J. Phys. Mater. 2020, 3, 022003. [Google Scholar] [CrossRef]
- Devi, P.; Saini, S.; Kim, K.H. The advanced role of carbon quantum dots in nanomedical applications. Biosens. Bioelectron. 2019, 141, 111158. [Google Scholar] [CrossRef]
- Cui, L.; Ren, X.; Wang, J.; Sun, M. Synthesis of homogeneous carbon quantum dots by ultrafast dual-beam pulsed laser ablation for bioimaging. Mater. Today Nano 2020, 2, 100091. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, T.; Li, X.; Xu, J.; Zeng, H. Progress of Carbon Quantum Dots in Photocatalysis Applications. Part. Part. Syst. Charact. 2016, 33, 457–472. [Google Scholar] [CrossRef]
- Tian, L.; Ghosh, D.; Chen, W.; Pradhan, S.; Chang, X.; Chen, S. Nanosized Carbon Particles from Natural Gas Soot. Chem. Mater. 2009, 21, 2803–2809. [Google Scholar] [CrossRef]
- Liu, R.; Wu, D.; Liu, S.; Koynov, K.; Knoll, W.; Li, Q. An Aqueous Route to Multicolor Photoluminescent Carbon Dots Using Silica Spheres as Carriers. Angew. Chem. 2009, 48, 4598–4601. [Google Scholar] [CrossRef]
- Ray, S.C.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent Carbon Nanoparticles: Synthesis, Characterization, and Bioimaging Application. J. Phys. Chem. C 2009, 113, 18546–18551. [Google Scholar] [CrossRef]
- Qiao, Z.A.; Wang, Y.; Gao, Y.; Li, H.; Dai, T.; Liua, Y.; Huo, Q. Commercially activated carbon as the source for producing multicolor photoluminescent carbon dots by chemical oxidation. Chem. Commun. 2010, 46, 8812–8814. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Lu, Q.; Mi, N.; Li, H.; Liu, M.; Xu, M.; Tan, L.; Xie, Q.; Zhang, Y.; Yao, S. Electrochemical Synthesis of Carbon Nanodots Directly from Alcohols. Chem. Eur. J. 2014, 20, 4993–4999. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Ding, H.; Xu, M.; Hu, X.; Li, Z.; Wang, J.; Liu, L.; Jiang, L.; Wang, D.; Dong, C.; et al. Recent Advances in Synthesis, Optical Properties, and Biomedical Applications of Carbon Dots. ACS Appl. Bio. Mater. 2019, 2, 2317–2338. [Google Scholar] [CrossRef]
- Pan, M.; Xie, X.; Liu, K.; Yang, J.; Hong, L.; Wang, S. Fluorescent Carbon Quantum Dots—Synthesis, Functionalization and Sensing Application in Food Analysis. Nanomaterials 2020, 10, 930. [Google Scholar] [CrossRef]
- Chen, Z.H.; Han, X.Y.; Lin, Z.Y.; Fan, Y.L.; Shi, G.; Zhang, S.; Zhang, M. Facile reflux synthesis of polyethyleneimine-capped fluorescent carbon dots for sequential bioassays toward Cu2+/H2S and its application for a logic system. Biotechnol. Appl. Biochem. 2019, 66, 426–433. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Y.; Niu, F.; Gooding, J.J.; Liu, J. Carbon quantum dots directly generated from electrochemical oxidation of graphite electrodes in alkaline alcohols and the applications for specific ferric ion detection and cell imaging. Analyst 2016, 141, 2657–2664. [Google Scholar] [CrossRef]
- Hou, Y.; Lu, Q.; Deng, J.; Li, H.; Zhang, Y. One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Anal. Chim. Acta 2015, 866, 69–74. [Google Scholar] [CrossRef]
- Farshbaf, M.; Davaran, S.; Rahimi, F.; Annabi, N.; Salehi, R.; Akbarzadeh, A. Carbon quantum dots: Recent progresses on synthesis, surface modification and applications. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1331–1348. [Google Scholar] [CrossRef]
- Shen, T.Y.; Jia, P.Y.; Chen, D.S.; Wang, L.N. Hydrothermal synthesis of N-doped carbon quantum dots and their application in ion-detection and cell-imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 248, 119282. [Google Scholar] [CrossRef]
- Crista, D.M.A.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Evaluation of Different Bottom-up Routes for the Fabrication of Carbon Dots. Nanomaterials 2020, 10, 1316. [Google Scholar] [CrossRef]
- Chu, K.W.; Lee, S.L.; Chang, C.-H.; Liu, L. Recent Progress of Carbon Dot Precursors and Photocatalysis Applications. Polymers 2019, 11, 689. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Sun, Z.; Zhou, W.; Mo, F.; Wu, Z.C.; Zhang, X. Hydrothermal synthesis of bright blue-emitting carbon dots for bioimaging and fluorescent determination of baicalein. Opt. Mater. 2021, 113, 110796. [Google Scholar] [CrossRef]
- Zheng, M.; Ruan, S.; Liu, S.; Sun, T.; Qu, D.; Zhao, H.; Xie, Z.; Gao, H.; Jing, X.; Sun, Z. Self-targeting fluorescent carbon dots for diagnosis of brain cancer cells. ACS Nano 2015, 9, 11455–11461. [Google Scholar] [CrossRef]
- Canevari, T.C.; Nakamura, M.; Cincotto, F.H.; de Melo, F.M.; Toma, H.E. High performance electrochemical sensors for dopamine and epinephrine using nanocrystalline carbon quantum dots obtained under controlled chronoamperometric conditions. Electrochim. Acta 2016, 209, 464–470. [Google Scholar] [CrossRef]
- Eskalen, H.; Uruş, S.; Cömertpay, S.; Kurt, A.H.; Özgan, S. Microwave-assisted ultra-fast synthesis of carbon quantum dots from linter: Fluorescence cancer imaging and human cell growth inhibition properties. Ind. Crop. Prod. 2020, 147, 112209. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, R.; Singh, D.P.; Savu, R.; Moshkalev, S.A. Progress in microwave-assisted synthesis of quantum dots (graphene/carbon/semiconducting) for bioapplications: A review. Mater. Today Chem. 2019, 12, 282–314. [Google Scholar] [CrossRef]
- Wan, J.Y.; Yang, Z.; Liu, Z.G.; Wang, H.X. Ionic liquid-assisted thermal decomposition synthesis of carbon dots and graphene-like carbon sheets for optoelectronic application. RSC Adv. 2016, 6, 61292–61300. [Google Scholar] [CrossRef]
- Shang, W.; Cai, T.; Zhang, Y.; Liu, D.; Liu, S. Facile one pot pyrolysis synthesis of carbon quantum dots and graphene oxide nanomaterials: All carbon hybrids as eco-environmental lubricants for low friction and remarkable wear-resistance. Tribol. Int. 2018, 118, 373–380. [Google Scholar] [CrossRef]
- Sagar Mittal, S.; Ramadas, G.; Vasanthmurali, N.; Madaneshwar, V.; Sathish Kumar, M.; Kothurkar, N. Carbon Quantum Dot-Polypyrrole Nanocomposite for Supercapacitor Electrodes. IOP Conf. Ser. Mater. Sci. Eng. 2019, 577, 012194. [Google Scholar] [CrossRef]
- Nguyen, H.; Richtera, L.; Moulick, A.; Xhaxhiu, K.; Kudr, J.; Cernei, N.; Polanska, H.; Heger, Z.; Masarik, M.; Kopel, P.; et al. Electrochemical sensing of etoposide using carbon quantum dot modified glassy carbon electrode. Analyst 2016, 141, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, W.; Świergosz, T.; Bednarz, S.; Walas, K.; Bashmakova, N.V.; Bogdał, D. Luminescence phenomena of carbon dots derived from citric acid and urea—A molecular insight. Nanoscale 2018, 10, 13889–13894. [Google Scholar] [CrossRef] [PubMed]
- Baragau, I.A.; Power, N.P.; Morgan, D.J.; Lobo, R.A.; Roberts, C.S.; Titirici, M.M.; Middelkoop, V.; Diaz, A.; Dunn, S.; Kellici, S. Efficient Continuous Hydrothermal Flow Synthesis of Carbon Quantum Dots from a Targeted Biomass Precursor for On–Off Metal Ions Nanosensing. ACS Sustain. Chem. Eng. 2021, 9, 2559–2569. [Google Scholar] [CrossRef]
- Qi, H.; Teng, M.; Liu, M.; Liu, S.; Li, J.; Yu, H.; Teng, C.; Huang, Z.; Liu, H.; Shao, Q.; et al. Biomass-derived nitrogen-doped carbon quantum dots: Highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J. Colloid Interface Sci. 2019, 539, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.T.; Lin, Y.S.; Chen, T.Y.; Chyueh, S.C.; Chang, H.T. Carbon dots functionalized papers for high-throughput sensing of 4-chloroethcathinone and its analogues in crime sites. R. Soc. Open Sci. 2019, 6, 191017. [Google Scholar] [CrossRef] [Green Version]
- Achadu, O.J.; Lioe, D.X.; Kagawa, K.; Kawahito, S.; Park, E.Y. Fluoroimmunoassay of influenza virus using sulfur-doped graphitic carbon nitride quantum dots coupled with Ag2S nanocrystals. Microchim. Acta 2020, 187, 466. [Google Scholar] [CrossRef]
- Kalaiyarasan, G.; Joseph, J.; Kumar, P. Phosphorus-Doped Carbon Quantum Dots as Fluorometric Probes for Iron Detection. ACS Omega 2020, 5, 22278–22288. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Liang, S.; Hou, S.; Chu, T.; Ma, J.; Chen, X.; Zhou, J.; Suna, R. Preparation of sulfur-doped carbon quantum dots from lignin as a sensor to detect Sudan I in an acidic environment. J. Mater. Chem. B 2020, 8, 10788–10796. [Google Scholar] [CrossRef]
- Kou, X.; Jiang, S.; Park, S.J.; Meng, L.Y. A review: Recent advances in preparations and applications of heteroatom-doped carbon quantum dots. Dalton Trans. 2020, 49, 6915–6938. [Google Scholar] [CrossRef]
- Talwatkar, S.S.; Sunatkari, A.L.; Tamgadge, Y.S.; Pahurkar, V.G.; Muley, G.G. Surface passivation by L-arginine and enhanced optical properties of CdS quantum dots co-doped with Nd3+–Li+. J. Nanostruct. Chem. 2015, 5, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Lai, Z.; Guo, X.; Cheng, Z.; Ruan, G.; Du, F. Green Synthesis of Fluorescent Carbon Dots from Cherry Tomatoes for Highly Effective Detection of Trifluralin Herbicide in Soil Samples. ChemistrySelect 2020, 5, 1956–1960. [Google Scholar] [CrossRef]
- Liu, W.; Li, C.; Sun, X.; Pan, W.; Yu, G.; Wang, J. Highly crystalline carbon dots from fresh tomato: UV emission and quantum confinement. Nanotechnology 2017, 28, 485705. [Google Scholar] [CrossRef]
- Qiang, R.; Yang, S.; Hou, K.; Wang, J. Synthesis of carbon quantum dots with green luminescence from potato starch. New J. Chem. 2019, 43, 10826–10833. [Google Scholar] [CrossRef]
- Su, R.; Wang, D.; Liu, M.; Yan, J.; Wang, J.; Zhan, Q.; Pu, Y.; Foster, N.R.; Chen, J.-F. Subgram-Scale Synthesis of Biomass Waste-Derived Fluorescent Carbon Dots in Subcritical Water for Bioimaging, Sensing, and Solid-State Patterning. ACS Omega 2018, 3, 13211–13218. [Google Scholar] [CrossRef]
- Tadesse, A.; Hagos, M.; RamaDevi, D.; Basavaiah, K.; Belachew, N. Fluorescent-Nitrogen-Doped Carbon Quantum Dots Derived from Citrus Lemon Juice: Green Synthesis, Mercury(II) Ion Sensing, and Live Cell Imaging. ACS Omega 2020, 5, 3889–3898. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Liang, J.; Zhao, L.; Wang, Y.; Zheng, Y.; Wu, Y.; Jiang, L. Synthesis of Novel Fluorescent Carbon Quantum Dots from Rosa roxburghii for Rapid and Highly Selective Detection of o-nitrophenol and Cellular Imaging. Front. Chem. 2020, 8, 665. [Google Scholar] [CrossRef]
- Jin, H.; Gui, R.; Wang, Y.; Sun, J. Carrot-derived carbon dots modified with polyethyleneimine and nile blue for ratiometric two-photon fluorescence turn-on sensing of sulfide anion in biological fluids. Talanta 2017, 169, 141–148. [Google Scholar] [CrossRef]
- Ren, G.; Tang, M.; Chai, F.; Wu, H. One-Pot Synthesis of Highly Fluorescent Carbon Dots from Spinach and Multipurpose Applications. Eur. J. Inor. Chem. 2018, 2018, 153–158. [Google Scholar] [CrossRef]
- Monte-Filho, S.S.; Andrade, S.I.E.; Lima, M.B.; Araujo, M.C.U. Synthesis of highly fluorescent carbon dots from lemon and onion juices for determination of riboflavin in multivitamin/mineral supplements. J. Pharm. Anal. 2019, 9, 209–216. [Google Scholar] [CrossRef]
- Zulfajri, M.; Gedda, G.; Chang, C.J.; Chang, Y.P.; Huang, G.G. Cranberry Beans Derived Carbon Dots as a Potential Fluorescence Sensor for Selective Detection of Fe3+ Ions in Aqueous Solution. ACS Omega 2019, 4, 15382–15392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, D.D.; Mukherjee, P.; Ghosh, D.; Banerjee, D. Carbon quantum dots prepared from onion extract as fluorescence turn-on probes for selective estimation of Zn2+ in blood plasma. Colloids Surf. A Physicochem. Eng. Asp. 2021, 61, 125781. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Yang, M.; Yan, Y.; Liu, E.; Ji, Z.; Fan, J. Facile synthesis of novel carbon quantum dots from biomass waste for highly sensitive detection of iron ions. Mater. Res. Bull. 2020, 124, 110730. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.; Singh, N.; Choudhary, S.; Gupta, R. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Su, A.; Wang, D.; Shu, X.; Zhong, Q.; Chen, Y.; Liu, J.; Wang, Y. Synthesis of Fluorescent Carbon Quantum Dots from Dried Lemon Peel for Determination of Carmine in Drinks. Chem. Res. Chin. Univ. 2018, 34, 164–168. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Y.; Niu, N.; Chen, L. Synthesis of molecularly imprinted fluorescent probe based on biomass-derived carbon quantum dots for detection of mesotrione. Anal. Bioanal. Chem. 2019, 411, 5519–5530. [Google Scholar] [CrossRef] [PubMed]
- Bandi, R.; Gangapuram, B.R.; Dadigala, R.; Eslavath, R.; Singh, S.S.; Guttena, V. Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents. RSC Adv. 2016, 6, 28633–28639. [Google Scholar] [CrossRef]
- Vandarkuzhali, S.A.A.; Natarajan, S.; Jeyabalan, S.; Sivaraman, G.; Singaravadivel, S.; Muthusubramanian, S.; Viswanathan, B. Pineapple Peel-Derived Carbon Dots: Applications as Sensor, Molecular Keypad Lock, and Memory Device. ACS Omega 2018, 3, 12584–12592. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, V.; Jhonsi, M.A.; Kathiravan, A.; Ashokkumar, M. Fuel waste to fluorescent carbon dots and its multifarious applications. Sens. Actuators B Chem. 2019, 282, 972–983. [Google Scholar] [CrossRef]
- Boruah, A.; Saikia, M.; Das, T.; Goswamee, R.L.; Saikia, B.K. Blue-emitting fluorescent carbon quantum dots from waste biomass sources and their application in fluoride ion detection in water. J. Photochem. Photobiol. B 2020, 209, 111940. [Google Scholar] [CrossRef]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A Review of Carbon Dots Produced from Biomass Wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef]
- Carbonaro, C.M.; Corpino, R.; Salis, M.; Mocci, F.; Thakkar, S.V.; Olla, C.; Ricci, P.C. On the Emission Properties of Carbon Dots: Reviewing Data and Discussing Models. J. Carbon Res. 2019, 5, 60. [Google Scholar] [CrossRef] [Green Version]
- Dimos, K. Carbon Quantum Dots: Surface Passivation and Functionalization. Curr. Org. Chem. 2016, 20, 682–695. [Google Scholar] [CrossRef]
- Koutsogiannis, P.; Thomou, E.; Stamatis, H.; Gournis, D.; Rudolf, P. Advances in fluorescent carbon dots for biomedical applications. Adv. Phys. X 2020, 5, 1758592. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Shim, J.-J.; Lee, Y.R. Microwave assisted green synthesis of fluorescent N-doped carbon dots: Cytotoxicity and bio-imaging applications. J. Photochem. Photobiol. B 2016, 161, 154–161. [Google Scholar] [CrossRef]
- Jing, S.; Zhao, Y.; Sun, R.; Zhong, L.; Peng, X. Facile and High-Yield Synthesis of Carbon Quantum Dots from Biomass-Derived Carbons at Mild Condition. ACS Sustain. Chem. Eng. 2019, 7, 7833–7843. [Google Scholar] [CrossRef]
- Lv, X.; Gao, C.; Han, T.; Shi, H.; Guo, W. Improving the quantum yields of fluorophores by inhibiting twisted intramolecular charge transfer using electron-withdrawing group-functionalized piperidine auxochromes. Chem. Commun. 2020, 56, 715–718. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Huang, M.; Liang, X.; Zhang, Z.; Wang, J.; Fei, Y.; Ma, J.; Qu, S.; Mi, L. Carbon Dots for Intracellular pH Sensing with Fluorescence Lifetime Imaging Microscopy. Nanomaterials 2020, 10, 604. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Dong, T. Photoluminescence tuning in carbon dots: Surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C 2018, 6, 7944–7970. [Google Scholar] [CrossRef]
- Li, P.; Li, S.F.Y. Recent advances in fluorescence probes based on carbon dots for sensing and speciation of heavy metals. Nanophotonics 2021, 10, 877–908. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Miao, S.; Liang, K.; Kong, B. Förster resonance energy transfer (FRET) paired carbon dot-based complex nanoprobes: Versatile platforms for sensing and imaging applications. Mater. Chem. Front. 2020, 4, 128–139. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Issa, M.A.; Abidin, Z.Z.; Sobri, S.; Rashid, S.A.; Mahdi, M.A.; Ibrahim, N.A. Fluorescent recognition of Fe3+ in acidic environment by enhanced-quantum yield N-doped carbon dots: Optimization of variables using central composite design. Sci. Rep. 2020, 10, 11710. [Google Scholar] [CrossRef]
- Yang, G.; Wan, X.; Su, Y.; Zenga, X.; Tang, J. Acidophilic S-doped carbon quantum dots derived from cellulose fibers and their fluorescence sensing performance for metal ions in an extremely strong acid environment. J. Mater. Chem. A 2016, 4, 12841–12849. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.; Zheng, X.; Li, H.; Chen, Q.; Qin, K.; Dinga, Y.; Wei, Y. Carbon dots derived from Fusobacterium nucleatum for intracellular determination of Fe3+ and bioimaging both in vitro and in vivo. Anal. Methods 2021, 13, 1121–1131. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Pan, W.; Yu, G.; Wang, J. Fe3+-Sensitive Carbon Dots for Detection of Fe3+ in Aqueous Solution and Intracellular Imaging of Fe3+ Inside Fungal Cells. Front. Chem. 2020, 7, 911. [Google Scholar] [CrossRef]
- Pu, Z.F.; Wen, Q.L.; Yang, Y.J.; Cui, X.M.; Ling, J.; Liu, P.; Cao, Q.E. Fluorescent carbon quantum dots synthesized using phenylalanine and citric acid for selective detection of Fe3+ ions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 15, 117944. [Google Scholar] [CrossRef]
- Mohammed, L.J.; Omer, K.M. Dual functional highly luminescence B, N Co-doped carbon nanodots as nanothermometer and Fe3+/Fe2+ sensor. Sci. Rep. 2020, 10, 3028. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Xu, C.; Tian, Z.; Lina, Y.; Shia, Z. Facilely synthesized N-doped carbon quantum dots with high fluorescent yield for sensing Fe3+. New J. Chem. 2016, 40, 2083–2088. [Google Scholar] [CrossRef]
- Wu, P.; Li, W.; Wu, Q.; Liu, Y.; Liu, S. Hydrothermal synthesis of nitrogen-doped carbon quantum dots from microcrystalline cellulose for the detection of Fe3+ ions in an acidic environment. RSC Adv. 2017, 7, 44144–44153. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Jiang, J.; Gu, X.; Tong, C. Nitrogen and sulfur co-doped carbon quantum dots for highly selective and sensitive fluorescent detection of Fe(III) ions and L-cysteine. Microchim. Acta 2017, 184, 2291–2298. [Google Scholar] [CrossRef]
- Deng, X.; Feng, Y.; Li, H.; Du, Z.; Teng, Q.; Wang, H. N-doped carbon quantum dots as fluorescent probes for highly selective and sensitive detection of Fe3+ ions. Particuology 2018, 41, 94–100. [Google Scholar] [CrossRef]
- Lu, M.; Duan, Y.; Song, Y.; Tan, J.; Zhou, L. Green preparation of versatile nitrogen-doped carbon quantum dots from watermelon juice for cell imaging, detection of Fe3+ ions and cysteine, and optical thermometry. J. Mol. Liquids 2018, 269, 766–774. [Google Scholar] [CrossRef]
- Kwonbeen, K.; Jongsung, K. Synthesis of Carbon Quantum Dots from Jujubes for Detection of Iron(III) Ions. J. Nanosci. Nanotechnol. 2018, 18, 1320–1322. [Google Scholar] [CrossRef]
- Jiang, X.; Shi, Y.; Liu, X.; Wang, M.; Song, P.; Xu, F.; Zhang, X. Synthesis of Nitrogen-Doped Lignin/DES Carbon Quantum Dots as a Fluorescent Probe for the Detection of Fe3+ Ions. Polymers 2018, 10, 1282. [Google Scholar] [CrossRef] [Green Version]
- Shi, B.; Su, Y.; Zhang, L.; Huang, M.; Liu, R.; Zhao, S. Nitrogen and Phosphorus Co-Doped Carbon Nanodots as a Novel Fluorescent Probe for Highly Sensitive Detection of Fe3+ in Human Serum and Living Cells. ACS Appl. Mater. Interfaces 2016, 8, 10717–10725. [Google Scholar] [CrossRef]
- Zhu, J.; Chu, H.; Wang, T.; Wang, C.; Wei, Y. Fluorescent probe based nitrogen doped carbon quantum dots with solid-state fluorescence for the detection of Hg2+ and Fe3+ in aqueous solution. Microchem. J. 2020, 158, 105142. [Google Scholar] [CrossRef]
- Huang, H.; Ge, H.; Ren, Z.; Huang, Z.; Xu, M.; Wang, X. Controllable Synthesis of Biocompatible Fluorescent Carbon Dots from Cellulose Hydrogel for the Specific Detection of Hg2+. Front. Bioeng. Biotechnol. 2021, 9, 617097. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Wang, X.; Pan, W.; Yu, G.; Wang, J. Carbon dots with red emission for bioimaging of fungal cells and detecting Hg2+ and ziram in aqueous solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 233, 118230. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, L.; Cao, F.; Leng, Y. Thermal treatment of hair for the synthesis of sustainable carbon quantum dots and the applications for sensing Hg2+. Sci. Rep. 2016, 6, 35795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Wang, Y.; Wang, P.; Di, J.; Yang, J.; Wu, Y. Synthesis of strongly fluorescent carbon quantum dots modified with polyamidoamine and a triethoxysilane as quenchable fluorescent probes for mercury(II). Microchim. Acta 2016, 183, 2571–2578. [Google Scholar] [CrossRef]
- Wan, X.; Li, S.; Zhuang, L.; Tang, J. L-Tryptophan-capped carbon quantum dots for the sensitive and selective fluorescence detection of mercury ion in aqueous solution. J. Nanopart. Res. 2016, 18, 202. [Google Scholar] [CrossRef]
- Huang, H.; Weng, Y.; Zheng, L.; Yao, B.; Weng, W.; Lin, X. Nitrogen-doped carbon quantum dots as fluorescent probe for ‘‘off-on” detection of mercury ions, L-cysteine and iodide ions. J. Colloid Interface Sci. 2017, 506, 373–378. [Google Scholar] [CrossRef]
- Liu, T.; Xue Dong, J.; Liu, G.S.; Li, N.; Lin, M.S.; Fan, Y.Z.; Lei, J.L.; Luo, H.Q.; Li, N.B. Carbon quantum dots prepared with polyethyleneimine as both reducing agent and stabilizer for synthesis of Ag/CQDs composite for Hg2+ ions detection. J. Hazard. Mater. 2017, 322, 430–436. [Google Scholar] [CrossRef]
- Ye, Q.; Yan, F.; Luo, Y.; Wang, Y.; Zhou, X.; Chen, L. Formation of N, S-codoped fluorescent carbon dots from biomass and their application for the selective detection of mercury and iron ion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 854–862. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Wang, N.; Lv, J.; Wang, H.; Choi, M.M.F.; Bian, W. Graphitic carbon nitride quantum dots as an “off-on” fluorescent switch for determination of mercury(II) and sulfide. Microchim. Acta 2018, 185, 471. [Google Scholar] [CrossRef]
- Bano, D.; Kumar, V.; Singh, V.K.; Hasan, S.H. Green synthesis of fluorescent carbon quantum dots for the detection of mercury(II) and glutathione. New J. Chem. 2018, 42, 5814–5821. [Google Scholar] [CrossRef]
- Meng, A.; Xu, Q.; Zhao, K.; Li, Z.; Liang, J.; Li, Q. Highly Selective and Sensitive “on-off-on” Fluorescent Probe for Detecting Hg(II) Based on Au/N-doped Carbon Quantum Dots. Sens. Actuators B Chem. 2018, 255, 657–665. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Omer, K.H.; Hamarawfa, R.F. Lowering the detection limit towards nanomolar mercury ion detection via surface modification of N-doped carbon quantum dots. New J. Chem. 2019, 43, 8677–8683. [Google Scholar] [CrossRef]
- Pourreza, N.; Ghomi, M. Green synthesized carbon quantum dots from Prosopis juliflora leaves as a dual off-on fluorescence probe for sensing mercury (II) and chemet drug. Mater. Sci. Eng. C 2019, 98, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Yahyazadeh, E.; Shemirani, F. Easily synthesized carbon dots for determination of mercury(II) in water samples. Heliyon 2019, 5, e01596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Tang, Q.; Meng, Y.; Liu, J.; Feng, T.; Zhao, X.; Zhu, S.; Yu, W.; Yang, B. Reversible “Off–On” Fluorescence of Zn2+-Passivated Carbon Dots: Mechanism and Potential for the Detection of EDTA and Zn2+. Langmuir 2018, 34, 7767–7775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pei, K.; Yang, Q.; Dong, J.; Yan, Z.; Chen, J. A nanosensor made of sulfur–nitrogen co-doped carbon dots for “off–on” sensing of hypochlorous acid and Zn(II) and its bioimaging properties. New J. Chem. 2018, 42, 15895–15904. [Google Scholar] [CrossRef]
- Arumugam, N.; Kim, J. Synthesis of carbon quantum dots from Broccoli and their ability to detect silver ions. Mater. Lett. 2018, 219, 37–40. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Kennedy, S.R.; Steed, J.W.; Valcárcel, M. Fluorescent carbon quantum dot hydrogels for direct determination of silver ions. Talanta 2016, 151, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Qin, D.; Jiang, X.; Zheng, X.; Deng, B. N-doped carbon quantum dots from osmanthus fragrans as a novel off-on fluorescent nanosensor for highly sensitive detection of quercetin and aluminium ion, and cell imaging. J. Pharm Biomed. Anal. 2021, 192, 113673. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Panneerselvam, P. Green synthesis of surface-passivated carbon dots from the prickly pear cactus as a fluorescent probe for the dual detection of arsenic(III) and hypochlorite ions from drinking water. RSC Adv. 2018, 8, 30455–30467. [Google Scholar] [CrossRef] [Green Version]
- Raji, K.; Ramanan, V.; Ramamurthy, P. Facile and green synthesis of highly fluorescent nitrogen-doped carbon dots from jackfruit seeds and its applications towards the fluorimetric detection of Au3+ ions in aqueous medium and in in vitro multicolor cell imaging. New J. Chem 2019, 43, 11710–11719. [Google Scholar] [CrossRef]
- Yue, J.; Li, L.; Cao, L.; Zan, M.; Yang, D.; Wang, Z.; Chang, Z.; Mei, Q.; Miao, P.; Dong, W.F. Two-Step Hydrothermal Preparation of Carbon Dots for Calcium Ion Detection. ACS Appl Mater. Interfaces 2019, 11, 44566–44572. [Google Scholar] [CrossRef]
- Ankireddy, S.R.; Kim, J. Highly Selective and Sensitive Detection of Calcium(II) Ions in Human Serum Using Novel Fluorescent Carbon Dots. Sens. Actuators B Chem 2018, 255, 3425–3433. [Google Scholar] [CrossRef]
- Niu, W.J.; Shan, D.; Zhu, R.H.; Deng, S.Y.; Cosnier, S.; Zhang, X.J. Dumbbell-Shaped Carbon Quantum Dots/AuNCs Nanohybrid as an Efficient Ratiometric Fluorescent Probe for Sensing Cadmium(II) Ions and L-ascorbic Acid. Carbon 2016, 96, 1034–1042. [Google Scholar] [CrossRef]
- Bano, D.; Kumar, V.; Chandra, S.; Kumar Singh, V.; Mohan, S.; Kumar Singh, D.; Talat, M.; Hasan, S.H. Synthesis of highly fluorescent nitrogen-rich carbon quantum dots and their application for the turn-off detection of cobalt (II). Opt. Mater. 2019, 92, 311–318. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, S.; Kaura, B.; Mehtaa, S.K. Potential prospects for carbon dots as a fluorescence sensing probe for metal ions. RSC Adv. 2016, 6, 90526–90536. [Google Scholar] [CrossRef]
- Vaz, R.; Bettini, J.; Júnior, J.G.F.; Lima, E.D.S.; Botero, W.G.; Santos, J.C.C.; Schiavon, M.A. High luminescent carbon dots as an eco-friendly fluorescence sensor for Cr(VI) determination in water and soil samples. J. Photochem. Photobiol. A Chem. 2017, 346, 502–511. [Google Scholar] [CrossRef]
- Rooj, B.; Dutta, A.; Islam, S.; Mandal, U. Green Synthesized Carbon Quantum Dots from Polianthes tuberose L. Petals for Copper (II) and Iron (II) Detection. J. Fluoresc. 2018, 28, 1261–1267. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, A.; Kumar Sahu, S.; Kumar, S. Synthesis of green fluorescent carbon quantum dots using waste polyolefins residue for Cu2+ ion sensing and live cell imaging. Sens. Actuators B Chem. 2018, 254, 197–205. [Google Scholar] [CrossRef]
- Amjadi, M.; Manzoori, J.L.; Hallaj, T.; Azizi, N. Sulfur and nitrogen co-doped carbon quantum dots as the chemiluminescence probe for detection of Cu2+ ions. J. Lumin. 2017, 182, 246–251. [Google Scholar] [CrossRef]
- Murugan, N.; Prakash, M.; Jayakumar, M.; Sundaramurthy, A.; Sundramoorthy, A.K. Green synthesis of fluorescent carbon quantum dots from Eleusine coracana and their application as a fluorescence ‘turn-off’ sensor probe for selective detection of Cu2+. Appl. Surf. Sci. 2019, 476, 468–480. [Google Scholar] [CrossRef]
- Gedda, G.; Lee, C.Y.; Lin, Y.C.; Wu, H.F. Green synthesis of Carbon dots from prawn shells for highly selective and sensitive detection of copper ions. Sens. Actuators B Chem. 2016, 224, 396–403. [Google Scholar] [CrossRef]
- Jayaweera, S.; Yin, K.; Hu, X.; Ng, W.J. Fluorescent N/Al Co-Doped Carbon Dots from Cellulose Biomass for Sensitive Detection of Manganese (VII). J. Fluoresc. 2019, 29, 1291–1300. [Google Scholar] [CrossRef]
- Gong, Y.; Liang, H. Nickel ion detection by imidazole modified carbon dots. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 211, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Q.; Yuan, Y.; Wu, Y. Hydrothermal synthesis of fluorescent carbon dots from sodium citrate and polyacrylamide and their highly selective detection of lead and pyrophosphate. Carbon 2017, 115, 550–560. [Google Scholar] [CrossRef]
- Hoan, B.T.; Thanh, T.T.; Tam, P.D.; Trung, N.N.; Cho, S.; Pham, V.H. A green luminescence of lemon derived carbon quantum dots and their applications for sensing of V5+ ions. Mater. Sci. Eng. B 2019, 251, 114455. [Google Scholar] [CrossRef]
- Chandra, S.; Mahto, T.K.; Chowdhuri, A.R.; Das, B.; Sahu, S.K. One step synthesis of functionalized carbon dots for the ultrasensitive detection of Escherichia coli and iron (III). Sens. Actuators B Chem. 2017, 245, 835–844. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Alhakamy, N.A.; Md, S.; Nair, A.B.; Deb, P.K. Exploring the Potential of Carbon Dots to Combat COVID-19. Front. Mol. Biosci. 2020, 7, 616575. [Google Scholar] [CrossRef] [PubMed]
- Qaddare, S.H.; Salimi, A. Amplified fluorescent sensing of DNA using luminescent carbon dots and AuNPs/GO as a sensing platform: A novel coupling of FRET and DNA hybridization for homogeneous HIV-1 gene detection at femtomolar level. Biosens. Bioelectron. 2017, 89, 773–780. [Google Scholar] [CrossRef]
- Wang, J.; Lu, T.; Hu, Y.; Wang, X.; Yuangen, W. A label-free and carbon dots based fluorescent aptasensor for the detection of kanamycin in milk. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 226, 117651. [Google Scholar] [CrossRef]
- Li, H.; Su, D.; Gao, H.; Yan, X.; Kong, D.; Jin, R.; Liu, X.; Wang, C.; Lu, G. Design of Red Emissive Carbon Dots: Robust Performance for Analytical Applications in Pesticide Monitoring. Anal. Chem. 2020, 92, 3198–3205. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, S.; Huo, D.; Hou, D.; Yang, M.; Li, J.; Bian, M. Simple and sensitive fluorescence sensor for methotrexate detection based on the inner filter effect of N, S co-doped carbon quantum dots. Anal. Chim. Acta 2019, 1047, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.H.; Li, Y.L.; Jiang, X.; Zheng, X.F.; Wang, Y.Y.; Huan, S.Y. Fluorescence Resonance Energy Transfer-based Biosensor Composed of Nitrogen-doped Carbon Dots and Gold Nanoparticles for the Highly Sensitive Detection of Organophosphorus Pesticides. Anal. Sci. 2016, 32, 951–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.L.; Su, L.X.; Zang, J.H.; Li, X.J.; Lou, Q.; Shan, C.X. Carbon Nanodots as Dual-Mode Nanosensors for Selective Detection of Hydrogen Peroxide. Nanoscale Res. Lett. 2017, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Hu, X.; Li, W.; Mwakosya, A.W.; Guo, Z.; Xu, Y.; Huang, X.; Li, Z.; Zhang, X.; Zou, X.; et al. Fluorescence and colorimetric dual-mode sensor for visual detection of malathion in cabbage based on carbon quantum dots and gold nanoparticles. Food Chem. 2021, 343, 128494. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lu, Y.; Shi, L.; Yang, Y. β-Cyclodextrin functionalized N,Zn codoped carbon dots for specific fluorescence detection of fluoroquinolones in milk samples. Microchem. J. 2020, 153, 104517. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Mondal, S.; Bose, M.; Kumar Das, A.; Banerjee, S.; Das, N.C. Heteroatom doped photoluminescent carbon dots for sensitive detection of acetone in human fluids. Sens. Actuators B Chem. 2018, 266, 583–593. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Chen, B.B.; Zou, H.Y.; Li, Y.F.; Huang, C.Z. Inner filter with carbon quantum dots: A selective sensing platform for detection of hematin in human red cells. Biosens. Bioelectron. 2018, 100, 148–154. [Google Scholar] [CrossRef]
- Mandal, P.; Sahoo, D.; Sarkar, P.; Chakraborty, K.; Das, S. Fluorescence turn-on and turn-off sensing of pesticides by carbon dot-based sensor. New J. Chem. 2019, 43, 12137–12151. [Google Scholar] [CrossRef]
- Wang, K.; Ji, Q.; Xu, J.; Li, H.; Zhang, D.; Liu, X.; Wu, Y.; Fan, H. Highly Sensitive and Selective Detection of Amoxicillin Using Carbon Quantum Dots Derived from Beet. J. Fluoresc. 2018, 28, 759–765. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Sun, Y. Aminophenol-based carbon dots with dual wavelength fluorescence emission for determination of heparin. Microchim. Acta 2017, 184, 187–193. [Google Scholar] [CrossRef]
- Achadu, O.J.; Takemura, K.; Khoris, I.M.; Park, E.Y. Plasmonic/magnetic molybdenum trioxide and graphitic carbon nitride quantum dots-based fluoroimmunosensing system for influenza virus. Sens. Actuators B Chem. 2020, 15, 128494. [Google Scholar] [CrossRef]
- Xu, L.-D.; Zhang, Q.; Ding, S.-N.; Xu, J.-J.; Chen, H.-Y. Ultrasensitive Detection of Severe Fever with Thrombocytopenia Syndrome Virus Based on Immunofluorescent Carbon Dots/SiO2 Nanosphere-Based Lateral Flow Assay. ACS Omega 2019, 4, 21431–21438. [Google Scholar] [CrossRef] [Green Version]
- Anju; Rais, A.; Rawat, K.; Prasad, T.; Bohidar, H.B. Boron-doped carbon quantum dots: A ‘turn-off’ fluorescent probe for dopamine detection. Nanotechnology 2020, 32, 025501. [Google Scholar] [CrossRef]
- Song, Z.; Quan, F.; Xu, Y.; Liu, M.; Cui, L.; Liu, J. Multifunctional N,S co-doped carbon quantum dots with pH- and thermo-dependent switchable fluorescent properties and highly selective detection of glutathione. Carbon 2016, 104, 169–178. [Google Scholar] [CrossRef]
- Issa, M.A.; Abidin, Z.Z.; Sobri, S.; Rashid, S.; Mahdi, M.A.; Ibrahim, N.A.; Pudza, M.Y. Facile Synthesis of Nitrogen-Doped Carbon Dots from Lignocellulosic Waste. Nanomaterials 2019, 9, 1500. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fang, X.; Zhao, H.; Li, Z. A highly sensitive and selective detection of Cr(VI) and ascorbic acid based on nitrogen-doped carbon dots. Talanta 2018, 181, 318–325. [Google Scholar] [CrossRef]
- Loo, A.H.; Sofer, Z.; Bouša, D.; Ulbrich, P.; Bonanni, A.; Pumera, M. Carboxylic Carbon Quantum Dots as a Fluorescent Sensing Platform for DNA Detection. ACS Appl. Mater. Interfaces 2016, 8, 1951–1957. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Weng, R.; Qiang, T.; Wang, W. Glucose assay based on a fluorescent multi-hydroxyl carbon dots reversible assembly with phenylboronic acid brush grafted magnetic nanoparticles. Sens. Actuators B Chem. 2020, 304, 127349. [Google Scholar] [CrossRef]
- Yan, F.; Zu, F.; Xu, J.; Zhou, X.; Bai, Z.; Ma, C.; Luo, Y.; Chen, L. Fluorescent carbon dots for ratiometric detection of curcumin and ferric ion based on inner filter effect, cell imaging and PVDF membrane fouling research of iron flocculants in wastewater treatment. Sens. Actuators B Chem. 2019, 287, 231–240. [Google Scholar] [CrossRef]
- Jung Lai, I.; Harroun, S.G.; Chen, S.Y.; Unnikrishnan, B.; Li, Y.J.; Huang, C.C. Solid-state synthesis of self-functional carbon quantum dots for detection of bacteria and tumor cells. Sens. Actuators B Chem. 2016, 228, 465–470. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Y.; Wu, Y.; Weng, B.; Liu, Y.; Lu, Z.; Li, C.M.; Yu, C. Aptamer induced assembly of fluorescent nitrogen-doped carbon dots on gold nanoparticles for sensitive detection of AFB1. Biosens. Bioelectron. 2016, 78, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.R.; Vignesh Kumar, T.H.; Sundramoorthy, A.K. Electrochemically Exfoliated Carbon Quantum Dots Modified Electrodes for Detection of Dopamine Neurotransmitter. J. Electrochem. Soc. 2018, 165. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Pan, C. Ionic liquid-assisted electrochemical exfoliation of carbon dots of different size for fluorescent imaging of bacteria by tuning the water fraction in electrolyte. Microchim. Acta 2016, 183, 2525–2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, L.; Li, X.; Fan, S.; Li, J.; Mu, J.; Qin, M.; Wang, L.; Gan, G.; Tadé, M.; Liu, S. The bioelectrochemical synthesis of high-quality carbon dots with strengthened electricity output and excellent catalytic performance. Nanoscale 2019, 11, 4428–4437. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xie, Y. Enhanced electrochemical performance of carbon quantum dots-polyaniline hybrid. J. Power Sources 2016, 337, 54–64. [Google Scholar] [CrossRef]
- Niu, F.; Xu, A.; Liu, J.; Song, Z.; Liu, M.; Liu, J. Controllable electrochemical/electroanalytical approach to generate nitrogen-doped carbon quantum dots from varied amino acids: Pinpointing the utmost quantum yield and the versatile photoluminescent and electrochemiluminescent applications. Electrochim. Acta 2017, 236, 239–251. [Google Scholar] [CrossRef]
- Tan, S.C.; Chin, S.F.; Pang, S.C. Disposable carbon dots modified screen printed carbon electrode electrochemical sensor strip for selective detection of ferric ions. J. Sens. 2017, 2017, 7576345. [Google Scholar] [CrossRef]
- Fares, N.V.; Medhat, P.M.; El Maraghy, C.M.; Okeil, S.; Ayad, M.F. Influence of Nitrogen-Doped Carbon Dot and Silver Nanoparticle Modified Carbon Paste Electrodes on the Potentiometric Determination of Tobramycin Sulfate: A Comparative Study. Chemosensors 2021, 9, 52. [Google Scholar] [CrossRef]
- Algarra, M.; González-Calabuig, A.; Radotić, K.; Mutavdzic, D.; Ania, C.; Lázaro-Martínez, J.; Jiménez-Jiménez, J.; Rodríguez-Castellón, E.; del Valle, M. Enhanced electrochemical response of carbon quantum dot modified electrodes. Talanta 2018, 178, 679–685. [Google Scholar] [CrossRef]
- Pandey, P.K.; Preeti; Rawat, K.; Prasad, T.; Bohidar, H.B. Ultrasensitive Detection of Severe Fever with Thrombocytopenia Syndrome Virus Based on Immunofluorescent Carbon Dots/SiO2 Nanosphere-Based Lateral Flow Assay. J. Mater. Chem. B 2020, 8, 1277–1289. [Google Scholar] [CrossRef]
- Sharath Shankar, S.; Shereema, R.; Ramachandran, V.; Sruthi, T.V.; Sameer Kumar, V.B.; Rakhi, R.B. Carbon Quantum Dot-Modified Carbon Paste Electrode-Based Sensor for Selective and Sensitive Determination of Adrenaline. ACS Omega 2019, 4, 7903–7910. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Zhang, D.; Fang, Y.; Wang, H.; Liu, Y.; Xu, Z.; Wang, S.; Guo, Y. Detection of Ascorbic Acid Using Green Synthesized Carbon Quantum Dots. J. Sens. 2019, 2019, 9869682. [Google Scholar] [CrossRef]








| Method | Advantages (Pros) | Disadvantages (Cons) | References |
|---|---|---|---|
| “Top–Down” Approach | [7,22,51] | ||
| Arc discharge |
|
| |
| Laser ablation | |||
| Chemical oxidation | |||
| Electrochemical oxidation | |||
| “Bottom–Up” Approach | |||
| Hydrothermal/solvothermal treatment |
|
| [33,51] |
| Carbonization | |||
| Microwave-assisted synthesis | |||
| Thermal decomposition and pyrolysis |
| Raw Material | Synthesis | Modification | QY | Application | Reference |
|---|---|---|---|---|---|
| Biomass | |||||
| Glucose | Continuous hydrothermal flow synthesis (CHFS) | ND 1 | 0.3% | Detection of Cr6+ and Fe2+ ions | [45] |
| Rice residue | Hydrothermal | Lysine | 23.48% | Detection of Fe3+ ions and tetracycline–antibiotic | [46] |
| Cherry tomato | Hydrothermal | ND 1 | 9.7% | Detection of trifluralin–herbicide | [53] |
| Fresh tomato | Microwave-assisted synthesis | EDA 2, urea | 1.77% (raw material), 7.9% for EDA, and 8.5% for urea (modified) | Vanillin detection | [54] |
| Potato starch | Acid-assisted ultrasonic route | Acid oxidation | 10.0% (standard: Rhodamine B) | Detection of Zn2+ ions | [55] |
| Expired milk | Subcritical water synthesis | ND 1 | 8.64% | Detection of Fe3+ ions | [56] |
| Citrus lemon juice | Hydrothermal | EDA 2 | 31.0% | Detection of Hg2+ ions | [57] |
| Rosa roxburghii | Hydrothermal | ND 1 | 24.8% | Detection of o-nitrophenol | [58] |
| Carrot | Hydrothermal | PEI 3 | 11.5% | Detection of S2− ions | [59] |
| Spinach | Hydrothermal | ND 1 | 53.0% | Detection of 2-nitrophenol (2-NP) and 4-nitrophenol (4-NP) | [60] |
| Mixture of lemon and onion juice | Microwave-assisted carbonization | NH4OH | 23.6% | Riboflavin | [61] |
| Cranberry beans | Hydrothermal | ND 1 | 10.85% | Detection of Fe3+ ions | [62] |
| Onion extract | Hydrothermal | EDA 2 | 6.21% | Detection of Zn2+ ions in blood plasma | [63] |
| Waste | |||||
| Orange peel (OP), ginkgo biloba leaves (GB), paulownia leaves (PL), and magnolia flower (MF) | Hydrothermal | ND 1 | 4.29% (OP-CQDs), 7.72% (GB-CQDs), 4.74% (PL-CQDs), 8.13% (MF-CQDs) | Detection of Fe3+ ions | [64] |
| Lemon peel | Hydrothermal | Acid oxidation (0.1 M H2SO4/TiO2) | 14.0% | Detection of Cr6+ | [65] |
| Hydrothermal | ND 1 | 11.0% | Detection of carmine in beverages (E120) | [66] | |
| Mango peel | Hydrothermal | Toluene, APTES 4 | ND | Detection of mesotrione–herbicide | [67] |
| Onion waste | Carbonization | EDA 2 | 28.0% | Detection of Fe3+ ions | [68] |
| Pineapple peel | Hydrothermal | Ethanol | 42.0% | Detection of Hg2+ ions | [69] |
| Fuel waste | Chemical oxidation | HNO3 | 3.0% | Detection of picric acid, Cu2+ and Fe3+ ions | [70] |
| Taro peel | Carbonization | H2O2; Eu3+ | 13.80% | Detection of F− ions | [71] |
| CQDs-Based Sensor | Type of CQDs | QY (%) | λEX/λEMmax | Linear Range | LOD | Reference |
|---|---|---|---|---|---|---|
| CQDs/rice residue–lysine | N-CQDs | 23.48% | 360/440 nm | 3.32–32.26 µM | 0.7462 µM | [46] |
| CQDs/trisodium citrate–phosphoric acid | P-CQDs | 16.1% | 310/440 nm | 0.02–3 µM | 9.5 nM | [49] |
| CQDs from cranberry | Pristine CQDs | 10.85% | 380/450 nm | 30–600 µM | 9.55 µM | [62] |
| CQDs from magnolia flower | N-CQDs | 8.13% | 350/435 nm | 0.2–100 µM | 0.073 µM | [64] |
| CQDs/carboxymethylcellulose–LPEI 1 | N-CQDs | 44.0% | 350/465.5 ± 3 nm | 1–400 µM | 0.14 µM | [86] |
| CQDs/cellulose fiber–sulfuric acid | S-CQDs | 32.0% | 360/435 nm | 25–250 µM | 0.96 µM | [87] |
| CQDs from Fusobacterium nucleatum | N-CQDs | 9.99% | 360/450 nm | 20–180 µM | 0.82 µM | [88] |
| CQDs/glutamic acid–EDA 2 | N-CQDs | 12.45% | 360/459 nm | 8–80 µM | 3.8 µM | [89] |
| CQDs/phenylalanine–citric acid | N-CQDs | ND 3 | 335/440 nm | 5–500 µM | 0.720 µM | [90] |
| CQDs/Trisodium citrate–urea–boric acid | B,N-co-doped CQDs | 70.0% | 340/450 nm | 0–100 µM | 80.0 ± 0.5 nM | [91] |
| CQDs/glutamic acid | N-CQDs | 17.8% | 370/440 nm | 0–50 µM | 4.67 µM | [92] |
| CQDs/EDA–microcrystalline cellulose | N-CQDs | 51.0% | 360/436 nm | 10–240 µM | 0.21 nM | [93] |
| CQDs/citric acid–L-cysteine | N,S-co-doped CQDs | 69.0% | 345/415 nm | 1–500 µM | 0.014 µM | [94] |
| CQDs/biomass tar–EDA | N-CQDs | 26.1% | 340/420 nm | 0.06–1400 µM | 60 nM | [95] |
| CQDs/watermelon juice–ethanol | N-CQDs | 10.6% | 355/439 nm | 0–300 µM | 0.16 µM | [96] |
| CQDs/jujube fruit | N-CQDs | ND 3 | 370/440 nm | 0–200 µM | ND3 | [97] |
| CQDs/lignin in DES 4 (betaine and lactic acid) | N-CQDs | 7.95% | 300/400 nm | 0–500 µM | 0.44 µM | [98] |
| CQDs/glucose–ammonia–phosphoric acid | N,P-co-doped CQDs | 30.0% | 336/437 nm | 5–100 nM | 1.8 nM | [99] |
| CQDs/tartaric acid–L-arginine | N-CQDs | 8.3% | 350/425 nm | 0–70 µM | 0.50 µM | [100] |
| CQDs-Based Sensor | Type of CQDs | QY | λEX/λEMmax | Linear Range | LOD | Reference |
|---|---|---|---|---|---|---|
| CQDs from lemon juice/EDA | N-CQDs | 31.0% | 360/452 nm | 0.001–1 µM | 5.3 nM | [57] |
| CQDs/tartaric acid–L-arginine | N-CQDs | 8.3% | 350/425 nm | 0–5 µM | 0.017 µM | [100] |
| CQDs/cellulose hydrogel | N-CQDs | 18.3% | 370/450 nm | (i) 0.2 to 10 µM (ii) 10 to 100 µM | 0.2 μM | [101] |
| CQDs/citric acid–acrylamide–formamide | N-CQDs | ND 1 | 560/644 nm | 0–40 µM | 0.19 µM | [102] |
| CQDs/hair | N-CQDs | 10.75% | 330/415 nm | 0–75 µM | 0.01 µM | [103] |
| CQDs/PAMAM 2–APTES 3 | N-CQDs | 52.6% | 354/442 nm | 0.2–15 nM | 0.087 nM | [104] |
| CQDs/L-tryptophan | N-CQDs | 32.0% | 350/440 nm | 0–1.8 µM | 10 nM (in tap water) | [105] |
| CQDs/citric acid–tartaric acid–ethanediamine | N-CQDs | 42.2% | 360/460 nm | 0–18 µM | 83.5 nM | [106] |
| Ag/CQDs composite | Ag/CQDs | ND 1 | ND 1 | 0.5–50 µM | 85 nM | [107] |
| CQDs/pigeon feathers (i), pigeon egg yolk (ii), and egg white (iii) | N,S-co-doped CQDs | (i) 24.8 %, (ii) 17.48%, (iii) 16.34% | (i) 330/415 nm, (ii) 340/410 nm, (iii) 340/420 nm | (i) 0 to 1.2 µM (pigeon feathers), (ii) 0.05 to 1.2 µM (pigeon egg white), (iii) 0 to 1.6 µM (pigeon egg yolk) | (i) 10.3 nM, (ii) 34.6 nM, (iii) 34.9 nM | [108] |
| CQDs/sodium citrate–urea | N-CQDs | ND 1 | 390/450 nm | 0.20–21 µM | 3.3 nM | [109] |
| CQDs/Tamarindus indica leaves | N-CQDs | 46.6% | 320/420 nm | 0–0.1 µM | 6 nM | [110] |
| CQDs/folic acid–glycerol–chloroauric acid | Au/N-CQDs | 8.6% | 355/450 nm | 0–41.9 µM | 0.118 µM | [111] |
| CQDs/glucose–aspartic acid–branched polyethyleneimine | N-CQDs | 45.0% | 365/440 nm | 20–800 nM | 10 nM | [112] |
| CQDs/Prosopis juliflora leaves | N-CQDs | 5.0% | (i) 325/396 nm(ii) 350/437 nm | 5–500 ng/mL | 1.26 ng mL−1 | [113] |
| CQDs/citric acid–glycine | N-CQDs | ND 1 | 350/432 nm | 0.12–2 ppm | 38 ppb | [114] |
| CQDs-Based Sensor | Type of CQDs | QY | λEX/λEMmax | Target Metal Ion | Linear Range | LOD | Reference |
|---|---|---|---|---|---|---|---|
| CQDs/potato starch | Pristine CQDs | 10.0% | 365/515 nm | Zn2+ | 0–20 µM | 1 nM | [55] |
| CQDs/onion extract | N-CQDs | 10.85% | 380/450 nm | Zn2+ | - | 6.4 μM | [63] |
| CQDs/zinc gluconate | Zn-CQDs | 13.89% | 365/460 nm | Zn2+ | 2–15 μM | 0.51 μM | [115] |
| CQDs/p-phenylenediamine and cysteamine | N,S-co-doped CQDs | ND 1 | 420/550 nm | Zn2+ | 0.18–4.22 μM | 0.021 μM | [116] |
| CQDs/broccoli | N-CQDs | ND 1 | 355/450 nm | Ag+ | 0–600 µM | 0.5 μM | [117] |
| CQDs/MWCNTs 2-amine and thiol modifications | CQDs hydrogels | ND 1 | 350/430 nm | Ag+ | 0.8–20 μg mL−1 | 0.55 μg mL−1 | [118] |
| CQDs/osmanthus fragrans | N-CQDs | 21.9% | 350/435 nm | Al3+ | 0.1–100 µM | 26 nM | [119] |
| CQDs/prickly pear cactus juice | GSH 3-CQDs | 12.7% | 355/446 nm | As3+ | 2–12 nM | 2.3 nM | [120] |
| CQDs/jackfruit seeds–o-phosphoric acid | N-CQDs | 17.91% | 360/437 nm | Au3+ | 0–100 µM | 239 nM | [121] |
| CQDs/citric acid–EDA 4-EGTA 5 | N-CQDs | ND 1 | 360/460 nm | Ca2+ | 15–300 µM | 0.38 µM | [122] |
| CQDs/EDTA 6 | N-CQDs | 22.0% | 353/450 nm | Ca2+ | 1–10 nM | 77 pM | [123] |
| CQDs/alanine–histidine | CQDs-AuNCs 7 | 20.02% | 340/438 nm | Cd2+ | 0.4–15 µM | 32.5 nM | [124] |
| CQDs/glycine–PEI 8 | N-CQDs | 57.0% | 365/464 nm | Co2+ | 0.5–3 µM | 0.12 µM | [125] |
| CQDs/edible seeds | Pristine CQDs | 41.8% (the best performing CQDs) | 347/421 nm | Cr3+ | 1–14 µM | 1.3 µM | [126] |
| CQDs/citric acid–L-reduced GSH 3 | N-CQDs | 69.0% | 350/418 nm | Cr6+ | 0.10–12 μg mL−1 | 0.03 μg mL−1 | [127] |
| CQDs/tuberose (Polianthes tuberose L.) | N-CQDs | 3.0% | 330/430 nm | Cu2+ | 0–70 µM | 200 nM | [128] |
| CQDs/waste polyolefin | Pristine CQDs | 4.84% | 490/540 nm | Cu2+ | 1–8 µM | 6.33 nM | [129] |
| CQDs/citric acid–L-cysteine | N,S-co-doped CQDs | 82.0% | 375/430 nm | Cu2+ | 0.01–0.5 μg L−1 | 2.1 μg L−1 | [130] |
| CQDs/Finger millet ragi (Eleusine coracana) | N-CQDs | ND 1 | 340/425 nm | Cu2+ | 0–100 µM | 10 nM | [131] |
| CQDs/prawn shell | N-CQDs | 9.0% | 330/405 nm | Cu2+ | 0.1–5 µM | 5 nM | [132] |
| CQDs/durian shell waste–urea–aluminum nitrate | N/Al-CQDs | 28.7% | 345/415 nm | Mn7+ | 0–100 µM | 46.8 nM | [133] |
| CQDs/citric acid–urea-imidazole | CQDs-imidazole | ND 1 | 360/435 nm | Ni2+ | 6–100 mM | 0.93 mM | [134] |
| CQDs/sodium citrate–polyacrylamide | N-CQDs | 18.0% | 343/434 nm | Pb2+ | 0.0167–1 µM | 4.6 nM | [135] |
| CQDs/lemon juice | Pristine CQDs | 21.0% | 420/540 nm | V5+ | 0–8 ppm | 3.2 ppm | [136] |
| Raw Material | Synthesis/Modification | QY | λEX/λEMmax | Analyte | Linear Range | LOD | Reference |
|---|---|---|---|---|---|---|---|
| Rice residue | Hydrothermal/lysine | 23.48% | 360/440 nm | Tetracycline–antibiotic | 3.32–32.26 µM | 0.7462 µM | [46] |
| Arginine | Hydrothermal | 48.0% | 360/430 nm | 4-chloroethcathinone–psychoactive substance | 2000–12500 ng mL−1 | 1300 ng mL−1 | [47] |
| Fresh tomato | Microwave-assisted synthesis/urea | 8.5% | 300/356 nm | Vanillin | 3–55 μM | 24.9 mg kg−1 | [54] |
| Mixture of lemon and onion juice | Microwave-assisted Carbonization/NH4OH | 23.6% | 380/440 nm | Riboflavin | 0.10–3.0 mg/mL | 1.0 ng/mL | [61] |
| Citric acid monohydrate and tartaric acid | One-step solvothermal | 42.2% | 360/460 nm | L-cysteine | 0–40 μM | 45.8 nM | [106] |
| Prosopis juliflora leaves | Carbonization | 5.0% | 350/437 nm | Chemet (antipoisoning drug for heavy metal ions) | 2.5–22.5 ng mL−1 | 1.4 ng mL−1 | [113] |
| Osmanthus fragrans | Hydrothermal | 21.9% | 350/435 nm | Quercetin (QT) | 0.003–80 μM | 1 nM | [119] |
| Histidine in NaOH | Hydrothermal | ND 1 | 350/460 nm | HIV DNA | 50.0 fM–1.0 nM | 15 fM | [139] |
| Citric acid | Hydrothermal | ND 1 | 320/430 nm | Kanamycin–antibiotic | 0.04–0.24 mM | 18 nM | [140] |
| Hydrothermal/thiourea | 24.0% | 550/610 nm | Parathion–insecticide | 0.1–10 mU mL−1 | 0.0625 pg mL−1 | [141] | |
| Hydrothermal/cysteine | 57.2% | 355/450 nm | Methotrexate–chemotherapy agent | 0.4–41.3 µg/mL | 12 ng/mL | [142] | |
| Microwave-assisted synthesis/EDA-AuNPs 2 | ND 1 | 360/455 nm | Paraoxon–insecticide | (i) 0.01–1 µg L−1 and (ii) 1.0–0.001 ng L−1 | 1.0 ng L−1 | [143] | |
| Citric acid–urea–DMF | Solvothermal | 5.5% | 365/450 and 500 nm | H2O2 | 0.05–0.5 M | 14 mM | [144] |
| Sucrose–H2SO4–PEG 200 3 | Reduction/CQDs-AuNPs 1 | ND 1 | 420/527 nm | Malathion in cabbage | 1 × 10−9–1 × 10−2 M | 0.13 × 10−9 M | [145] |
| Ethylenediamine (EDA) | Microwave-assisted synthesis/β-cyclodextrin functionalized N,Zn-co-doped CQD | 14.26% | 350/480 nm | Ofloxacin–antibiotic | 0.027–1.3537 μg mL−1 | 0.0181 μg mL−1 | [146] |
| κ-Carrageenan and urea | Hydrothermal | 69.27% | 360/432 nm | Acetone in human fluids | 0–0.05 M (in blood) and 0–0.01 M (in urine) | 0.72 µM | [147] |
| p-aminobenzoic acid and ethanol | Solvothermal | ND 1 | 350/460 nm | Hematin in human red cells | 0.5–10 µM | 0.25 µM | [148] |
| Gelatin | Hydrothermal | 26.9% | 330/436 nm | Atrazine, chlorpyrifos, imidacloprid, lindane and tetradifon–insecticides | Best performance for imidacloprid 0–17 mM | 0.013 mM | [149] |
| Beet | Hydrothermal | ND 1 | ND 1 | Amoxicillin–antibiotic | 0–400 μM | 0.475 μM | [150] |
| o-aminophenol | Hydrothermal | 40.0% | 300/410 nm | Heparin–anticoagulant | 10–100 nM | 8.2 nM | [151] |
| Melamine | Hydrothermal/solvothermal treatment/molybdenum trioxide (MP-MoO3 CQDs) | 44.0% | 500/652 nm | Influenza A virus (H1N1) | 45–25000 PFU/mL | 45 PFU/mL | [152] |
| 4-amino-3- hydrazino-5-mercapto-1,2,4-triazole | Solvothermal synthesis/N-,S-doped CQDs | 33.0% | 400/512 nm | 10 fg/mL to 1.0 ng/mL | 5.5 fg/mL | [48] | |
| n-(2-aminoethyl)-3-aminopropyl-trimethoxysilane | Co-hydrolysis/silanized CQDs with TEOS 4 | 56.3% | 360/459 nm | SFTS 5 virus | Visual detection | 10 pg/mL | [153] |
| 4-hydroxy phenylboronic acid | Hydrothermal/B-doped CQDs | 30.0% | 295/398 nm | Dopamine | (i) 0.28–1.5 mM and (ii) 1.32–2.5 mM | 6 µM | [154] |
| L-cysteine and ammonia | Hydrothermal/N,S-doped CQDs | 17.2% | 410/505 nm | Glutathione | (i) 0–50 μM, and (ii) 50–100 μM | 6.7 μM | [155] |
| Lignin | Hydrothermal | 44.0% | 350/465.5 nm | Ascorbic Acid (AA) and Fe3+ | For AA (i) 0–350 μM, and for Fe3+ (ii) 50–650 μM | (i) LOD = 5.34 μM, and (ii) LOD = 196 nM | [156] |
| Glutamic acid and citric acid | One-step pyrolysis | 16.2% | 360/448 nm | Ascorbic Acid (AA) and Cr6+ | For AA (i) 1.0–750 μM, and for Cr6+ (ii) 0.01 to 250 μM | (i) LOD = 0.3 μM, and (ii) LOD = 5 nM | [157] |
| (i) Citric acid–NaOH; (ii) malic acid–NaOH | Chemical oxidation | ND1 | (i) 370/465 nm, (ii) 370/430 nm | DNA | (i) 0.4–400 nM, and (ii) 0.04–400 nM | (i) 45.6 nM, and (ii) 17.4 nM | [158] |
| Tris(hydroxymethyl)aminomethane and citric acid | Pyrolysis/composite of CQDs/Fe3O4@APBA 6 | 58.7% | 332/407 nm | Glucose | 0.2–20 mM | 0.15 µM | [159] |
| Hydrothermal | ND 1 | 350/420 nm | Curcumin | 5–30 μM | 21.79 nM | [160] | |
| di-Ammonium hydrogen citrate | Pyrolysis/ CQDs@colistin | 7.56 % | 360/450 nm | Escherichia coli (E. coli) | 3.81 × 102–2.44 × 104 CFU mL−1 | 460 CFU mL−1 | [137] |
| Ammonium citrate-mannose | Solid-state synthesis | 9.0 % | 365/460 nm | 0–108 CFU mL−1 | 100 CFU mL−1 | [161] | |
| Pancreatin | Hydrothermal/N-CQDs/aptamer/AuNPs 2 | 26.8% | 370/445 nm | Aflatoxin B1 (AFB1) | 0.005–2 ng mL−1 | 5 pg mL−1 | [162] |
| Precursor | Electrochemical Synthesis—Conditions | QY (λEX/λEMmax) | Modification Method (CQDs/Electrode) | Applications | References |
|---|---|---|---|---|---|
| Graphite rod | Electrochemical synthesis; two graphite rods as working and counter electrode in electrolyte of ionic liquid (IL) | 10.0% (360/440 nm) | - | Bacteria cell imaging | [164] |
| Electrochemical synthesis; two graphite rods were used as anode and cathode in 0.1 M NaOH/EtOH electrolyte, constant current 50 mA was applied for 3 h | ND 1 (ND 1) | Glassy carbon electrode (GCE) and screen-printed electrode (SPCE) modified with CQDs dispersion–drop casting | Electrochemical sensor for dopamine, LOD = 0.099 μM | [163] | |
| Electrochemical synthesis; two graphite rods were used as anode and cathode in mineral water (Wahaha water) as electrolyte | ND 1 (500/558 nm) | GCE prepared by CQDs drop-casting on the electrode surface | Photocatalytic activity for H2 production, contaminant elimination activity at solid-solid interface | [165] | |
| Graphite electrode | Electrochemical synthesis; graphite working electrode, platinum foil counter electrode and Ag/AgCl reference electrode in EtOH/NaOH electrolyte, applied voltage 5 V for 3 h in nitrogen atmosphere | 4.6–11.2% (365/436–438 nm) | - | Determination of Fe3+ ion in tap water (LOD = 1.8 µM) by measuring PL intensity and cell imaging | [29] |
| Electrochemical synthesis; electrooxidation of graphite column electrode at 3 V in 0.1 M KH2PO4 | ND 1 (ND 1) | CQDs incorporated in PANI 2, photo-assisted cyclic voltammetry electrodeposition | All-solid-state flexible supercapacitor | [166] | |
| Amino acids (Cys, Asp, Ser, Met, His, Arg) | Electrochemical synthesis; Pt working and auxiliary electrode and Ag/AgCl reference electrode, potential range from 1 V to 10 V | 46.2–Asp (360/430 nm) | - | Cell imaging, fiber staining, and sensitive detection of Fe3+ ions (LOD = 0.5 μM) | [167] |
| Carbon rod | Electrochemical synthesis; two carbon rods were used as anode and cathode in ultrapure water, constant voltage of 50 V was applied, electrolyte was stirred for 96 h | ND 1 (ND 1) | Screen-printed electrode (SPCE) modified with CQDs dispersion–drop casting; dried in oven at 90 °C for 10 min | Electrochemical sensor for ferric ions (Fe3+), LOD = 0.44 ± 0.04 ppm | [168] |
| Precursor | Preparation of CQDs | Modification Method (CQDs/Electrode) | Applications | References |
|---|---|---|---|---|
| Citric acid | Solvothermal bottom–up synthesis | CQD–polypyrrole composite | Supercapacitor electrode material | [42] |
| Pyrolysis; temperature of 300 °C for 3 h under nitrogen flow | PVP 1; GCE modified with CQDs capped with PVP | Electrochemical sensor for etoposide (ETO), LOD = 5 nm, determination of ETO in real samples under optimal conditions | [43] | |
| Citric acid and urea | Microwave-assisted method | Carbon paste electrode modified with nitrogen-doped CQDs | Electrochemical sensor for TOBRA 2, LOD = 3.2 nM | [169] |
| Sucrose | Hydrothermal synthesis; sucrose, and NaOH in a Teflon-lined stainless-steel autoclave at 160 °C for 4 h | |||
| Graphite | Green modification of Hummers method | Glassy carbon electrode modified with CQDs, 40 µL of suspension (γ = 1 mg mL−1) placed on the electrode surface and dried at 40 °C for 1 h | Electrochemical sensor for dopamine (LOD = 2.7 µM) and uric acid (LOD = 1.3 µM) | [170] |
| Salmon DNA | Hydrothermal/DNA hydrogel | Drop-casting sol on ITO plate | Dopamine detection LOD = 5 µM | [171] |
| Carbon soot | Mixed with NaOH, followed by centrifugation and multiple sonication | Carbon paste prepared by CQDs drop casting on the electrode surface | Electrochemical sensor for adrenaline, LOD = 6 nM | [172] |
| Glucose | Microwave synthesis; glucose mixes with PEG-200 3 | GCE modified with CQDs with electropolymerization (cyclic voltammetry, 20 cycles, −1 to 2 V, scan rate 50 mV/s | Electrochemical sensor for ascorbic acid (AA), LOD = 10 µM | [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šafranko, S.; Goman, D.; Stanković, A.; Medvidović-Kosanović, M.; Moslavac, T.; Jerković, I.; Jokić, S. An Overview of the Recent Developments in Carbon Quantum Dots—Promising Nanomaterials for Metal Ion Detection and (Bio)Molecule Sensing. Chemosensors 2021, 9, 138. https://doi.org/10.3390/chemosensors9060138
Šafranko S, Goman D, Stanković A, Medvidović-Kosanović M, Moslavac T, Jerković I, Jokić S. An Overview of the Recent Developments in Carbon Quantum Dots—Promising Nanomaterials for Metal Ion Detection and (Bio)Molecule Sensing. Chemosensors. 2021; 9(6):138. https://doi.org/10.3390/chemosensors9060138
Chicago/Turabian StyleŠafranko, Silvija, Dominik Goman, Anamarija Stanković, Martina Medvidović-Kosanović, Tihomir Moslavac, Igor Jerković, and Stela Jokić. 2021. "An Overview of the Recent Developments in Carbon Quantum Dots—Promising Nanomaterials for Metal Ion Detection and (Bio)Molecule Sensing" Chemosensors 9, no. 6: 138. https://doi.org/10.3390/chemosensors9060138
APA StyleŠafranko, S., Goman, D., Stanković, A., Medvidović-Kosanović, M., Moslavac, T., Jerković, I., & Jokić, S. (2021). An Overview of the Recent Developments in Carbon Quantum Dots—Promising Nanomaterials for Metal Ion Detection and (Bio)Molecule Sensing. Chemosensors, 9(6), 138. https://doi.org/10.3390/chemosensors9060138