Pharmacokinetics of Antibacterial Agents in the Elderly: The Body of Evidence
Abstract
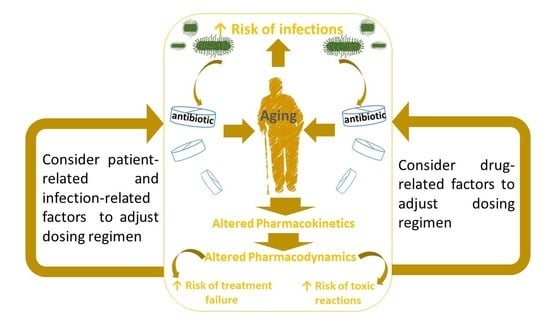
:1. Introduction
2. Factors Influencing AB Prescribing in the Elderly
3. General Considerations on AB Pharmacokinetics in the Elderly
- time-dependent (β-lactams, natural macrolides, lincosamides, oxazolidinones),
- concentration-dependent (aminoglycosides, fluoroquinolones, nitroimidazoles, daptomycin, quinupristin/dalfopristin),
- concentration-dependent with time-dependence (tetracyclines, glycylcyclines, glycopeptides, semisynthetic macrolides).
3.1. Absorption
3.2. Distribution
3.3. Metabolism
3.4. Excretion
4. AB Dosing Regimens in the Elderly
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinzowu, T.; Roy, S.; Nishtala, P.S. Antimicrobial-associated organ injury among the elderly: A systematic review and meta-analysis protocol. BMJ Open 2022, 11, e055210. [Google Scholar] [CrossRef] [PubMed]
- Sabri, S.M.; Annuar, N.; Rahman, N.L.A.; Musairah, S.K.; Mutalib, H.A.; Subagja, I.K. Major Trends in Ageing Population Research: A Bibliometric Analysis from 2001 to 2021. Proceedings 2022, 82, 19. [Google Scholar] [CrossRef]
- Veimer Jensen, M.L.; Aabenhus, R.M.; Holzknecht, B.J.; Bjerrum, L.; Jensen, J.N.; Siersma, V.; Córdoba, G. Antibiotic prescribing in Danish general practice in the elderly population from 2010 to 2017. Scand. J. Prim. Health Care 2021, 39, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, N.; Cataldo, M.A.; Pea, F. Treatment options for community-acquired pneumonia in the elderly people. Expert Rev. Anti-Infect. Ther. 2015, 13, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.F. Principles of Antimicrobial Therapy in Older Adults. Clin. Geriatr. Med. 2016, 32, 443–457. [Google Scholar] [CrossRef]
- Bouza, E.; Brenes, F.J.; Díez Domingo, J.; Eiros Bouza, J.M.; González, J.; Gracia, D.; Juárez González, R.; Muñoz, P.; Petidier Torregrossa, R.; Casado, J.M.R.; et al. The situation of infection in the elderly in Spain: A multidisciplinary opinion document. Rev. Española Quimioter. 2020, 33, 327–349. [Google Scholar] [CrossRef]
- Barber, K.E.; Bell, A.M.; Stover, K.R.; Wagner, J.L. Intravenous Vancomycin Dosing in the Elderly: A Focus on Clinical Issues and Practical Application. Drugs Aging 2016, 33, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Giarratano, A.; Green, S.E.; Nicolau, D.P. Review of antimicrobial use and considerations in the elderly population. Clin. Interv. Aging 2018, 13, 657–667. [Google Scholar] [CrossRef] [Green Version]
- Pagani, L. Appropriate antimicrobial therapy in the elderly: When half-size does not fit all frail patients. Clin. Microbiol. Infect. 2015, 21, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Cristina, M.L.; Spagnolo, A.M.; Giribone, L.; Demartini, A.; Sartini, M. Epidemiology and Prevention of Healthcare-Associated Infections in Geriatric Patients: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 5333. [Google Scholar] [CrossRef]
- Sanz, F.; Morales-Suarez-Varela, M.; Fernandez, E.; Force, L.; Perez-Lozano, M.J.; Martin, V.; Egurrola, M.; Castilla, J.; Astray, J.; Toledo, D.; et al. A Composite of Functional Status and Pneumonia Severity Index Improves the Prediction of Pneumonia Mortality in Older Patients. J. Gen. Intern. Med. 2018, 33, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.X.; Yong, Y.; Tan, W.C.; Shen, L.; Ng, H.S.; Fong, K.Y. Prognostic factors for mortality due to pneumonia among adults from different age groups in Singapore and mortality predictions based on PSI and CURB-65. Singap. Med. J. 2018, 59, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Prim. 2021, 7, 25. [Google Scholar] [CrossRef]
- Galimberti, F.; Casula, M.; Olmastroni, E.; Catapano, A.L.; Tragni, E.; On Behalf Of Edu Re Drug Group. Antibiotic Prescription in the Community-Dwelling Elderly Population in Lombardy, Italy: A Sub-Analysis of the EDU.RE.DRUG Study. Antibiotics 2022, 11, 1369. [Google Scholar] [CrossRef]
- Kusuma, I.Y.; Matuz, M.; Bordás, R.; Juhasz Haverinen, M.; Bahar, M.A.; Hajdu, E.; Visnyovszki, Á.; Ruzsa, R.; Doró, P.; Engi, Z.; et al. Antibiotic use in elderly patients in ambulatory care: A comparison between Hungary and Sweden. Front. Pharmacol. 2022, 13, 1042418. [Google Scholar] [CrossRef]
- Cruz, S.P.; Cebrino, J. Prevalence and Determinants of Antibiotic Consumption in the Elderly during 2016–2017. Int. J. Environ. Res. Public Health 2020, 17, 3243. [Google Scholar] [CrossRef]
- Insani, W.N.; Whittlesea, C.; Alwafi, H.; Man, K.K.C.; Chapman, S.; Wei, L. Prevalence of adverse drug reactions in the primary care setting: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0252161. [Google Scholar] [CrossRef]
- Vrdoljak, D.; Borovac, J.A. Medication in the elderly—Considerations and therapy prescription guidelines. Acta Med. Acad. 2015, 44, 159–168. [Google Scholar] [CrossRef]
- Lexow, M.; Wernecke, K.; Schmid, G.L.; Sultzer, R.; Bertsche, T.; Schiek, S. Considering additive effects of polypharmacy: Analysis of adverse events in geriatric patients in long-term care facilities. Wien. Klin. Wochenschr. 2021, 133, 816–824. [Google Scholar] [CrossRef]
- Dovjak, P. Polypharmacy in elderly people. Wien. Med. Wochenschr. 2022, 172, 109–113. [Google Scholar] [CrossRef]
- Kim, J.; Parish, A.L. Polypharmacy and Medication Management in Older Adults. Nurs. Clin. N. Am. 2017, 52, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Yang-Huang, J.; Franse, C.B.; Rukavina, T.; Vasiljev, V.; Mattace-Raso, F.; Verma, A.; Borrás, T.A.; Rentoumis, T.; Raat, H. Factors associated with polypharmacy and the high risk of medication-related problems among older community-dwelling adults in European countries: A longitudinal study. BMC Geriatr. 2022, 22, 841. [Google Scholar] [CrossRef] [PubMed]
- Corsonello, A.; Abbatecola, A.M.; Fusco, S.; Luciani, F.; Marino, A.; Catalano, S.; Maggio, M.G.; Lattanzio, F. The impact of drug interactions and polypharmacy on antimicrobial therapy in the elderly. Clin. Microbiol. Infect. 2015, 21, 20–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pea, F. Pharmacokinetics and drug metabolism of antibiotics in the elderly. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Hoff, B.M.; Maker, J.H.; Dager, W.E.; Heintz, B.H. Antibiotic Dosing for Critically Ill Adult Patients Receiving Intermittent Hemodialysis, Prolonged Intermittent Renal Replacement Therapy, and Continuous Renal Replacement Therapy: An Update. Ann. Pharmacother. 2020, 54, 43–55. [Google Scholar] [CrossRef]
- Tannenbaum, C.; Day, D.; on behalf of the Matera Alliance. Age and sex in drug development and testing for adults. Pharmacol. Res. 2017, 121, 83–93. [Google Scholar] [CrossRef]
- Appaneal, H.J.; Shireman, T.I.; Lopes, V.V.; Mor, V.; Dosa, D.M.; LaPlante, K.L.; Caffrey, A.R. Poor clinical outcomes associated with suboptimal antibiotic treatment among older long-term care facility residents with urinary tract infection: A retrospective cohort study. BMC Geriatr. 2021, 21, 436. [Google Scholar] [CrossRef]
- Simonetti, A.F.; Viasus, D.; Garcia-Vidal, C.; Carratalà, J. Management of community-acquired pneumonia in older adults. Ther. Adv. Infect. Dis. 2014, 2, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Compté, N.; Dumont, L.; Bron, D.; De Breucker, S.; Praet, J.P.; Bautmans, I.; Pepersack, T. White blood cell counts in a geriatric hospitalized population: A poor diagnostic marker of infection. Exp. Gerontol. 2018, 114, 87–92. [Google Scholar] [CrossRef]
- Chong, C.P.; Street, P.R. Pneumonia in the elderly: A review of the epidemiology, pathogenesis, microbiology, and clinical features. South Med. J. 2008, 101, 1141–1145; quiz 1132, 1179. [Google Scholar] [CrossRef]
- Davies, E.A.; O’Mahony, M.S. Adverse drug reactions in special populations—The elderly. Br. J. Clin. Pharmacol. 2015, 80, 796–807. [Google Scholar] [CrossRef] [Green Version]
- Völter, C.; Götze, L.; Dazert, S.; Wirth, R.; Thomas, J.P. Impact of Hearing Loss on Geriatric Assessment. Clin. Interv. Aging 2020, 15, 2453–2467. [Google Scholar] [CrossRef]
- Kim, L.D.; Koncilja, K.; Nielsen, C. Medication management in older adults. Clevel. Clin. J. Med. 2018, 85, 129–135. [Google Scholar] [CrossRef]
- Zullo, A.R.; Gray, S.L.; Holmes, H.M.; Marcum, Z.A. Screening for Medication Appropriateness in Older Adults. Clin. Geriatr. Med. 2018, 34, 39–54. [Google Scholar] [CrossRef]
- Morrill, H.J.; Caffrey, A.R.; Jump, R.L.; Dosa, D.; LaPlante, K.L. Antimicrobial Stewardship in Long-Term Care Facilities: A Call to Action. J. Am. Med. Dir. Assoc. 2016, 17, 183.e1–183.e16. [Google Scholar] [CrossRef]
- Janssen, M.W.H.; de Bont, E.G.P.M.; Hoebe, C.J.P.A.; Cals, J.W.L.; den Heijer, C.D.J. Trends in antibiotic prescribing in Dutch general practice and determinants of nonprudent antibiotic prescriptions. Fam. Pract. 2023, 40, 61–67. [Google Scholar] [CrossRef]
- Palmer, M.E.; Andrews, L.J.; Abbey, T.C.; Dahlquist, A.E.; Wenzler, E. The importance of pharmacokinetics and pharmacodynamics in antimicrobial drug development and their influence on the success of agents developed to combat resistant gram negative pathogens: A review. Front. Pharmacol. 2022, 13, 888079. [Google Scholar] [CrossRef]
- Póvoa, P.; Moniz, P.; Pereira, J.G.; Coelho, L. Optimizing Antimicrobial Drug Dosing in Critically Ill Patients. Microorganisms 2021, 9, 1401. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Pruskowski, K.A. Pharmacokinetics and Pharmacodynamics of Antimicrobial Agents in Burn Patients. Surg. Infect. 2021, 22, 77–82. [Google Scholar] [CrossRef]
- Asín-Prieto, E.; Rodríguez-Gascón, A.; Isla, A. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J. Infect. Chemother. 2015, 21, 319–329. [Google Scholar] [CrossRef]
- Butranova, O.I.; Ushkalova, E.A.; Zyryanov, S.K.; Chenkurov, M.S. Developmental Pharmacokinetics of Antibiotics Used in Neonatal ICU: Focus on Preterm Infants. Biomedicines 2023, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Verrest, L.; Wilthagen, E.A.; Beijnen, J.H.; Huitema, A.D.R.; Dorlo, T.P.C. Influence of Malnutrition on the Pharmacokinetics of Drugs Used in the Treatment of Poverty-Related Diseases: A Systematic Review. Clin. Pharmacokinet. 2021, 60, 1149–1169. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Bunachita, S.; Agarwal, A.A.; Bhamidipati, A.; Patel, U.K. A Comprehensive Overview of Antibiotic Selection and the Factors Affecting It. Cureus 2021, 13, e13925. [Google Scholar] [CrossRef] [PubMed]
- Dumic, I.; Nordin, T.; Jecmenica, M.; Stojkovic Lalosevic, M.; Milosavljevic, T.; Milovanovic, T. Gastrointestinal Tract Disorders in Older Age. Can. J. Gastroenterol. Hepatol. 2019, 2019, 6757524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Izumi, N.; Funayama, S.; Nohno, K.; Katsura, K.; Kaneko, N.; Inoue, M. Characteristics of medication-induced xerostomia and effect of treatment. PLoS ONE 2023, 18, e0280224. [Google Scholar] [CrossRef]
- Tan, E.C.K.; Lexomboon, D.; Sandborgh-Englund, G.; Haasum, Y.; Johnell, K. Medications That Cause Dry Mouth as an Adverse Effect in Older People: A Systematic Review and Metaanalysis. J. Am. Geriatr. Soc. 2018, 66, 76–84. [Google Scholar] [CrossRef]
- Abdi, S.; Masbough, F.; Nazari, M.; Abbasinazari, M. Drug-induced esophagitis and helpful management for healthcare providers. Gastroenterol. Hepatol. Bed Bench 2022, 15, 219–224. [Google Scholar] [CrossRef]
- Hughes, J.; Lockhart, J.; Joyce, A. Do calcium antagonists contribute to gastro-oesophageal reflux disease and concomitant noncardiac chest pain? Br. J. Clin. Pharmacol. 2007, 64, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Dai, Y.; Lu, L.; Fu, Z. Dabigatran-induced esophagitis: A case report. Medicine 2020, 99, e19890. [Google Scholar] [CrossRef]
- Kono, Y.; Miyahara, K.; Nakagawa, M. A case of esophagitis induced by apixaban. J. Gastrointest. Liver Dis. 2020, 29, 471. [Google Scholar] [CrossRef]
- Nagata, K.; Akazawa, Y. Exfoliative Esophagitis Induced By Sunitinib. Mayo Clin. Proc. 2019, 94, 557–558. [Google Scholar] [CrossRef] [Green Version]
- Kooshkaki, O.; Derakhshani, A.; Hosseinkhani, N.; Torabi, M.; Safaei, S.; Brunetti, O.; Racanelli, V.; Silvestris, N.; Baradaran, B. Combination of Ipilimumab and Nivolumab in Cancers: From Clinical Practice to Ongoing Clinical Trials. Int. J. Mol. Sci. 2020, 21, 4427. [Google Scholar] [CrossRef]
- Endo, R.; Nakamura, Y.; Ishizuki, S.; Ishitsuka, Y.; Watanabe, R.; Okiyama, N.; Ito, Y.; Nagafuchi, M.; Mizokami, Y.; Fujisawa, Y. Ulcerative esophagitis associated with combined nivolumab and ipilimumab therapy. J. Dermatol. 2020, 47, e299–e300. [Google Scholar] [CrossRef]
- Nasir, U.M.; Rodgers, B.; Panchal, D.; Choi, C.; Ahmed, S.; Ahlawat, S. Ferrous Sulfate-Induced Esophageal Injury Leading to Esophagitis Dissecans Superficialis. Case Rep. Gastroenterol. 2020, 14, 172–177. [Google Scholar] [CrossRef]
- Lanas, A.; Boers, M.; Nuevo, J. Gastrointestinal events in at-risk patients starting non-steroidal anti-inflammatory drugs (NSAIDs) for rheumatic diseases: The EVIDENCE study of European routine practice. Ann. Rheum. Dis. 2015, 74, 675–681. [Google Scholar] [CrossRef]
- Helgadottir, H.; Bjornsson, E.S. Problems Associated with Deprescribing of Proton Pump Inhibitors. Int. J. Mol. Sci. 2019, 20, 5469. [Google Scholar] [CrossRef] [Green Version]
- Xun, X.; Yin, Q.; Fu, Y.; He, X.; Dong, Z. Proton Pump Inhibitors and the Risk of Community-Acquired Pneumonia: An Updated Meta-analysis. Ann. Pharmacother. 2022, 56, 524–532. [Google Scholar] [CrossRef]
- Lin, S.H.; Chang, Y.S.; Lin, T.M.; Hu, L.-F.; Hou, T.-Y.; Hsu, H.-C.; Shen, Y.-C.; Kuo, P.-I.; Chen, W.-S.; Lin, Y.-C.; et al. Proton Pump Inhibitors Increase the Risk of Autoimmune Diseases: A Nationwide Cohort Study. Front. Immunol. 2021, 12, 736036. [Google Scholar] [CrossRef]
- Ariel, H.; Cooke, J.P. Cardiovascular Risk of Proton Pump Inhibitors. Methodist Debakey Cardiovasc. J. 2019, 15, 214–219. [Google Scholar] [CrossRef]
- Novotny, M.; Klimova, B.; Valis, M. PPI Long Term Use: Risk of Neurological Adverse Events? Front. Neurol. 2019, 9, 1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briganti, S.I.; Naciu, A.M.; Tabacco, G.; Cesareo, R.; Napoli, N.; Trimboli, P.; Castellana, M.; Manfrini, S.; Palermo, A. Proton Pump Inhibitors and Fractures in Adults: A Critical Appraisal and Review of the Literature. Int. J. Endocrinol. 2021, 2021, 8902367. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Fukahori, M.; Ikemura, A.; Kubota, A.; Higashino, H.; Sakuma, S.; Yamashita, S. Effects of gastric pH on oral drug absorption: In vitro assessment using a dissolution/permeation system reflecting the gastric dissolution process. Eur. J. Pharm. Biopharm. 2016, 101, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; De Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.G.; Fitzgerald, G.F.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4586–4591. [Google Scholar] [CrossRef] [Green Version]
- Walrath, T.; Dyamenahalli, K.U.; Hulsebus, H.J.; McCullough, R.L.; Idrovo, J.-P.; Boe, D.M.; McMahan, R.H.; Kovacs, E.J. Age-related changes in intestinal immunity and the microbiome. J. Leukoc. Biol. 2021, 109, 1045–1061. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, Y.; Shen, W.; Xu, J.; Wang, L.; Chen, S.; Hou, T.; Si, J. The Aged Intestine: Performance and Rejuvenation. Aging Dis. 2021, 12, 1693–1712. [Google Scholar] [CrossRef]
- Brechmann, T.; Günther, K.; Neid, M.; Schmiegel, W.; Tannapfel, A. Triggers of histologically suspected drug-induced colitis. World J. Gastroenterol. 2019, 25, 967–979. [Google Scholar] [CrossRef]
- Herlihy, N.; Feakins, R. Gut inflammation induced by drugs: Can pathology help to differentiate from inflammatory bowel disease? United Eur. Gastroenterol. J. 2022, 10, 451–464. [Google Scholar] [CrossRef]
- Tawam, D.; Baladi, M.; Jungsuwadee, P.; Earl, G.; Han, J. The Positive Association between Proton Pump Inhibitors and Clostridium Difficile Infection. Innov. Pharm. 2021, 12, 21. [Google Scholar] [CrossRef]
- Rémond, D.; Shahar, D.R.; Gille, D.; Pinto, P.; Kachal, J.; Peyron, M.-A.; dos Santos, C.N.; Walther, B.; Bordoni, A.; Dupont, D.; et al. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 2015, 6, 13858–13898. [Google Scholar] [CrossRef] [Green Version]
- Löhr, J.M.; Panic, N.; Vujasinovic, M.; Verbeke, C.S. The ageing pancreas: A systematic review of the evidence and analysis of the consequences. J. Intern. Med. 2018, 283, 446–460. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, D.; Kanji, S.; Yazdi, F.; Barbeau, P.; Rice, D.; Beck, A.; Butler, C.; Esmaeilisaraji, L.; Skidmore, B.; Moher, D.; et al. Drug induced pancreatitis: A systematic review of case reports to determine potential drug associations. PLoS ONE 2020, 15, e0231883. [Google Scholar] [CrossRef] [Green Version]
- Olesen, A.E.; Brokjaer, A.; Fisher, I.W.; Larsen, I.M. Pharmacological challenges in chronic pancreatitis. World J. Gastroenterol. 2013, 19, 7302–7307. [Google Scholar] [CrossRef]
- Kim, I.H.; Kisseleva, T.; Brenner, D.A. Aging and liver disease. Curr. Opin. Gastroenterol. 2015, 31, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Björnsson, E.S.; Stephens, C.; Atallah, E.; Robles-Diaz, M.; Alvarez-Alvarez, I.; Gerbes, A.; Weber, S.; Stirnimann, G.; Kullak-Ublick, G.; Cortez-Pinto, H.; et al. A new framework for advancing in drug-induced liver injury research. The Prospective European DILI Registry. Liver Int. 2023, 43, 115–126. [Google Scholar] [CrossRef]
- Drenth-van Maanen, A.C.; Wilting, I.; Jansen, P.A.F. Prescribing medicines to older people-How to consider the impact of ageing on human organ and body functions. Br. J. Clin. Pharmacol. 2020, 86, 1921–1930. [Google Scholar] [CrossRef] [Green Version]
- Jubeh, B.; Breijyeh, Z.; Karaman, R. Antibacterial Prodrugs to Overcome Bacterial Resistance. Molecules 2020, 25, 1543. [Google Scholar] [CrossRef] [Green Version]
- Evans, L.E.; Krishna, A.; Ma, Y.; Webb, T.E.; Marshall, D.C.; Tooke, C.L.; Spencer, J.; Clarke, T.B.; Armstrong, A.; Edwards, A.M. Exploitation of antibiotic resistance as a novel drug target: Development of a β-lactamase-activated antibacterial prodrug. J. Med. Chem. 2019, 62, 4411–4425. [Google Scholar] [CrossRef] [Green Version]
- Sousa, P.; Bertani, L.; Rodrigues, C. Management of inflammatory bowel disease in the elderly: A review. Dig. Liver Dis. 2023. advance online publication. [Google Scholar] [CrossRef]
- Alrubia, S.; Mao, J.; Chen, Y.; Barber, J.; Rostami-Hodjegan, A. Altered Bioavailability and Pharmacokinetics in Crohn’s Disease: Capturing Systems Parameters for PBPK to Assist with Predicting the Fate of Orally Administered Drugs. Clin. Pharmacokinet. 2022, 61, 1365–1392. [Google Scholar] [CrossRef]
- Rattanacheeworn, P.; Kerr, S.J.; Kittanamongkolchai, W.; Townamchai, N.; Udomkarnjananun, S.; Praditpornsilpa, K.; Thanusuwannasak, T.; Udomnilobol, U.; Jianmongkol, S.; Ongpipattanakul, B.; et al. Quantification of CYP3A and Drug Transporters Activity in Healthy Young, Healthy Elderly and Chronic Kidney Disease Elderly Patients by a Microdose Cocktail Approach. Front. Pharmacol. 2021, 12, 726669. [Google Scholar] [CrossRef] [PubMed]
- Riches, Z.; Abanda, N.; Collier, A.C. BCRP protein levels do not differ regionally in adult human livers, but decline in the elderly. Chem. Biol. Interact. 2015, 242, 203–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viennois, E.; Pujada, A.; Zen, J.; Merlin, D. Function, Regulation, and Pathophysiological Relevance of the POT Superfamily, Specifically PepT1 in Inflammatory Bowel Disease. Compr. Physiol. 2018, 8, 731–760. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chu, C.; Ji, X.; Luo, G.; Xu, C.; He, H.; Yao, J.; Wu, J.; Hu, J.; Jin, Y. Biology of Peptide Transporter 2 in Mammals: New Insights into Its Function, Structure and Regulation. Cells 2022, 11, 2874. [Google Scholar] [CrossRef] [PubMed]
- Dücker, C.M.; Brockmöller, J. Genomic Variation and Pharmacokinetics in Old Age: A Quantitative Review of Age- vs. Genotype-Related Differences. Clin. Pharmacol. Ther. 2019, 105, 625–640. [Google Scholar] [CrossRef] [Green Version]
- Prasad, B.; Evers, R.; Gupta, A.; Hop, C.E.C.A.; Salphati, L.; Shukla, S.; Ambudkar, S.V.; Unadkat, J.D. Interindividual variability in hepatic organic anion-transporting polypeptides and P-glycoprotein (ABCB1) protein expression: Quantification by liquid chromatography tandem mass spectroscopy and influence of genotype, age, and sex. Drug Metab. Dispos. 2014, 42, 78–88. [Google Scholar] [CrossRef]
- Hou, W.Y.; Xu, S.F.; Zhu, Q.N.; Lu, Y.F.; Cheng, X.G.; Liu, J. Age- and sex-related differences of organic anion-transporting polypeptide gene expression in livers of rats. Toxicol. Appl. Pharmacol. 2014, 280, 370–377. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Saupe, K.W.; Klaassen, C.D. Energy restriction does not compensate for the reduced expression of hepatic drug-processing genes in mice with aging. Drug Metab. Dispos. 2010, 38, 1122–1131. [Google Scholar] [CrossRef] [Green Version]
- Rosati, A.; Maniori, S.; Decorti, G.; Candussio, L.; Giraldi, T.; Bartoli, F. Physiological regulation of P-glycoprotein, MRP1, MRP2 and cytochrome P450 3A2 during rat ontogeny. Dev. Growth Differ. 2003, 45, 377–387. [Google Scholar] [CrossRef]
- Qian, X.; Cheng, Y.H.; Mruk, D.D.; Cheng, C.Y. Breast cancer resistance protein (Bcrp) and the testis—An unexpected turn of events. Asian J. Androl. 2013, 15, 455–460. [Google Scholar] [CrossRef] [Green Version]
- DrugBank Online. BCRP/ABCG2 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002663 (accessed on 10 April 2023).
- Brenner, S.S.; Klotz, U. P-glycoprotein function in the elderly. Eur. J. Clin. Pharmacol. 2004, 60, 97–102. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Aleksunes, L.M. Xenobiotic, bile acid, and cholesterol transporters: Function and regulation. Pharmacol. Rev. 2010, 62, 1–96. [Google Scholar] [CrossRef] [Green Version]
- Sugie, M.; Asakura, E.; Zhao, Y.L.; Torita, S.; Nadai, M.; Baba, K.; Kitaichi, K.; Takagi, K.; Takagi, K.; Hasegawa, T. Possible involvement of the drug transporters P glycoprotein and multidrug resistance-associated protein Mrp2 in disposition of azithromycin. Antimicrob. Agents Chemother. 2004, 48, 809–814. [Google Scholar] [CrossRef] [Green Version]
- Sakaeda, T.; Nakamura, T.; Okumura, K. MDR1 genotype-related pharmacokinetics and pharmacodynamics. Biol. Pharm. Bull. 2002, 25, 1391–1400. [Google Scholar] [CrossRef] [Green Version]
- Putnam, W.S.; Woo, J.M.; Huang, Y.; Benet, L.Z. Effect of the MDR1 C3435T variant and P-glycoprotein induction on dicloxacillin pharmacokinetics. J. Clin. Pharmacol. 2005, 45, 411–421. [Google Scholar] [CrossRef]
- Stage, T.B.; Graff, M.; Wong, S.; Rasmussen, L.L.; Nielsen, F.; Pottegård, A.; Brøsen, K.; Kroetz, D.L.; Khojasteh, S.C.; Damkier, P. Dicloxacillin induces CYP2C19, CYP2C9 and CYP3A4 in vivo and in vitro. Br. J. Clin. Pharmacol. 2018, 84, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Human Transporters MRP2. Available online: https://www.solvobiotech.com/transporters/mrp2 (accessed on 10 April 2023).
- Maeda, T.; Takahashi, K.; Ohtsu, N.; Oguma, T.; Ohnishi, T.; Atsumi, R.; Tamai, I. Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol. Pharm. 2007, 4, 85–94. [Google Scholar] [CrossRef]
- Franke, R.M.; Baker, S.D.; Mathijssen, R.H.; Schuetz, E.G.; Sparreboom, A. Influence of solute carriers on the pharmacokinetics of CYP3A4 probes. Clin. Pharmacol. Ther. 2008, 84, 704–709. [Google Scholar] [CrossRef]
- Mahalingam, A.; Shenoy, B. Tebipenem: A Novel Oral Carbapenem. Pediatr. Infect. Dis. 2020, 2, 25–28. [Google Scholar] [CrossRef]
- Badée, J.; Achour, B.; Rostami-Hodjegan, A.; Galetin, A. Meta-analysis of expression of hepatic organic anion-transporting polypeptide (OATP) transporters in cellular systems relative to human liver tissue. Drug Metab. Dispos. Biol. Fate Chem. 2015, 43, 424–432. [Google Scholar] [CrossRef] [Green Version]
- Nakakariya, M.; Shimada, T.; Irokawa, M.; Maeda, T.; Tamai, I. Identification and species similarity of OATP transporters responsible for hepatic uptake of beta-lactam antibiotics. Drug Metab. Pharmacokinet. 2008, 23, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I.; Nezu, J.; Uchino, H.; Sai, Y.; Oku, A.; Shimane, M.; Tsuji, A. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem. Biophys. Res. Commun. 2000, 273, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Shirasaka, Y.; Kuraoka, E.; Kikuchi, A.; Iguchi, M.; Suzuki, H.; Shibasaki, S.; Kurosawa, T.; Tamai, I. Intestinal absorption mechanism of tebipenem pivoxil, a novel oral carbapenem: Involvement of human OATP family in apical membrane transport. Mol. Pharm. 2010, 7, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, O.A.; King, N.; Andronicos, N.M.; Jones, G.L.; Chami, B.; Witting, P.K.; Moens, P.D.J. Molecular changes to the rat renal cotransporters PEPT1 and PEPT2 due to ageing. Mol. Cell. Biochem. 2019, 452, 71–82. [Google Scholar] [CrossRef]
- Lu, X.; Chan, T.; Xu, C.; Zhu, L.; Zhou, Q.T.; Roberts, K.D.; Chan, H.K.; Li, J.; Zhou, F. Human oligopeptide transporter 2 (PEPT2) mediates cellular uptake of polymyxins. J. Antimicrob. Chemother. 2016, 71, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Shekhawat, P.B.; Pokharkar, V.B. Understanding peroral absorption: Regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles. Acta Pharm. Sin. B 2017, 7, 260–280. [Google Scholar] [CrossRef] [Green Version]
- Rangaraj, N.; Sampathi, S.; Junnuthula, V.; Kolimi, P.; Mandati, P.; Narala, S.; Nyavanandi, D.; Dyawanapelly, S. Fast-Fed Variability: Insights into Drug Delivery, Molecular Manifestations, and Regulatory Aspects. Pharmaceutics 2022, 14, 1807. [Google Scholar] [CrossRef]
- Genser, D. Food and drug interaction: Consequences for the nutrition/health status. Ann. Nutr. Metab. 2008, 52 (Suppl. S1), 29–32. [Google Scholar] [CrossRef]
- Thambavita, D.D.; Galappatthy, P.; Jayakody, R.L. Pharmacokinetics and Bioequivalence of Two Amoxicillin 500 mg Products: Effect of Food on Absorption and Supporting Scientific Justification for Biowaiver. J. Pharm. Sci. 2021, 110, 3735–3741. [Google Scholar] [CrossRef]
- Weitschies, W.; Friedrich, C.; Wedemeyer, R.S.; Schmidtmann, M.; Kosch, O.; Kinzig, M.; Trahms, L.; Sörgel, F.; Siegmund, W.; Horkovics-Kovats, S.; et al. Bioavailability of amoxicillin and clavulanic acid from extended release tablets depends on intragastric tablet deposition and gastric emptying. Eur. J. Pharm. Biopharm. 2008, 70, 641–648. [Google Scholar] [CrossRef]
- Gardiner, S.J.; Drennan, P.G.; Begg, R.; Zhang, M.; Green, J.K.; Isenman, H.L.; Everts, R.J.; Chambers, S.T.; Begg, E.J. In healthy volunteers, taking flucloxacillin with food does not compromise effective plasma concentrations in most circumstances. PLoS ONE 2018, 13, e0199370. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Deng, Q.; Li, Y.; Li, P.; Liu, G.; Wang, Y.; Liu, Z.; Yu, S.; Cheng, Y.; Zhou, Y.; et al. Pharmacokinetics and safety of the two oral cefaclor formulations in healthy chinese subjects in the fasting and postprandial states. Front. Pharmacol. 2022, 13, 1012294. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Omata, H.; Nishimura, H.; Tanaka, S.; Matsumoto, H.; Fujii, A.; Akimoto, Y.; Komiya, M. Cefpodoxime Concentrations in Human Serum and Oral Tissues Following a Single Oral Administration of Cefpodoxime Proxetil. Int. J. Oral-Med. Sci. 2015, 14, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Borin, M.T.; Forbes, K.K. Effect of food on absorption of cefpodoxime proxetil oral suspension in adults. Antimicrob. Agents Chemother. 1995, 39, 273–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borin, M.T.; Ferry, J.J.; Forbes, K.K.; Hughes, G.S. Pharmacokinetics of cefpodoxime proxetil in healthy young and elderly volunteers. J. Clin. Pharmacol. 1994, 34, 774–781. [Google Scholar] [CrossRef]
- Curatolo, W.; Foulds, G.; Labadie, R. Mechanistic study of the azithromycin dosage-form-dependent food effect. Pharm. Res. 2010, 27, 1361–1366. [Google Scholar] [CrossRef]
- Kshirsagar, A.S.; Patil, V.M.; Patil, S. A Study on Effect of Food on Pharmacokinetics of Clindamycin: A Research. Int. J. Sci. Res. 2022, 11, 304–307. [Google Scholar] [CrossRef]
- Stalker, D.J.; Jungbluth, G.L. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin. Pharmacokinet. 2003, 42, 1129–1140. [Google Scholar] [CrossRef]
- Imaoka, A.; Abiru, K.; Akiyoshi, T.; Ohtani, H. Food intake attenuates the drug interaction between new quinolones and aluminum. J. Pharm. Health Care Sci. 2018, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Williams, N.T. Medication administration through enteral feeding tubes. Am. J. Health Syst. Pharm. 2008, 65, 2347–2357. [Google Scholar] [CrossRef]
- Lee, L.J.; Hafkin, B.; Lee, I.D.; Hoh, J.; Dix, R. Effects of food and sucralfate on a single oral dose of 500 milligrams of levofloxacin in healthy subjects. Antimicrob. Agents Chemother. 1997, 41, 2196–2200. [Google Scholar] [CrossRef] [Green Version]
- Amsden, G.W.; Whitaker, A.M.; Johnson, P.W. Lack of bioequivalence of levofloxacin when coadministered with a mineral-fortified breakfast of juice and cereal. J. Clin. Pharmacol. 2003, 43, 990–995. [Google Scholar] [CrossRef]
- Taubel, J.; Ferber, G.; Lorch, U.; Batchvarov, V.; Savelieva, I.; Camm, A.J. Pharmacokinetics of 400 mg oral moxifloxacin in the fed and fasted state in TQT studies. Br. J. Clin. Pharmacol. 2014, 77, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; Matzneller, P.; Gautam, A.; Österreicher, Z.; Wulkersdorfer, B.; Reiter, B.; Stimpfl, T.; Zeitlinger, M. Target site pharmacokinetics of doxycycline for rosacea in healthy volunteers is independent of the food effect. Br. J. Clin. Pharmacol. 2018, 84, 2625–2633. [Google Scholar] [CrossRef] [Green Version]
- McCormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: Changes to life-style as a therapy. Biogerontology 2018, 19, 519–536. [Google Scholar] [CrossRef] [Green Version]
- Mangoni, A.A.; Jarmuzewska, E.A. The influence of heart failure on the pharmacokinetics of cardiovascular and non-cardiovascular drugs: A critical appraisal of the evidence. Br. J. Clin. Pharmacol. 2019, 85, 20–36. [Google Scholar] [CrossRef]
- Kaestli, L.Z.; Wasilewski-Rasca, A.F.; Bonnabry, P.; Vogt-Ferrier, N. Use of transdermal drug formulations in the elderly. Drugs Aging 2008, 25, 269–280. [Google Scholar] [CrossRef]
- Cho, S.J.; Stout-Delgado, H.W. Aging and Lung Disease. Annu. Rev. Physiol. 2020, 82, 433–459. [Google Scholar] [CrossRef] [Green Version]
- Wallin, M.; Tagami, T.; Chen, L.; Yang, M.; Chan, H.K. Pulmonary drug delivery to older people. Adv. Drug Deliv. Rev. 2018, 135, 50–61. [Google Scholar] [CrossRef]
- Singam, N.S.V.; Fine, C.; Fleg, J.L. Cardiac changes associated with vascular aging. Clin. Cardiol. 2020, 43, 92–98. [Google Scholar] [CrossRef]
- Falcone, M.; Paul, M.; Tiseo, G.; Yahav, D.; Prendki, V.; Friberg, L.E.; Guerri, R.; Gavazzi, G.; Mussini, C.; Tinelli, M.; et al. Considerations for the optimal management of antibiotic therapy in elderly patients. J. Glob. Antimicrob. Resist. 2020, 22, 325–333. [Google Scholar] [CrossRef]
- Wicha, S.G.; Märtson, A.G.; Nielsen, E.I.; Koch, B.C.; Friberg, L.E.; Alffenaar, J.; Minichmayr, I.K. From Therapeutic Drug Monitoring to Model-Informed Precision Dosing for Antibiotics. Clin. Pharmacol. Ther. 2021, 109, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Ponti, F.; Santoro, A.; Mercatelli, D.; Gasperini, C.; Conte, M.; Martucci, M.; Sangiorgi, L.; Franceschi, C.; Bazzocchi, A. Aging and Imaging Assessment of Body Composition: From Fat to Facts. Front. Endocrinol. 2020, 10, 861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra-Prat, M.; Lorenzo, I.; Palomera, E.; Ramírez, S.; Yébenes, J.C. Total Body Water and Intracellular Water Relationships with Muscle Strength, Frailty and Functional Performance in an Elderly Population. A Cross-Sectional Study. J. Nutr. Health Aging 2019, 23, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Smit, C.; De Hoogd, S.; Brüggemann, R.J.M.; Knibbe, C.A.J. Obesity and drug pharmacology: A review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert Opin. Drug Metab. Toxicol. 2018, 14, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Roberts, J.A.; Lipman, J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 2013, 39, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Hurkacz, M.; Dobrek, L.; Wiela-Hojeńska, A. Antibiotics and the Nervous System-Which Face of Antibiotic Therapy Is Real, Dr. Jekyll (Neurotoxicity) or Mr. Hyde (Neuroprotection)? Molecules 2021, 26, 7456. [Google Scholar] [CrossRef]
- Smith, S.A.; Waters, N.J. Pharmacokinetic and Pharmacodynamic Considerations for Drugs Binding to Alpha-1-Acid Glycoprotein. Pharm. Res. 2018, 36, 30. [Google Scholar] [CrossRef]
- Celestin, M.N.; Musteata, F.M. Impact of Changes in Free Concentrations and Drug-Protein Binding on Drug Dosing Regimens in Special Populations and Disease States. J. Pharm. Sci. 2021, 110, 3331–3344. [Google Scholar] [CrossRef]
- Brock, F.; Bettinelli, L.A.; Dobner, T.; Stobbe, J.C.; Pomatti, G.; Telles, C.T. Prevalence of hypoalbuminemia and nutritional issues in hospitalized elders. Rev. Lat. Am. Enfermagem. 2016, 24, e2736. [Google Scholar] [CrossRef] [Green Version]
- Moramarco, S.; Morciano, L.; Morucci, L.; Messinese, M.; Gualtieri, P.; Carestia, M.; Ciccacci, F.; Orlando, S.; Buonomo, E.; Legramante, J.M.; et al. Epidemiology of Hypoalbuminemia in Hospitalized Patients: A Clinical Matter or an Emerging Public Health Problem? Nutrients 2020, 12, 3656. [Google Scholar] [CrossRef]
- Frith, E.; Loprinzi, P.D. Physical Activity and Cognitive Function among Older Adults with an Elevated Gamma Gap. Med. Princ. Pract. 2018, 27, 531–536. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Roberts, J.A.; Rello, J.; Paterson, D.L.; Lipman, J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin. Pharmacokinet. 2011, 50, 99–110. [Google Scholar] [CrossRef]
- Pereira, J.G.; Fernandes, J.; Duarte, A.R.; Fernandes, S.M. β-Lactam Dosing in Critical Patients: A Narrative Review of Optimal Efficacy and the Prevention of Resistance and Toxicity. Antibiotics 2022, 11, 1839. [Google Scholar] [CrossRef]
- Mizuno, T.; Mizokami, F.; Fukami, K.; Ito, K.; Shibasaki, M.; Nagamatsu, T.; Furuta, K. The influence of severe hypoalbuminemia on the half-life of vancomycin in elderly patients with methicillin-resistant Staphylococcus aureus hospital-acquired pneumonia. Clin. Interv. Aging 2013, 8, 1323–1328. [Google Scholar] [CrossRef] [Green Version]
- Zusman, O.; Farbman, L.; Tredler, Z.; Daitch, V.; Lador, A.; Leibovici, L.; Paul, M. Association between hypoalbuminemia and mortality among subjects treated with ertapenem versus other carbapenems: Prospective cohort study. Clin. Microbiol. Infect. 2014, 21, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Heffernan, A.J.; Sime, F.B.; Kumta, N.; Wallis, S.C.; McWhinney, B.; Ungerer, J.; Wong, G.; Joynt, G.M.; Lipman, J.; Roberts, J.A. Multicenter Population Pharmacokinetic Study of Unbound Ceftriaxone in Critically Ill Patients. Antimicrob. Agents Chemother. 2022, 66, e0218921. [Google Scholar] [CrossRef]
- Baalbaki, N.; Blum, S.; Akerman, M.; Johnson, D. Ceftriaxone 1 g Versus 2 g Daily for the Treatment of Enterobacterales Bacteremia: A Retrospective Cohort Study. J. Pharm. Technol. 2022, 38, 326–334. [Google Scholar] [CrossRef]
- Allegaert, K.; Muller, A.E.; Russo, F.; Schoenmakers, S.; Deprest, J.; Koch, B.C.P. Pregnancy-related pharmacokinetics and antimicrobial prophylaxis during fetal surgery, cefazolin and clindamycin as examples. Prenat. Diagn. 2020, 40, 1178–1184. [Google Scholar] [CrossRef]
- Tucker, L.A.; Parker, K. 10-Year Weight Gain in 13,802 US Adults: The Role of Age, Sex, and Race. J. Obes. 2022, 2022, 7652408. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, T.; Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of obesity among older adults in the United States, 2007–2010. Natl. Center Health Stat. Data Brief. 2012, 106, 1–8. [Google Scholar]
- Meng, L.; Mui, E.; Holubar, M.K.; Deresinski, S.C. Comprehensive Guidance for Antibiotic Dosing in Obese Adults. Pharmacotherapy 2017, 37, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.E.; Lockwood, A.M.; Nguyen, N.H. Antibiotic dosing in obesity: The search for optimum dosing strategies. Clin. Obes. 2014, 4, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.; Shield, A.; Peterson, G.M.; Hussain, Z. Prophylactic Cefazolin Dosing in Obesity-a Systematic Review. Obes. Surg. 2022, 32, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Janson, B.; Thursky, K. Dosing of antibiotics in obesity. Curr. Opin. Infect. Dis. 2012, 25, 634–649. [Google Scholar] [CrossRef] [Green Version]
- Donini, L.M.; Stephan, B.C.M.; Rosano, A.; Molfino, A.; Poggiogalle, E.; Lenzi, A.; Siervo, M.; Muscaritoli, M. What Are the Risk Factors for Malnutrition in Older-Aged Institutionalized Adults? Nutrients 2020, 12, 2857. [Google Scholar] [CrossRef]
- Alvis, B.D.; Hughes, C.G. Physiology Considerations in Geriatric Patients. Anesthesiol. Clin. 2015, 33, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.L.; Eastment, J.G.; Poudel, A.; Hubbard, R.E. Age-Related Changes in Hepatic Function: An Update on Implications for Drug Therapy. Drugs Aging 2015, 32, 999–1008. [Google Scholar] [CrossRef]
- Kaburaki, S.; Yoshimura, E.; Miyamoto, Y.; Imai, S.; Kashiwagi, H.; Ueno, H.; Sugawara, M.; Takekuma, Y. Hepatic drug metabolism in older people with body composition changes. Geriatr. Gerontol. Int. 2022, 22, 449–454. [Google Scholar] [CrossRef]
- Drozdzik, M.; Lapczuk-Romanska, J.; Wenzel, C.; Szelag-Pieniek, S.; Post, M.; Skalski, Ł.; Kurzawski, M.; Oswald, S. Gene Expression and Protein Abundance of Hepatic Drug Metabolizing Enzymes in Liver Pathology. Pharmaceutics 2021, 13, 1334. [Google Scholar] [CrossRef]
- Trobec, K.; Kerec Kos, M.; von Haehling, S.; Springer, J.; Anker, S.D.; Lainscak, M. Pharmacokinetics of drugs in cachectic patients: A systematic review. PLoS ONE 2013, 8, e79603. [Google Scholar] [CrossRef] [Green Version]
- Obach, R.S. Linezolid Metabolism Is Catalyzed by Cytochrome P450 2J2, 4F2, and 1B1. Drug Metab. Dispos. 2022, 50, 413–421. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P450 1A1. Available online: https://go.drugbank.com/polypeptides/P04798 (accessed on 13 April 2023).
- Lu, J.; Shang, X.; Zhong, W.; Xu, Y.; Shi, R.; Wang, X. New insights of CYP1A in endogenous metabolism: A focus on single nucleotide polymorphisms and diseases. Acta Pharm. Sin. B 2020, 10, 91–104. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P-450 CYP1A2 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002609 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP1A2. Available online: Inhibitorshttps://go.drugbank.com/categories/DBCAT000402 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP1A2 Inducers. Available online: https://go.drugbank.com/categories/DBCAT000614 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2A6 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002613 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2A6 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT002614 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2A6 Inducers. Available online: https://go.drugbank.com/categories/DBCAT002617 (accessed on 13 April 2023).
- Tanner, J.A.; Prasad, B.; Claw, K.G.; Stapleton, P.; Chaudhry, A.; Schuetz, E.G.; Thummel, K.E.; Tyndale, R.F. Predictors of Variation in CYP2A6 mRNA, Protein, and Enzyme Activity in a Human Liver Bank: Influence of Genetic and Nongenetic Factors. J. Pharmacol. Exp. Ther. 2017, 360, 129–139. [Google Scholar] [CrossRef]
- Li, F.; Zhu, W.; Gonzalez, F.J. Potential role of CYP1B1 in the development and treatment of metabolic diseases. Pharmacol. Ther. 2017, 178, 18–30. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P-450 CYP2B6 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT001285 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2B6 Inducers. Available online: https://go.drugbank.com/categories/DBCAT001015 (accessed on 13 April 2023).
- Torgersen, J.; Bellamy, S.L.; Ratshaa, B.; Han, X.; Mosepele, M.; Zuppa, A.F.; Vujkovic, M.; Steenhoff, A.P.; Bisson, G.P.; Gross, R. Impact of Efavirenz Metabolism on Loss to Care in Older HIV+ Africans. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 179–187. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P-450 CYP2C8 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002642 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2C8 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT000868 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2C8 Inducers. Available online: https://go.drugbank.com/categories/DBCAT001247 (accessed on 13 April 2023).
- Liu, W.; Wang, B.; Ding, H.; Wang, D.W.; Zeng, H. A potential therapeutic effect of CYP2C8 overexpression on anti-TNF-α activity. Int. J. Mol. Med. 2014, 34, 725–732. [Google Scholar] [CrossRef] [Green Version]
- DrugBank Online. Cytochrome P-450 CYP2C9 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002634 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P450 2C9. Available online: https://go.drugbank.com/polypeptides/P11712 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2C19 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT000403 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2C19 Inducers. Available online: https://go.drugbank.com/categories/DBCAT001246 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2D6 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002623 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2D6 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT000911 (accessed on 13 April 2023).
- Waade, R.B.; Hermann, M.; Moe, H.L.; Molden, E. Impact of age on serum concentrations of venlafaxine and escitalopram in different CYP2D6 and CYP2C19 genotype subgroups. Eur. J. Clin. Pharmacol. 2014, 70, 933–940. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P-450 CYP2E1 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002628 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2E1 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT002629 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2E1 Inducers. Available online: https://go.drugbank.com/categories/DBCAT002632 (accessed on 13 April 2023).
- DrugBank Online. Erythromycin. Available online: https://go.drugbank.com/drugs/DB00199 (accessed on 13 April 2023).
- Wynalda, M.A.; Hutzler, J.M.; Koets, M.D.; Podoll, T.; Wienkers, L.C. In Vitro Metabolism Of Clindamycin in Human Liver and Intestinal Microsomes. Drug Metab. Dispos. 2003, 31, 878–887. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P-450 CYP3A4 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002646 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP3A4 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT003232 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP3A4 Inducers. Available online: https://go.drugbank.com/categories/DBCAT003896 (accessed on 13 April 2023).
- Álvarez, L.A.; Van de Sijpe, G.; Desmet, S.; Metsemakers, W.-J.; Spriet, I.; Allegaert, K.; Rozenski, J. Ways to Improve Insights into Clindamycin Pharmacology and Pharmacokinetics Tailored to Practice. Antibiotics 2022, 11, 701. [Google Scholar] [CrossRef] [PubMed]
- DrugBank Online. Cytochrome P-450 CYP3A5 Substrates. Available online: https://go.drugbank.com/categories/DBCAT003807 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP3A5 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT003893 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP3A5 Inducers. Available online: https://go.drugbank.com/categories/DBCAT004489 (accessed on 13 April 2023).
- Williams, J.A.; Ring, B.J.; Cantrell, V.E.; Jones, D.R.; Eckstein, J.; Ruterbories, K.; Hamman, M.A.; Hall, S.D.; Wrighton, S.A. Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab. Dispos. 2002, 30, 883–891. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P-450 CYP3A7 Substrates. Available online: https://go.drugbank.com/categories/DBCAT003800 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP3A7 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT003892 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP3A7 Inducers. Available online: https://go.drugbank.com/categories/DBCAT004499 (accessed on 13 April 2023).
- Sim, S.C.; Edwards, R.J.; Boobis, A.R.; Ingelman-Sundberg, M. CYP3A7 protein expression is high in a fraction of adult human livers and partially associated with the CYP3A7*1C allele. Pharm. Genom. 2005, 15, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, A.S.; Kruer, R.M.; Johnson, D.; Lipsett, P.A. Antimicrobial Pharmacokinetics and Pharmacodynamics. Surg. Infect. 2015, 16, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Karam, Z.; Tuazon, J. Anatomic and physiologic changes of the aging kidney. Clin. Geriatr. Med. 2013, 29, 555–564. [Google Scholar] [CrossRef]
- Lerma, E.V. Anatomic and physiologic changes of the aging kidney. Clin. Geriatr. Med. 2009, 25, 325–329. [Google Scholar] [CrossRef]
- Fang, Y.; Gong, A.Y.; Haller, S.T.; Dworkin, L.D.; Liu, Z.; Gong, R. The ageing kidney: Molecular mechanisms and clinical implications. Ageing Res. Rev. 2020, 63, 101151. [Google Scholar] [CrossRef]
- Kampmann, J.D.; Heaf, J.G.; Mogensen, C.B.; Mickley, H.; Wolff, D.L.; Brandt, F. Prevalence and incidence of chronic kidney disease stage 3–5—Results from KidDiCo. BMC Nephrol. 2023, 24, 17. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. The aging kidney and the nephrotoxic effects of mercury. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 55–80. [Google Scholar] [CrossRef]
- Benson, J.M. Antimicrobial Pharmacokinetics and Pharmacodynamics in Older Adults. Infect. Dis. Clin. N. Am. 2017, 31, 609–617. [Google Scholar] [CrossRef]
- Otobe, Y.; Rhee, C.M.; Nguyen, M.; Kalantar-Zadeh, K.; Kopple, J.D. Current status of the assessment of sarcopenia, frailty, physical performance and functional status in chronic kidney disease patients. Curr. Opin. Nephrol. Hypertens. 2022, 31, 109–128. [Google Scholar] [CrossRef]
- Chinzowu, T.; Chyou, T.Y.; Nishtala, P.S. Antibacterial-associated acute kidney injury among older adults: A post-marketing surveillance study using the FDA adverse events reporting system. Pharmacoepidemiol. Drug Saf. 2022, 31, 1190–1198. [Google Scholar] [CrossRef]
- Liu, J.; Tong, S.Y.C.; Davis, J.S.; Rhodes, N.J.; Scheetz, M.H.; CAMERA2 Study Group. Vancomycin Exposure and Acute Kidney Injury Outcome: A Snapshot From the CAMERA2 Study. Open Forum Infect. Dis. 2020, 7, ofaa538. [Google Scholar] [CrossRef]
- Robertson, A.D.; Li, C.; Hammond, D.A.; Dickey, T.A. Incidence of Acute Kidney Injury among Patients Receiving the Combination of Vancomycin with Piperacillin-Tazobactam or Meropenem. Pharmacotherapy 2018, 38, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, B.; Davies, M.R.P.; Trubiano, J.A.; Pellicano, R. Time to Acute Kidney Injury in β-Lactam-Induced Acute Interstitial Nephritis. Kidney Int. Rep. 2020, 5, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Crochette, R.; Ravaiau, C.; Perez, L.; Coindre, J.P.; Piccoli, G.B.; Blanchi, S. Incidence and Risk Factors for Acute Kidney Injury during the Treatment of Methicillin-Sensitive Staphylococcus aureus Infections with Cloxacillin Based Antibiotic Regimens: A French Retrospective Study. J. Clin. Med. 2021, 10, 2603. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Chan-Tompkins, N.H.; Como, J.; Guarascio, A.J. Retrospective Analysis of Adverse Drug Events between Nafcillin Versus Cefazolin for Treatment of Methicillin-Susceptible Staphylococcus aureus Infections. Ann. Pharmacother. 2020, 54, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Bairami, S.; Kargar, M. Antibiotics induced acute kidney injury: Incidence, risk factors, onset time and outcome. Acta Med. Iran. 2013, 51, 871–878. [Google Scholar]
- Bausch, S.; Araschmid, L.J.; Hardmeier, M.; Osthoff, M. Cefepime-Induced Neurotoxicity in the Setting of Acute Kidney Injury: A Case Series and Discussion of Preventive Measures. Cureus 2022, 14, e26392. [Google Scholar] [CrossRef]
- Billups, K.B.; Reed, E.E.; Phillips, G.S.; Stevenson, K.B.; Steinberg, S.M.; Murphy, C.V. Risk of acute kidney injury in critically ill surgical patients with presumed pneumonia is not impacted by choice of methicillin-resistant staphylococcus aureus therapy. Int. J. Crit. Illn. Inj. Sci. 2018, 8, 22–27. [Google Scholar] [CrossRef]
- Perazella, M.A. Drug-induced acute kidney injury: Diverse mechanisms of tubular injury. Curr. Opin. Crit. Care 2019, 25, 550–557. [Google Scholar] [CrossRef]
- Kan, W.C.; Chen, Y.C.; Wu, V.C.; Shiao, C.C. Vancomycin-Associated Acute Kidney Injury: A Narrative Review from Pathophysiology to Clinical Application. Int. J. Mol. Sci. 2022, 23, 2052. [Google Scholar] [CrossRef]
- Ergün, B.; Esenkaya, F.; Küçük, M.; Yakar, M.N.; Uzun, Ö.; Heybeli, C.; Hanci, V.; Ergan, B.; Cömert, B.; Gökmen, A.N. Amikacin-induced acute kidney injury in mechanically ventilated critically ill patients with sepsis. J. Chemother. 2022, 1–9. [Google Scholar] [CrossRef]
- Russell, W.; Smith, W. Clarithromycin-induced acute interstitial nephritis and minimal change disease. NDT Plus 2009, 2, 382–383. [Google Scholar] [CrossRef] [Green Version]
- Mishima, E.; Maruyama, K.; Nakazawa, T.; Abe, T.; Ito, S. Acute Kidney Injury from Excessive Potentiation of Calcium-channel Blocker via Synergistic CYP3A4 Inhibition by Clarithromycin Plus Voriconazole. Intern. Med. 2017, 56, 1687–1690. [Google Scholar] [CrossRef] [Green Version]
- Nolin, T.D.; Himmelfarb, J. Mechanisms of drug-induced nephrotoxicity. Handb. Exp. Pharmacol. 2010, 196, 111–130. [Google Scholar] [CrossRef]
- Hajji, M.; Jebali, H.; Mrad, A.; Blel, Y.; Brahmi, N.; Kheder, R.; Beji, S.; Ben Fatma, L.; Smaoui, W.; Krid, M.; et al. Nephrotoxicity of Ciprofloxacin: Five Cases and a Review of the Literature. Drug Saf. Case Rep. 2018, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Ansari, F.A.; Manuel, S.; Dwivedi, R.; Boraiah, S.K.; Raju, S.B.; Uppin, M.; Sharma, A. A Rare Case of Acute Kidney Injury Due to Levofloxacin-induced Crystal Nephropathy. Indian J. Nephrol. 2019, 29, 424–426. [Google Scholar] [CrossRef]
- Shimizu, Y.; Hirai, T.; Ogawa, Y.; Yamada, C.; Kobayashi, E. Characteristics of risk factors for acute kidney injury among inpatients administered sulfamethoxazole/trimethoprim: A retrospective observational study. J. Pharm. Health Care Sci. 2022, 8, 20. [Google Scholar] [CrossRef]
- Fraser, T.N.; Avellaneda, A.A.; Graviss, E.A.; Musher, D.M. Acute kidney injury associated with trimethoprim/sulfamethoxazole. J. Antimicrob. Chemother. 2012, 67, 1271–1277. [Google Scholar] [CrossRef] [Green Version]
- Sepúlveda, R.A.; Anghileri, F.; Huidobro, E.J.P.; Julio, R.; Ávila, E.; Figueroa, C. Acute kidney injury associated to sulfamethoxazole urine crystal: The importance of clinical suspicion. Clin. Nephrol. Case Stud. 2022, 10, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Arrayasillapatorn, N.; Promsen, P.; Kritmetapak, K.; Anunnatsiri, S.; Chotmongkol, W.; Anutrakulchai, S. Colistin-Induced Acute Kidney Injury and the Effect on Survival in Patients with Multidrug-Resistant Gram-Negative Infections: Significance of Drug Doses Adjusted to Ideal Body Weight. Int. J. Nephrol. 2021, 2021, 7795096. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Samodelov, S.L.; Kullak-Ublick, G.A.; Visentin, M. Molecular Mechanisms of Colistin-Induced Nephrotoxicity. Molecules 2019, 24, 653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshehri, A.M.; Alzahrani, M.Y.; Abujamal, M.A.; Abdalla, M.H.; Alowais, S.A.; Alfayez, O.M.; Alyami, M.S.; Almutairi, A.R.; Almohammed, O.A. Comparative Risk of Acute Kidney Injury Following Concurrent Administration of Vancomycin with Piperacillin/Tazobactam or Meropenem: A Systematic Review and Meta-Analysis of Observational Studies. Antibiotics 2022, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Ide, N.; Sato, S.; Sawaguchi, K. Risk of Acute Kidney Injury in Patients Treated with Vancomycin and Piperacillin/Tazobactam Compared to Vancomycin and Meropenem or Doripenem: A Retrospective Cohort Study. Yakugaku Zasshi 2019, 139, 1609–1614. [Google Scholar] [CrossRef] [Green Version]
- Sussman, M.S.; Mulder, M.B.; Ryon, E.L.; Urrechaga, E.M.; Lama, G.A.; Bahga, A.; Eidelson, S.A.; Lieberman, H.M.; Schulman, C.I.; Namias, N.; et al. Acute Kidney Injury Risk in Patients Treated with Vancomycin Combined with Meropenem or Cefepime. Surg. Infect. 2021, 22, 415–420. [Google Scholar] [CrossRef]
- Le, P.; Navaneethan, S.D.; Yu, P.C.; Pallotta, A.M.; Rastogi, R.; Patel, P.; Brateanu, A.; Imrey, P.B.; Rothberg, M.B. Association of antibiotic use and acute kidney injury in patients hospitalized with community-acquired pneumonia. Curr. Med. Res. Opin. 2022, 38, 443–450. [Google Scholar] [CrossRef]
- Gaggl, M.; Pate, V.; Stürmer, T.; Kshirsagar, A.V.; Layton, J.B. The comparative risk of acute kidney injury of vancomycin relative to other common antibiotics. Sci. Rep. 2020, 10, 17282. [Google Scholar] [CrossRef]
- Cavalcanti, A.B.; Goncalves, A.R.; Almeida, C.S.; Bugano, D.D.; Silva, E. Teicoplanin versus vancomycin for proven or suspected infection. Cochrane Database Syst. Rev. 2010, CD007022. [Google Scholar] [CrossRef]
- Aslan, A.T.; Pashayev, T.; Dağ, O.; Akova, M. Comparison of teicoplanin versus vancomycin in combination with piperacillin-tazobactam or meropenem for the risk of acute kidney injury. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1953–1961. [Google Scholar] [CrossRef]
- Brown, M.L.; Motsch, J.; Kaye, K.S.; File, T.M.; Boucher, H.W.; Vendetti, N.; Aggrey, A.; Joeng, H.-K.; Tipping, R.W.; Du, J.; et al. Evaluation of Renal Safety between Imipenem/Relebactam and Colistin Plus Imipenem in Patients with Imipenem-Nonsusceptible Bacterial Infections in the Randomized, Phase 3 RESTORE-IMI 1 Study. Open Forum Infect. Dis. 2020, 7, ofaa054. [Google Scholar] [CrossRef] [Green Version]
- Mousavi Movahed, S.M.; Akhavizadegan, H.; Dolatkhani, F.; Nejadghaderi, S.A.; Aghajani, F.; Gangi, M.F.; Ghazi, Z.; Ghasemi, H. Different incidences of acute kidney injury (AKI) and outcomes in COVID-19 patients with and without non-azithromycin antibiotics: A retrospective study. J. Med. Virol. 2021, 93, 4411–4419. [Google Scholar] [CrossRef]
- Zoratti, C.; Moretti, R.; Rebuzzi, L.; Albergati, I.V.; Di Somma, A.; Decorti, G.; Di Bella, S.; Crocè, L.S.; Giuffrè, M. Antibiotics and Liver Cirrhosis: What the Physicians Need to Know. Antibiotics 2021, 11, 31. [Google Scholar] [CrossRef]
- Cotta, M.O.; Roberts, J.A.; Lipman, J. Antibiotic dose optimization in critically ill patients. Med. Intensiv. 2015, 39, 563–572. (In English)(In Spanish) [Google Scholar] [CrossRef]
- Halilovic, J.; Heintz, B.H. Antibiotic dosing in cirrhosis. Am. J. Health Syst. Pharm. 2014, 71, 1621–1634. [Google Scholar] [CrossRef]
- Lind, L.; Sundström, J.; Larsson, A.; Lampa, E.; Ärnlöv, J.; Ingelsson, E. Longitudinal effects of aging on plasma proteins levels in older adults—Associations with kidney function and hemoglobin levels. PLoS ONE 2019, 14, e0212060. [Google Scholar] [CrossRef]
- Drugbank Online. Narrow Therapeutic Index Drugs. Available online: https://go.drugbank.com/categories/DBCAT003972 (accessed on 16 April 2023).
- Hilmer, S.N. ADME-tox issues for the elderly. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1321–1331. [Google Scholar] [CrossRef]
- Cattaneo, D.; Falcone, M.; Gervasoni, C.; Marriott, D.J.E. Therapeutic Drug Monitoring of Antibiotics in the Elderly: A Narrative Review. Ther. Drug Monit. 2022, 44, 75–85. [Google Scholar] [CrossRef]
- Cattaneo, D.; Fusi, M.; Cozzi, V.; Baldelli, S.; Bonini, I.; Gervasoni, C.; Clementi, E. Supra-therapeutic Linezolid Trough Concentrations in Elderly Patients: A Call for Action? Clin. Pharmacokinet. 2021, 60, 603–609. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, N.; Wei, W.; Jiang, C. Outcomes and Nephrotoxicity Associated with Vancomycin Treatment in Patients 80 Years and Older. Clin. Interv. Aging 2021, 16, 1023–1035. [Google Scholar] [CrossRef]
- Yahav, D.; Abbas, M.; Nassar, L.; Ghrayeb, A.; Shepshelovich, D.; Kurnik, D.; Leibovici, L.; Paul, M. Attention to age: Similar dosing regimens lead to different vancomycin levels among older and younger patients. Age Ageing 2019, 49, 26–31. [Google Scholar] [CrossRef]
- Hatti, M.; Solomonidi, N.; Odenholt, I.; Tham, J.; Resman, F. Considerable variation of trough β-lactam concentrations in older adults hospitalized with infection–A prospective observational study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 485–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, N.; Nishimura, N.; Ikawa, K.; Karino, F.; Miura, K.; Tamaki, H.; Yano, T.; Isobe, T.; Morikawa, N.; Naora, K. Population Pharmacokinetic Modeling and Pharmacodynamic Target Attainment Simulation of Piperacillin/Tazobactam for Dosing Optimization in Late Elderly Patients with Pneumonia. Antibiotics 2020, 9, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, M.; Inui, N.; Suda, T.; Nakamura, Y.; Wajima, T.; Matsuo, Y.; Chida, K. Pharmacokinetic analysis of doripenem in elderly patients with nosocomial pneumonia. Int. J. Antimicrob. Agents 2013, 42, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Frey, O.R.; Hempel, G. Population pharmacokinetics of meropenem in elderly patients: Dosing simulations based on renal function. Eur. J. Clin. Pharmacol. 2017, 73, 333–342. [Google Scholar] [CrossRef]
- Medellín-Garibay, S.E.; Romano-Aguilar, M.; Parada, A.; Suárez, D.; Romano-Moreno, S.; Barcia, E.; Cervero, M.; García, B. Amikacin pharmacokinetics in elderly patients with severe infections. Eur. J. Pharm. Sci. 2022, 175, 106219. [Google Scholar] [CrossRef]
- Cojutti, P.G.; Ramos-Martin, V.; Schiavon, I.; Rossi, P.; Baraldo, M.; Hope, W.; Pea, F. Population Pharmacokinetics and Pharmacodynamics of Levofloxacin in Acutely Hospitalized Older Patients with Various Degrees of Renal Function. Antimicrob. Agents Chemother. 2017, 61, e02134-16. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.H.; Nicolau, D.P. Augmented Renal Clearance and How to Augment Antibiotic Dosing. Antibiotics 2020, 9, 393. [Google Scholar] [CrossRef]
- Hefny, F.; Stuart, A.; Kung, J.Y.; Mahmoud, S.H. Prevalence and Risk Factors of Augmented Renal Clearance: A Systematic Review and Meta-Analysis. Pharmaceutics 2022, 14, 445. [Google Scholar] [CrossRef]
- Nazer, L.H.; AbuSara, A.K.; Kamal, Y. Augmented renal clearance in critically ill patients with cancer (ARCCAN Study): A prospective observational study evaluating prevalence and risk factors. Pharmacol. Res. Perspect. 2021, 9, e00747. [Google Scholar] [CrossRef]
- Mikami, R.; Hayakawa, M.; Imai, S.; Sugawara, M.; Takekuma, Y. Onset timing and duration of augmented renal clearance in a mixed intensive care unit. J. Intensive Care 2023, 11, 13. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Zhu, M. Effect of augmented renal clearance on the therapeutic drug monitoring of vancomycin in patients after neurosurgery. J. Int. Med. Res. 2020, 48, 300060520949076. [Google Scholar] [CrossRef]
- Yu, Y.X.; Lu, J.; Lu, H.D.; Li, L.; Li, J.J.; Shi, L.; Duan, L.F.; Zhuang, Z.W.; Xue, S.D.; Shen, Y.; et al. Predictive performance of reported vancomycin population pharmacokinetic model in patients with different renal function status, especially those with augmented renal clearance. Eur. J. Hosp. Pharm. Sci. Pract. 2022, 29, e6–e14. [Google Scholar] [CrossRef]
- Gijsen, M.; Elkayal, O.; Annaert, P.; Van Daele, R.; Meersseman, P.; Debaveye, Y.; Wauters, J.; Dreesen, E.; Spriet, I. Meropenem Target Attainment and Population Pharmacokinetics in Critically Ill Septic Patients with Preserved or Increased Renal Function. Infect. Drug Resist. 2022, 15, 53–62. [Google Scholar] [CrossRef]
- Barrasa, H.; Soraluce, A.; Usón, E.; Sainz, J.; Martín, A.; Sánchez-Izquierdo, J.Á.; Maynar, J.; Rodríguez-Gascón, A.; Isla, A. Impact of augmented renal clearance on the pharmacokinetics of linezolid: Advantages of continuous infusion from a pharmacokinetic/pharmacodynamic perspective. Int. J. Infect. Dis. 2020, 93, 329–338. [Google Scholar] [CrossRef]
- Janknegt, R.; Boogaard-Van den Born, J.; Hameleers, B.A.; Hooymans, P.M.; Rang, J.; Smits, C.A.; Willems-Thissen, M.E. Pharmacokinetics of amoxycillin in elderly in-patients. Pharm. Weekbl. Sci. 1992, 14, 27–29. [Google Scholar] [CrossRef]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Møller, N.; Muller, A.E.; Paccaud, J.P.; Mouton, J.W. Oral amoxicillin and amoxicillin-clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef]
- Meyers, B.R.; Wilkinson, P.; Mendelson, M.H.; Walsh, S.; Bournazos, C.; Hirschman, S.Z. Pharmacokinetics of ampicillin-sulbactam in healthy elderly and young volunteers. Antimicrob. Agents Chemother. 1991, 35, 2098–2101. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, K.; Matsumoto, K.; Yokoyama, Y.; Watanabe, E.; Enoki, Y.; Shigemi, A.; Ikawa, K.; Terazono, H.; Morikawa, N.; Ohshige, T.; et al. Dosing Optimization of Ampicillin-Sulbactam Based on Cystatin C in Elderly Patients with Pneumonia. Biol. Pharm. Bull. 2021, 44, 732–736. [Google Scholar] [CrossRef]
- Riccobene, T.; Jakate, A.; Rank, D. A series of pharmacokinetic studies of ceftaroline fosamil in select populations: Normal subjects, healthy elderly subjects, and subjects with renal impairment or end-stage renal disease requiring hemodialysis. J. Clin. Pharmacol. 2014, 54, 742–752. [Google Scholar] [CrossRef]
- Barbhaiya, R.H.; Knupp, C.A.; Pittman, K.A. Effects of age and gender on pharmacokinetics of cefepime. Antimicrob. Agents Chemother. 1992, 36, 1181–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pais, G.M.; Chang, J.; Barreto, E.F.; Stitt, G.; Downes, K.J.; Alshaer, M.H.; Lesnicki, E.; Panchal, V.; Bruzzone, M.; Bumanglag, A.V.; et al. Clinical Pharmacokinetics and Pharmacodynamics of Cefepime. Clin. Pharmacokinet. 2022, 61, 929–953. [Google Scholar] [CrossRef] [PubMed]
- Geny, F.; Costa, P.; Bressolle, F.; Galtier, M. Ceftriaxone pharmacokinetics in elderly subjects and penetration into epididymis. Biopharm. Drug Dispos. 1993, 14, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.H.; Chan, Y.P.; Arnold, K.; Sun, M. Single-dose pharmacokinetics of ceftriaxone in healthy Chinese adults. Antimicrob. Agents Chemother. 1985, 27, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Wong, G.; Briscoe, S.; Adnan, S.; McWhinney, B.; Ungerer, J.; Lipman, J.; Roberts, J.A. Protein binding of β-lactam antibiotics in critically ill patients: Can we successfully predict unbound concentrations? Antimicrob. Agents Chemother. 2013, 57, 6165–6170. [Google Scholar] [CrossRef] [Green Version]
- Drug Bank Online. Doripenem. Available online: https://go.drugbank.com/drugs/DB06211 (accessed on 15 April 2023).
- Paterson, D.L.; Depestel, D.D. Doripenem. Clin. Infect. Dis. 2009, 49, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Finch, R.G.; Craddock, C.; Kelly, J.; Deaney, N.B. Pharmacokinetic studies of imipenem/cilastatin in elderly patients. J. Antimicrob. Chemother. 1986, 18 (Suppl. E), 103–107. [Google Scholar] [CrossRef]
- Drusano, G.L.; Standiford, H.C.; Bustamante, C.; Forrest, A.; Rivera, G.; Leslie, J.; Tatem, B.; Delaportas, D.; MacGregor, R.R.; Schimpff, S.C. Multiple-dose pharmacokinetics of imipenem-cilastatin. Antimicrob. Agents Chemother. 1984, 26, 715–721. [Google Scholar] [CrossRef] [Green Version]
- DrugBank Online. Imipenem. Available online: https://go.drugbank.com/drugs/DB01598 (accessed on 13 April 2023).
- Ljungberg, B.; Nilsson-Ehle, I. Pharmacokinetics of meropenem and its metabolite in young and elderly healthy men. Antimicrob. Agents Chemother. 1992, 36, 1437–1440. [Google Scholar] [CrossRef] [Green Version]
- Cunha, B.A. Meropenem in elderly and renally impaired patients. Int. J. Antimicrob. Agents. 1998, 10, 107–117, Corrected in Int. J. Antimicrob. Agents. 1999, 11, 167–177. [Google Scholar] [CrossRef]
- Namkoong, H.; Kameyama, Y.; Yasuda, H.; Nakayama, S.; Kaneko, H.; Kawashima, C.; Terajima, T.; Maezawa, K.; Hayashi, T.; Sandoh, M.; et al. The efficacy, safety, and pharmacokinetics of biapenem administered thrice daily for the treatment of pneumonia in the elderly. J. Infect. Chemother. 2014, 20, 356–360. [Google Scholar] [CrossRef]
- Kozawa, O.; Uematsu, T.; Matsuno, H.; Niwa, M.; Takiguchi, Y.; Matsumoto, S.; Minamoto, M.; Niida, Y.; Yokokawa, M.; Nagashima, S.; et al. Pharmacokinetics and safety of a new parenteral carbapenem antibiotic, biapenem (L-627), in elderly subjects. Antimicrob. Agents Chemother. 1998, 42, 1433–1436. [Google Scholar] [CrossRef] [Green Version]
- Griffith, D.C.; Morgan, E.E.; Dudley, M.N.; Loutit, J.S. A Phase 1 Study of the Safety, Tolerability, and Pharmacokinetics of Biapenem in Healthy Adult Subjects. Antimicrob. Agents Chemother. 2023, 65, e02612-20. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, F.; Chen, C.; Ma, L.; Yang, T.; Liu, X.; Liu, Y.; Wang, X.; Zhao, X.; Que, C.; et al. Development of a Population Pharmacokinetic Model of Vancomycin and its Application in Chinese Geriatric Patients with Pulmonary Infections. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Guay, D.R.; Vance-Bryan, K.; Gilliland, S.; Rodvold, K.; Rotschafer, J. Comparison of vancomycin pharmacokinetics in hospitalized elderly and young patients using a Bayesian forecaster. J. Clin. Pharmacol. 1993, 33, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.J.; Germano, A.; Sime, F.B.; Roberts, J.A.; Kimura, E. Vancomycin population pharmacokinetics for adult patients with sepsis or septic shock: Are current dosing regimens sufficient? Eur. J. Clin. Pharmacol. 2019, 75, 1219–1226. [Google Scholar] [CrossRef]
- DrugBank Online. Vancomycin. Available online: https://go.drugbank.com/drugs/DB00512 (accessed on 13 April 2023).
- Patel, S.; Preuss, C.V.; Bernice, F. Vancomycin. [Updated 2023 Jan 14]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459263/ (accessed on 13 April 2023).
- Kasai, H.; Tsuji, Y.; Hiraki, Y.; Tsuruyama, M.; To, H.; Yamamoto, Y. Population pharmacokinetics of teicoplanin in hospitalized elderly patients using cystatin C as an indicator of renal function. J. Infect. Chemother. 2018, 24, 284–291. [Google Scholar] [CrossRef]
- Outman, W.R.; Nightingale, C.H.; Sweeney, K.R.; Quintiliani, R. Teicoplanin pharmacokinetics in healthy volunteers after administration of intravenous loading and maintenance doses. Antimicrob. Agents Chemother. 1990, 34, 2114–2117. [Google Scholar] [CrossRef] [Green Version]
- DrugBank Online. Teicoplanin. Available online: https://go.drugbank.com/drugs/DB06149 (accessed on 13 April 2023).
- Dvorchik, B.; Damphousse, D. Single-dose pharmacokinetics of daptomycin in young and geriatric volunteers. J. Clin. Pharmacol. 2004, 44, 612–620. [Google Scholar] [CrossRef]
- Goldberg, M.R.; Wong, S.L.; Shaw, J.P.; Kitt, M.M.; Barriere, S.L. Single-dose pharmacokinetics and tolerability of telavancin in elderly men and women. Pharmacotherapy 2010, 30, 806–811. [Google Scholar] [CrossRef]
- Das, B.; Sarkar, C.; Das, D.; Gupta, A.; Kalra, A.; Sahni, S. Telavancin: A novel semisynthetic lipoglycopeptide agent to counter the challenge of resistant Gram-positive pathogens. Ther. Adv. Infect. Dis. 2017, 4, 49–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisson, T.L.; Jungbluth, G.L.; Hopkins, N.K. Age and sex effects on the pharmacokinetics of linezolid. Eur. J. Clin. Pharmacol. 2002, 57, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Bouza, E.; Muñoz, P. Linezolid: Pharmacokinetic characteristics and clinical studies. Clin. Microbiol. Infect. 2001, 7 (Suppl. S4), 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flanagan, S.D.; Minassian, S.L.; Prokocimer, P. Pharmacokinetics, Safety, and Tolerability of Tedizolid Phosphate in Elderly Subjects. Clin. Pharmacol. Drug Dev. 2018, 7, 788–794. [Google Scholar] [CrossRef]
- Iqbal, K.; Milioudi, A.; Wicha, S.G. Pharmacokinetics and Pharmacodynamics of Tedizolid. Clin. Pharmacokinet. 2022, 61, 489–503. [Google Scholar] [CrossRef]
- Muralidharan, G.; Fruncillo, R.J.; Micalizzi, M.; Raible, D.G.; Troy, S.M. Effects of age and sex on single-dose pharmacokinetics of tigecycline in healthy subjects. Antimicrob. Agents Chemother. 2005, 49, 1656–1659. [Google Scholar] [CrossRef] [Green Version]
- Dorn, C.; Kratzer, A.; Liebchen, U.; Schleibinger, M.; Murschhauser, A.; Schlossmann, J.; Kees, F.; Simon, P.; Kees, M.G. Impact of Experimental Variables on the Protein Binding of Tigecycline in Human Plasma as Determined by Ultrafiltration. J. Pharm. Sci. 2018, 107, 739–744. [Google Scholar] [CrossRef]
- Chow, A.T.; Fowler, C.; Williams, R.R.; Morgan, N.; Kaminski, S.; Natarajan, J. Safety and pharmacokinetics of multiple 750-milligram doses of intravenous levofloxacin in healthy volunteers. Antimicrob. Agents Chemother. 2001, 45, 2122–2125. [Google Scholar] [CrossRef] [Green Version]
- Fish, D.N.; Chow, A.T. The clinical pharmacokinetics of levofloxacin. Clin. Pharmacokinet. 1997, 32, 101–119. [Google Scholar] [CrossRef]
- Sullivan, J.T.; Lettieri, J.T.; Liu, P.; Heller, A.H. The influence of age and gender on the pharmacokinetics of moxifloxacin. Clin. Pharmacokinet. 2001, 40 (Suppl. S1), 11–18. [Google Scholar] [CrossRef]
- Gai, X.; Shen, N.; He, B.; Zhou, Q.; Bo, S.; Li, X.; Zhai, S.; Yin, A.; Lu, W. Population pharmacokinetics of ciprofloxacin in Chinese elderly patients with lower respiratory tract infection. Zhonghua Yi Xue Za Zhi 2015, 95, 1581–1585. (In Chinese) [Google Scholar]
- Drug Bank Online. Ciprofloxacin. Available online: https://go.drugbank.com/drugs/DB00537 (accessed on 15 May 2023).
- Kato, H.; Parker, S.L.; Roberts, J.A.; Hagihara, M.; Asai, N.; Yamagishi, Y.; Paterson, D.L.; Mikamo, H. Population Pharmacokinetics Analysis of Amikacin Initial Dosing Regimen in Elderly Patients. Antibiotics 2021, 10, 100. [Google Scholar] [CrossRef]
- Ghaffari, S.; Hadi, A.M.; Najmeddin, F.; Shahrami, B.; Rouini, M.R.; Najafi, A.; Mojtahedzadeh, M. Evaluation of amikacin dosing schedule in critically ill elderly patients with different stages of renal dysfunction. Eur. J. Hosp. Pharm. 2022, 29, e67–e71. [Google Scholar] [CrossRef]
- Bauer, L.A.; Blouin, R.A. Influence of age on amikacin pharmacokinetics in patients without renal disease. Comparison with gentamicin and tobramycin. Eur. J. Clin. Pharmacol. 1983, 24, 639–642. [Google Scholar] [CrossRef]
- DrugBank Online. Amikacin. Available online: https://go.drugbank.com/drugs/DB00479 (accessed on 13 April 2023).
- Hilmer, S.N.; Tran, K.; Rubie, P.; Wright, J.; Gnjidic, D.; Mitchell, S.J.; Matthews, S.; Carroll, P.R. Gentamicin pharmacokinetics in old age and frailty. Br. J. Clin. Pharmacol. 2011, 71, 224–231. [Google Scholar] [CrossRef] [Green Version]
- Triggs, E.; Charles, B. Pharmacokinetics and therapeutic drug monitoring of gentamicin in the elderly. Clin. Pharmacokinet. 1999, 37, 331–341. [Google Scholar] [CrossRef]
- Wang, P.; Liu, D.; Sun, T.; Zhang, X.; Yang, J. Pharmacokinetics and pharmacodynamics of polymyxin B and proposed dosing regimens in elderly patients with multi-drug-resistant Gram-negative bacterial infections. Int. J. Antimicrob. Agents. 2022, 60, 106693. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, X.; Du, X.; Chen, H.; Zhao, F.; Zhou, Z.; Wang, Y.; Zheng, Y.; Bergen, P.J.; Li, X.; et al. Pharmacokinetics/pharmacodynamics of polymyxin B in patients with bloodstream infection caused by carbapenem-resistant Klebsiella pneumoniae. Front. Pharmacol. 2022, 13, 975066. [Google Scholar] [CrossRef]
- Avedissian, S.N.; Liu, J.; Rhodes, N.J.; Lee, A.; Pais, G.M.; Hauser, A.R.; Scheetz, M.H. A Review of the Clinical Pharmacokinetics of Polymyxin B. Antibiotics 2019, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Abodakpi, H.; Gohlke, J.; Chang, K.T.; Chow, D.S.; Tam, V.H. Analytical and functional determination of polymyxin B protein binding in serum. Antimicrob. Agents Chemother. 2015, 59, 7121–7123. [Google Scholar] [CrossRef] [Green Version]
- Majcher-Peszynska, J.; Loebermann, M.; Klammt, S.; Frimmel, S.; Mundkowski, R.G.; Welte, T.; Reisinger, E.C.; Drewelow, B.; CAPNETZ Study Group. Ampicillin/sulbactam in elderly patients with community-acquired pneumonia. Infection 2014, 42, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sugiyama, E.; Nozawa, K.; Tajima, M.; Takahashi, K.; Yoshii, M.; Suzuki, H.; Sato, V.H.; Sato, H. Effects of dosing frequency on the clinical efficacy of ampicillin/sulbactam in Japanese elderly patients with pneumonia: A single-center retrospective observational study. Pharmacol. Res. Perspect. 2021, 9, e00746. [Google Scholar] [CrossRef] [PubMed]
- Cojutti, P.G.; Morandin, E.; Baraldo, M.; Pea, F. Population pharmacokinetics of continuous infusion of piperacillin/tazobactam in very elderly hospitalized patients and considerations for target attainment against Enterobacterales and Pseudomonas aeruginosa. Int. J. Antimicrob. Agents. 2021, 58, 106408. [Google Scholar] [CrossRef] [PubMed]
- Ulldemolins, M.; Roberts, J.A.; Lipman, J.; Rello, J. Antibiotic dosing in multiple organ dysfunction syndrome. Chest 2011, 139, 1210–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinton, M.C.; Bodeau, S.; Kontar, L.; Zerbib, Y.; Maizel, J.; Slama, M.; Masmoudi, K.; Lemaire-Hurtel, A.S.; Bennis, Y. Neurotoxic Concentration of Piperacillin during Continuous Infusion in Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e00654-17. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Ramos, J.; Herrera-Mateo, S.; López-Vinardell, L.; Juanes-Borrego, A.; Puig-Campmany, M.; Mangues-Bafalluy, M.A. Cefepime Dosing Requirements in Elderly Patients Attended in the Emergency Rooms. Dose Response 2022, 20, 15593258221078393. [Google Scholar] [CrossRef]
- Boschung-Pasquier, L.; Atkinson, A.; Kastner, L.K.; Banholzer, S.; Haschke, M.; Buetti, N.; Furrer, D.I.; Hauser, C.; Jent, P.; Que, Y.A.; et al. Cefepime neurotoxicity: Thresholds and risk factors. A retrospective cohort study. Clin. Microbiol. Infect. 2020, 26, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.J.; Cockcroft, M.; Page-Sharp, M.; Arendts, G.; Davis, T.M.E.; Moore, B.R.; Batty, K.T.; Salman, S.; Manning, L. Population Pharmacokinetic Study of Ceftriaxone in Elderly Patients, Using Cystatin C-Based Estimates of Renal Function To Account for Frailty. Antimicrob. Agents Chemother. 2020, 64, e00874-20. [Google Scholar] [CrossRef]
- Jadot, L.; Judong, A.; Canivet, J.L.; Lorenzo-Villalba, N.; Damas, P. Ceftriaxone-induced Encephalopathy: A Pharmacokinetic Approach. Eur. J. Case Rep. Intern. Med. 2021, 8, 003011. [Google Scholar] [CrossRef]
- Veillette, J.J.; Truong, J.; Forland, S.C. Pharmacokinetics of Ceftazidime-Avibactam in Two Patients with KPC-Producing Klebsiella pneumoniae Bacteremia and Renal Impairment. Pharmacotherapy 2016, 36, e172–e177. [Google Scholar] [CrossRef]
- Pingue, V.; Penati, R.; Nardone, A.; Franciotta, D. Ceftazidime/avibactam neurotoxicity in an adult patient with normal renal function. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef]
- Aloy, B.; Launay-Vacher, V.; Bleibtreu, A.; Bortolotti, P.; Faure, E.; Filali, A.; Gauzit, R.; Gilbert, M.; Lesprit, P.; Mahieu, R.; et al. Antibiotics and chronic kidney disease: Dose adjustment update for infectious disease clinical practice. Med. Mal. Infect. 2020, 50, 323–331. [Google Scholar] [CrossRef]
- Musson, D.G.; Majumdar, A.; Holland, S.; Birk, K.; Xi, L.; Mistry, G.; Sciberras, D.; Muckow, J.; Deutsch, P.; Rogers, J.D. Pharmacokinetics of total and unbound ertapenem in healthy elderly subjects. Antimicrob. Agents Chemother. 2004, 48, 521–524. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Ueng, Y.F.; Wu, C.W.; Chou, Y.C.; Ng, Y.Y.; Yang, W.C. The recommended dose of ertapenem poses a potential risk for central nervous system toxicity in haemodialysis patients—Case reports and literature reviews. J. Clin. Pharm. Ther. 2015, 40, 240–244. [Google Scholar] [CrossRef]
- Steffens, N.A.; Zimmermann, E.S.; Nichelle, S.M.; Brucker, N. Meropenem use and therapeutic drug monitoring in clinical practice: A literature review. J. Clin. Pharm. Ther. 2021, 46, 610–621. [Google Scholar] [CrossRef]
- Karino, F.; Deguchi, N.; Kanda, H.; Ohe, M.; Kondo, K.; Tada, M.; Kuraki, T.; Isobe, T.; Nishimura, N.; Moriyama, H.; et al. Evaluation of the efficacy and safety of biapenem against pneumonia in the elderly and a study on its pharmacokinetics. J. Infect. Chemother. 2013, 19, 98–102. [Google Scholar] [CrossRef]
- Bourguignon, L.; Goutelle, S.; De Saint-Martin, J.B.; Maire, P.; Ducher, M. Evaluation of various gentamicin dosage regimens in geriatric patients: A simulation study. Fundam. Clin. Pharmacol. 2010, 24, 109–113. [Google Scholar] [CrossRef]
- Samura, M.; Takada, K.; Yamamoto, R.; Ito, H.; Nagumo, F.; Uchida, M.; Kurata, T.; Koshioka, S.; Enoki, Y.; Taguchi, K.; et al. Population Pharmacokinetic Analysis and Dosing Optimization Based on Unbound Daptomycin Concentration and Cystatin C in Nonobese Elderly Patients with Hypoalbuminemia and Chronic Kidney Disease. Pharm. Res. 2021, 38, 1041–1055. [Google Scholar] [CrossRef]
- Balice, G.; Passino, C.; Bongiorni, M.G.; Segreti, L.; Russo, A.; Lastella, M.; Luci, G.; Falcone, M.; Di Paolo, A. Daptomycin Population Pharmacokinetics in Patients Affected by Severe Gram-Positive Infections: An Update. Antibiotics 2022, 11, 914. [Google Scholar] [CrossRef]
- Ito, F.; Ohno, Y.; Toyoshi, S.; Kaito, D.; Koumei, Y.; Endo, J.; Kamamiya, F.; Mori, H.; Mori, M.; Morishita, M.; et al. Pharmacokinetics of consecutive oral moxifloxacin (400 mg/day) in patients with respiratory tract infection. Ther. Adv. Respir. Dis. 2016, 10, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Qi, T.T.; Qu, Q.; Long, W.M.; Chen, Y.; Luo, Y.; Wang, Y. Polymyxin B-Based Regimens for Patients Infected with Carbapenem-Resistant Gram-Negative Bacteria: Clinical and Microbiological Efficacy, Mortality, and Safety. Infect. Drug Resist. 2022, 15, 1205–1218. [Google Scholar] [CrossRef] [PubMed]

| Part of GIT | Age-Associated Changes | Drug-Induced Changes | PK Changes | References |
|---|---|---|---|---|
| Oral cavity | Xerostomia, dysgeusia and ageusia, oropharyngeal dysphagia | Xerostomia may be induced by cholinolytics, histamine H1 antagonists, α1 adrenergic antagonists, tricyclic antidepressants | Decreased absorption from the oral cavity | [45,46,47] |
| Esophagus | Esophageal dysphagia, odynophagia, increased risk of gastroesophageal reflux disease, Barrett’s columnar-lined esophagus, DIE | DIE may be induced by antibiotics (tetracycline, doxycycline, clindamycin), bisphosphonates (alendronate), calcium channel blockers (amlodipine), anti-coagulants (dabigatran, apixaban), Chemotherapeutic agents (sunitinib, doxorubicin, methotrexate, nivolumab, ipilimumab), ferrous sulfate, NSAIDs | Decreased absorption of weak acids and weak bases, high risk of drug interactions resulting in further PK changes | [45,48,49,50,51,52,53,54,55,56] |
| Stomach | Chronic atrophic gastritis, increased risks of hypochlorhydria and of hyperchlorhydria with peptic ulcer, decreased gastric motility. | Proton pump inhibitors (PPIs) contribute to the development of hypochlorhydria and may induce enterochromaffin-like cells hyperplasia, gastric polyp formation, and hypergastrinemia, PPI could increase the risk of community-acquired pneumonia, autoimmune diseases, cardiovascular diseases, onset of dementia and depression, fragility fractures, mainly hip fractures | Hypochlorhydria may result in the impaired drug dissolution and changed systemic exposure of poorly water-soluble drugs | [8,45,57,58,59,60,61,62,63] |
| Intestine | Malnutrition, chronic constipation, high risk of colorectal cancer, increased gut permeability, increased chronic and systemic mild inflammatory responses with risks for inflammatory bowel disease, dysbiosis (50% of microbiome in the elderly—Bacteroides, Alistipes, and Parabacteroides, versus 8–27% in a younger cohort), decreased small bowel surface area, increased rates of Clostridium difficile colitis, and diverticular disease | Drug-induced colitis may be caused by diuretics, dihydropyridines, glycosides, platelet aggregation inhibitors, NSAIDs, statins and fibrates, as well, as immune checkpoint inhibitors (ipilimumab and nivolumab), idelalisib, mycophenolate mofetil. PPIs are associated with the risk of developing Clostridium difficile infections | Decreased absorption mainly because of the decreased intestinal blood flow and decreased absorption area | [64,65,66,67,68,69] |
| Pancreas | Decreased pancreatic secretion (decreased lipase, chymotrypsin, amylase levels), pancreatic atrophy, lobulation, and fatty degeneration | The highest number of drug-induced pancreatitis cases were associated with the use of valproic acid, L-asparaginase, and 5-aminosalicylic acid | Fat malabsorption may alter absorption of lipophilic drugs | [70,71,72,73] |
| Liver | Liver volume decreases by 20–40% with aging, blood flow decrease by 35% compared with persons < 40 years old. Increased rates of oxidative stress and inflammatory response, high prevalence of liver fibrosis, NAFLD | Drug induced liver injury is commonly caused by antibacterials (amoxicillin-clavulanate, flucloxacillin, nitrofurantoin), statins (atorvastatin), immune checkpoints inhibitors (nivolumab, ipilimumab, infliximab) | Decreased first-pass metabolism with consequent increase of absorption and bioavailability of high-clearance drugs, decreased rates of formation of active drugs from prodrugs with consequent decrease of their plasma concentrations and possible failure of treatment | [74,75,76] |
| Transport Protein | Age-Dependent Change of Expression | Transported Antibiotics | References |
|---|---|---|---|
| BCRP | Decreased protein expression in the human intestine and liver in the elderly. Gene expression is significantly decreased in animal models. No marked changes in human with aging | Fluoroquinolones (delafloxacin, ciprofloxacin, enrofloxacin, norfloxacin, ofloxacin), nitrofurantoin | [81,82,85,89,90,91] |
| P-gp | No marked changes in human with aging. Significant reduction of activity in the elderly with renal failure. | Erythromycin, tetracycline Azithromycin Levofloxacin, sparfloxacin Dicloxacillin | [81,92,93,94,95,96,97] |
| MRP2 | No data available in human | Ampicillin, azithromycin, ceftriaxone, cefodizime, ceftriaxone | [98] |
| OATP1A2 | No data available in human | Most of fluoroquinolones (ciprofloxacin, enoxacin, gatifloxacin, levofloxacin, lomefloxacin, norfloxacin), erythromycin, tebipenem | [93,99,100,101] |
| OATP1B1 | No marked changes in human aging. A weak correlation was noted between OATP1B1 abundance and age of human donors. Significant reduction of activity in the elderly with renal failure | Benzylpenicillin, rifampicin, rifampin, rifampicin, cefazolin, cefditoren, cefoperazone, nafcillin | [81,85,86,93,102,103] |
| OATP1B3 | No marked changes in human aging | Rifampicin, rifampin, cefadroxil, cefazolin, cefditoren, cefmetazole, cefoperazone, cephalexin, nafcillin, erythromycin | [86,93,100,103] |
| OATP2B1 | No marked changes in human aging. Gene expression is significantly decreased in animal models | Benzylpenicillin, tebipenem pivoxil | [86,88,104,105] |
| PEPT1 | In diabetes mellitus—downregulation of PepT1. In obesity—leptin-dependent activation of PepT1 activity and expression | Penicillins (penicillin G, cyclacillin), cephalosporines (cefadroxil, ceftibuten, cefixime, cephradine, cephalexin, cefroxadine, loracarbef) | [83,103] |
| PEPT2 | Age-dependent changes were observed for different locations (heart, brain, nervous tissue, kidney) with increase of expression with aging | Colistin | [84,106,107] |
| Drug (Oral Administration) | Food Effect on PK Parameters | References |
|---|---|---|
| Ampicillin | Plasma concentration is decreased in fed state. Should not be taken with food to allow optimal absorption | [110] |
| Amoxicillin | Cmax decreased, Tmax prolonged under fed condition, but with no change of the AUC, thus use both under fasted and fed state is effective, since it is time-dependent AB | [111] |
| Amoxicillin-clavulanate | Decreased bioavailability of clavulanic acid after meal (extended-release tablets), so administration before meal is preferrable | [112] |
| Flucloxacillin | Reduced AUC, Cmax, and prolonged Tmax of both free and total concentrations compared with the fasting state. Achievement of free concentration targets associated with efficacy was in most circumstances equivalent, suggesting no negative association with the fed state | [113] |
| Cefaclor | Cmax decreased, Tmax delayed, but no AUC changes were reported for cefaclor granule and cefaclor suspension under fed state supposing effective use regardless of meal | [114] |
| Cefuroxime axetil | Positive food effect on absorption with AUC in fed state greater than in the fasted state, suggesting postprandial administration be more effective | [109] |
| Cefpodoxime proxetil | Achievement of proper Cmax and MIC values was reported in non-fasting patients. In the elderly patients, the absorption is approximately 30% lower compared to younger patients | [115,116,117] |
| Azithromycin | Capsules have delayed disintegration under fed state, resulting in the extended gastric residence and gastric degradation of azithromycin, thus capsules should be taken only in the fasted state. Tablets can be used regardless of meal | [118] |
| Clindamycin | The extent to systemic exposure was affected by the delay in absorption in the fed state, suggesting optimal dosing is in the fasted state | [119] |
| Linezolid | A slight decrease in Cmax, and delay in Tmax were observed in fed state, with no effect on AUC, suggesting effective use regardless of meal | [120] |
| Ofloxacin | Cmax and AUC were greater in the fasted state, significant decrease of absorption was observed with aluminum co-administration | [121] |
| Ciprofloxacin | Cmax and AUC were greater in the fasted state, significant decrease of absorption was observed with aluminum co-administration. Meal should be held for 1 h before and 2 h after fluoroquinolone administration | [121,122] |
| Levofloxacin | Slight delay of absorption with no alteration of the overall bioavailability after high fat meal. Food or drinks enriched with calcium may decrease Cmax and delay Tmax | [123,124] |
| Moxifloxacin | Considerable decrease of plasma concentrations in the fed state in comparison with the fasted state, so preprandial use is recommended | [125] |
| Doxycycline | Decrease of the Cmax and AUC in the fed state compared with the fasted state | [126] |
| Route of Administration | Age-Associated Change | Absorption Change | References |
|---|---|---|---|
| Intramuscular | Sarcopenia (the loss of muscle mass and function), fibrosis, infiltration of fat into skeletal muscle, increased inflammatory response | Some increase for depot preparations | [127] |
| Hypoperfusion of skeletal muscles | May decrease | [76,128] | |
| Percutaneous | Decreased hydration and changed lipid structure result in an increased barrier function of the stratum corneum | Some decrease for hydrophilic drugs | [129] |
| Inhalation | Lung function decline: increase in alveolar size and alveolar-capillary surface area, reduction of the elastic recoil of the lungs, increase in end-expiratory lung volume, increase of the functional residual capacity, reduction of the expiratory airflow, decline in forced expiratory volume by approximately 30 mL/year and forced vital capacity by approximately 20 mL/year, decrease of the blood flow rates | Variable effect on absorption, leading to increase, decrease, or no changes of AUC and Cmax compared to younger patients | [130,131] |
| CYP450 Isoenzyme | Antibiotic-Substrate | Antibiotic-Inducer or Antibiotic-Inhibitor | Age-Related Changes | References |
|---|---|---|---|---|
| CYP1A1 | Linezolid | Inhibitor-Norfloxacin | Certain change is unknown. CYP1A1 polymorphism is supposed to be related to the development of multiple age-associated diseases (cancers, chronic obstructive pulmonary disease, coronary artery diseases) | [165,166,167] |
| CYP1A2 | Grepafloxacin, Lomefloxacin | Inhibitors—quinolones and fluoroquinolones Inducers—Rifampicin, Nafcillin | Certain change is unknown. CYP1A2 polymorphism is supposed to be related to the development of multiple age-associated diseases (cancers, hypertension, chronic obstructive pulmonary disease, coronary artery diseases) | [167,168,169,170] |
| CYP2A6 | Metronidazole | Inhibitors—Isoniazid, Ethambutol Inducers—Rifampicin | Weak positive association of the age and CYP2A6 protein levels and enzyme activity (nicotine and coumarin metabolism studies) | [171,172,173,174] |
| CYP1B1 | Linezolid | NA | Age-related changes are supposed. High frequency expression along with polymorphism is specific for a variety of cancers, obesity, glucose intolerance. CYP1B1 is involved in hypertension development and progression | [165,166,167,168,169,170,171,172,173,174,175] |
| CYP2B6 | NA | Inhibitors—Rifamycin Inducers—Rifampicin, Rifabutin, Rifamycin, Rifapentine | Age modified the effect of CYP2B6 genotype on loss to care in older HIV positive Africans: older slow metabolizers were at over four-fold higher risk when compared to older intermediate metabolizers (OR: 4.06 95% CI: 1.38, 11.89) | [176,177,178] |
| CYP2C8 | Linezolid, Trimethoprim | Inhibitors—Trimethoprim, Metronidazole, Isoniazid, Rifampicin, Rifamycin, Amoxicillin Inducers—Rifampicin, Rifabutin, Rifamycin, Rifapentine, Rifaximin | Some decrease is supposed. CYP2C8 provides anti-inflammatory and anti-oxidative effects in the vessels, its induction leads to the suppression of TNF-α induced inflammatory cytokines | [165,178,179,180,181,182] |
| CYP2C9 | Sulfamethoxazole, Trimethoprim | Inhibitors—Metronidazole, Sulfamethoxazole, Isoniazid, Sulfadiazine, Sulfisoxazole, Sulfamethizole, Rifamycin, Oritavancin Inducers—Rifampicin, Rifapentine, Rifabutin, Rifamycin | Systemic celecoxib exposure suggests that for the elderly extensive metabolizers enzyme activity may exceed that of younger ones. For intermediate and poor elderly metabolizers activity is reduced compared to the young ones. Systemic warfarin exposure was higher in all types of elderly metabolizers compared to young ones | [85,183,184] |
| CYP2C19 | NA | Inhibitors—Chloramphenicol, Oritavancin, Isoniazid, Sulfanilamide, Rifamycin, Ethambutol Inducers—Rifampicin, Rifamycin, Rifapentine, Rifabutin, Rifaximin | A decline in CYP2C19 metabolic activity was associated with sarcopenia and fatty liver disease in the elderly | [162,185,186] |
| CYP2D6 | Linezolid, Fusidic acid | Inhibitors—Isoniazid, Fusidic acid, Rifamycin, Oritavancin, Ethambutol | Decrease is supposed due to 20% reduction for CYP2D6 substrates. Less activity of CYP2D6 was in poor metabolizers >65 years compared with those <40 years (p < 0.001) | [23,165,187,188,189] |
| CYP2E1 | Isoniazid | Inhibitors—Isoniazid Inducers—Delafloxacin, Isoniazid, Rifampicin | Significant reduction of the protein levels was observed in liver pathology | [163,190,191,192] |
| CYP3A4 | Erythromycin, Linezolid, Clindamycin, Telithromycin, Clarithromycin, Azithromycin, Rifabutin, Rifapentine, Rifaximin, Grepafloxacin, Roxithromycin, Cethromycin, Clindamycin, Tetracycline, Trimethoprim, Cephalexin, Sulfadiazine, Fusidic acid, Eravacycline, Flucloxacillin | Inhibitors—Macrolides, Isoniazid, Dalfopristin, Quinupristin, Chloramphenicol, Metronidazole, Fusidic acid, Clindamycin, Ciprofloxacin, Norfloxacin, Tetracycline, Doxycycline, Sulfamethoxazole, Sulfanilamide, Rifamycin, Oritavancin Inducers—Rifabutin, Rifampicin, Rifapentine, Rifaximin, Rifamycin, Nafcillin, Oritavancin, Flucloxacillin, Dicloxacillin, Cefradine, Delafloxacin | Decrease is supposed due to 30 to 50% clearance reduction for the CYP3A4 substrates Decline of CYP3A4 activity was associated with sarcopenia in the elderly | [24,162,165,193,194,195,196,197] |
| CYP3A5 | Linezolid, Clindamycin, Clarithromycin, Telithromycin, Cethromycin, Erythromycin, Metronidazole, Clindamycin | Inhibitors—Ciprofloxacin, Erythromycin, Clarithromycin, Telithromycin, Chloramphenicol, Inducers—Rifampicin | Excessive systemic substrate exposure suggests decline of activity in the elderly | [85,165,198,199,200,201] |
| CYP3A7 | Clarithromycin, Erythromycin, Telithromycin, Metronidazole | Inhibitors—Erythromycin, Ciprofloxacin, Norfloxacin, Chloramphenicol Inducers—Rifampicin | Primarily expressed in the fetus and newborn, with relative decline with aging | [202,203,204,205,206] |
| Antibacterial Agents | Mechanism of AKI | References |
|---|---|---|
| Amoxicillin, Flucloxacillin, Piperacillin−tazobactam, Cloxacillin, Nafcillin | AIN with a proposed role of allergic inflammation | [215,216,217,218,219,220] |
| Cefazolin, Ceftriaxone, Cefepime | AIN with a proposed role of allergic inflammation | [221,222] |
| Vancomycin | Dose-dependent induction of oxidative stress, complement activation, and mitochondrial damage resulting in the acute tubular injury/necrosis or acute tubulointerstitial nephritis. New mechanism—drug-induced obstructive tubular cast formation. Acute tubulointerstitial nephritis with significant eosinophil infiltration, suggesting allergic mechanism | [216,221,223,224,225] |
| Linezolid | AIN | [223] |
| Gentamicin, amikacin | Apical transport results in the accumulation of aminoglycosides within tubular cells leading to the cell injury and death (proximal tubulopathy) due to lysosomal accumulation, inhibition of lysosomal enzymes and formation of myelin bodies. direct proximal and distal tubule cytotoxicity | [221,226] |
| Clarithromycin | Cell-mediated hypersensitivity reaction resulting in acute kidney injury and nephrotic syndrome. Drug interaction: macrolides are CYP3A4 inhibitors, their concomitant use with calcium blockers may result in excessive hypotension leading to the ischemic acute kidney injury | [215,227,228] |
| Ciprofloxacin Levofloxacin | Crystal-induced acute kidney injury, damage of the collecting duct. Urine pH more than 6.0 mediates crystal precipitation within tubular lumens | [224,229,230,231] |
| Sulfamethoxazole and trimethoprim | Intrinsic renal impairment, sulfamethoxazole urine crystal formation | [215,232,233,234] |
| Colistin | Accumulation of colistin in the proximal tubule cells, direct targeting the mitochondria | [215,235,236] |
| Drug | Volume of Distribution, Vd | Plasma Protein Binding Rate, PPB Rate | Clearance, CL | Half-Life Period, T1/2 | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Elderly Patients | Adults | Elderly Patients | Adults | Elderly Patients | Adults | Elderly Patients | Adults | |||
| Penicillins | ||||||||||
| Amoxicillin | 21 ± 9 L (mean age 82 ± 6 years) | 20 L | NA | 20% | 109 ± 72 mL/min (mean age 82 ± 6 years) | 230–280 mL/min | 2.4 ± 0.6 h (i.v.), 2.0 ± 0.41 h (capsule), 1.9 ± 0.55 h (solutab) (mean age 82 ± 6 years) | 1 h | [270,271] | |
| Ampicillin/Sulbactam | Ampicillin | 26.33 ± 8.75 L (mean age 73.9 ± 5.1 years) 19.3 ± 0.2 L (mean age 85.7 ± 7.9 years) | 31.4 ± 13.12 L (mean age 30 ± 6.5 years) 31.29 + 8.72 L (mean age 51 ± 7.3 years) | NA | 18–28% | 198.02 ± 55.60 mL/min (mean age 73.9 ± 5.1 years) 6.5 ± 4.0 L/h (mean age 85.7 ± 7.9 years) | 289.15 ± 50.52 mL/min (mean age 30 ± 6.5 years) 281.29 ± 33.64 mL/min (mean age 51 ± 7.3 years) | 1.35 ± 0.29 h (mean age 73.9 ± 5.1 years) 2.7 ± 1.6 h, (mean age 85.7 ± 7.9 years) | 0.86 ± 0.15 h (mean age 30 ± 6.5 years) 1.09 ± 0.18 h (mean age 51 ± 7.3 years) | [272,273] |
| Sulbactam | 23.54 ± 7.71 L (mean age 73.9 ± 5.1 years) 18.6 ± 6.8 L (mean age 85.7 ± 7.9 years) | 24.98 ± 4.66 L; (mean age 30 ± 6.5 years) 29.76 + 10.01 L (mean age 51 ± 7.3 years) | 38% | 162.69 ± 46.21 mL/min (mean age 73.9 ± 5.1 years) 5.6 ± 3.3 L/h (mean age 85.7 ± 7.9 years) | 254.96 ± 53.04 mL/min (mean age 30 ± 6.5 years) 236.16 ± 26.98 mL/min (mean age 51 ± 7.3 years) | 1.58 ± 0.29 h (mean age 73.9 ± 5.1 years) 3.3 ± 3.3 h (mean age 85.7 ± 7.9 years) | 0.93 ± 0.15 h (mean age 30 ± 6.5 years) 1.19 + 0.17 h (mean age 51 ± 7.3 years) | |||
| Cephalosporins | ||||||||||
| Ceftaroline | Vss 17.9 ± 3.0 L (mean age 72.2 years) | Vss 15.8 ± 2.7 L (age range 18 to 45 years) | NA | 20% | 95.7 ± 13.4 mL/min (mean age 72.2 years) | 127.3 ± 15.0 L mL/min (age: 18 to 45 years) | 3.1 ± 0.4 h (mean age 72.2 years) | 2.2 h (age: 18 to 45 years) | [274] | |
| Cefepime | Vss 0.23 ± 0.03 L/kg (mean age 67 ± 2 years, (men)) Vss 0.24 ± 0.03 L/kg (mean age 69 ± 5 years, (women)) | Vss 0.21 ± 0.02 L/kg (mean age 30 ± 6 years, men) Vss 0.21 ± 0.02 L/kg (mean age 33 ± 5 years, women) | NA | 20% | 1.11 ± 0.12 mL/min/kg (mean age 67 ± 2 years, men) 1.22 ± 0.19 mL/min/kg (mean age 69 ± 5 years, women) | 1.54 ± 0.22 mL/min/kg (mean age 30 ± 6 years, men) 1.56 ± 0.22 mL/min/kg (mean age 33 ± 5 years, women) | 3.05 ± 0.50 h (mean age 67 ± 2 years, men) 2.92 ± 0.38 h (mean age 69 ± 5 years, women) | 2.26 ± 0.51 h (mean age 30 ± 6 years, men) 2.15 ± 0.33 h (mean age 33 ± 5 years, women) | [275,276] | |
| Ceftriaxone | 0.144 ± 0.018 L/ kg (mean age 69.6 ± 5.1 years) | 8.5 ± 1.3 L (age range 19 to 40 years) | NA | 83–96% | 1.17 ± 0.29 L/h (mean age 69.6 ± 5.1 years) | 0.68 ± 0.11 L/h (age 19 to 40 years) | 6.9 ± 1.7 h (mean age 69.6 ± 5.1 years) | 8.1 ± 0.3 h age 19 to 40 years: | [277,278,279] | |
| Carbapenems | ||||||||||
| Doripenem | median value 28.4 (IQR: 15.7–37.0) L (age >60 years) | 16.8 L | NA | 8.1% | median value 19.2 (IQR: 12.8–23.9) L/h (age > 60 years) | 16.0 L/h | 1.89 h (age >60 years) | 1 h | [258,280,281] | |
| Imipenem cilastatin | Imipenem | 0.33 ± 0.09 L/kg (age 68 to 83 years) | Vc 0.16 ± 0.05 L/kg (age 19 to 34 years) | NA | 20% | 159.20 ± 48.38 mL/min/kg (age 68 to 83 years) | 12.1 ± 0.06 L/h 1.73 m2 (age 19 to 34 years) | 1.6 ± 0.72 h (age 68 to 83 years) | 0.93 ± 0.09 h (age 19 to 34 years) | [282,283,284] |
| Cilastatin | 0.26 ± 0.07 L/kg (age 68 to 83 years) | Vc 0.14 ± 0.03 L/kg (age 19 to 34 years) | 138.96 ± 81.6 mL/min/kg (age 68 to 83 years) | 12.4 ± 1.1 L/h 1.73 m2 (age 19 to 34 years) | 2.1 ± 2.14 h (age 68 to 83 years) | 0.84 ± 0.11 h (age 19 to 34 years) | ||||
| Meropenem | Vc 17.2 ± 14 L Vp 10.6 ± 13 L (median age 75 (65–94) years) 13.2 ± 1.4 L/1.73 m2 (mean age 73 ± 4.6 years) | 11.7 ± 1.2 L/1.73 m2 (mean age 28 ± 5.2 years) | NA | 2% | 5.27 L/h (median age 75 (65–94) years) 139 ± 20.0 mL/min 1.73 m2 (mean age 73 ± 4.6 years) | 15.2 L/h | 1.27 h (age 65 to 80 years) 1.27 h (mean age 73 ± 4.6 years) | 0.81 h (mean age 28 ± 5.2 years) | [259,279,285,286] | |
| Biapenem | 4.19 ± 1.58 L (mean age 78.5 ± 5.3 years) Vss (dose 300 mg) 15.2 ± 4.1 L Vss (dose 600 mg) 15.1 ± 2.7 L (mean age 71.6 ± 2.7 years) Vss (dose 300 mg) 13.7 ± 2.7 L Vss (dose 600 mg) 13.4 ± 3.1 L (mean age 77.8 ± 1.9 years) | Vss 16.4 ± 2.64 L (dose 1250 mg) 15.3 ± 4.69 L (dose 1000 mg) 22.4 ± 8.55 L (dose 250 mg) (mean age 37.9 years) | NA | 7% | 6.22 ± 1.87 L/h (mean age 78.5 ± 5.3 years) 8.8 ± 1.1 L/h (dose 300 mg), 8.9 ± 1.9 L/h (dose 600 mg), (mean age 71.6 ± 2.7 years) 6.8 ± 0.9 L/h (dose 300 mg), 6.7 ± 1.2 L/h (dose 600 mg) (mean age 77.8 ± 1.9 years) | 8.73 ± 1.99 L/h (dose 1000 mg), 14.2 ± 1.22 L/h (dose 250 mg), (average age 37.9 years) | 1.82 ± 1.14 h (dose 300 mg), 1.45 ± 0.36 h (dose 600 mg), (mean age 71.6 ± 2.7 years) 1.75 ± 0.23 h (dose 300 mg), 1.59 ± 0.18 h (dose 600 mg), (mean age 77.8 ± 1.9 years) | 1.03 ± 0.03 h (dose 750 mg), 1.31 ± 0.31 h (dose 1250 mg), (average age 37.9 years) | [287,288,289] | |
| Drug | Volume of Distribution, Vd | Plasma Protein Binding Rate, PPB Rate | Clearance, CL | Half-Life Period, T1/2 | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Elderly Patients | Adults | Elderly Patients | Adults | Elderly Patients | Adults | Elderly Patients | Adults | ||
| Vancomycin | 154 L (mean age 78.3 ± 6.96 years) 74.2 ± 32.3 L (age ≥ 60 years) | 54.20 L (median age 37 (26–49.3) years) | NA | 50% | 2.45 L/h (mean age 78.3 ± 6.96 years) 0.71 ± 0.41 mL/min/kg (age ≥ 60 years) | 7.29 L/h median age 37 (26–49.3) years: | 17.8 ± 11.8 h (age ≥ 60 years) | 4–6 h | [290,291,292,293,294] |
| Teicoplanin | Vc 78.1 (18.2) L (mean age 77.1 ± 11.4 years, men) 80.1 ± 7.0 years, women) | Vss 1.21 ± 0.56 L/kg (age range 19 to 31 years) | NA | 90–95% | 0.51 ± 3.9 L/h (mean age 77.1 ± 11.4 years, men 80.1 ± 7.0 years, women) | 0.21 ± 0.018 mL/min/kg (age range 19 to 31 years) | 106.1 h (mean age 77.1 ± 11.4 years, men 80.1 ± 7.0 years, women) | 157 ± 92.8 h (age range 19 to 31) | [295,296,297] |
| Daptomycin | Vss 0.15 L/kg (age >75 years) | Vss 0.14 L/kg | NA | 87–92% | 9.86 mL/h/kg (age >75 years) | 15.09 mL/h/kg | 11.85 h (age >75 years) | 6.79 h | [298] |
| Telavancin | Vss 156 ± 12 mL/kg (mean age 70.7 ± 5.6 years) | 157 ± 19 mL/kg | NA | 93% | 12.2 ± 1.4 mL/min/kg (mean age 70.7 ± 5.6 years) | 12 ± 2 mL/h/kg | 9.3 ± 1.3 h (mean age 70.7 ± 5.6 years) | 9.6 ± 2.9 h | [299,300] |
| Drug | Volume of Distribution, Vd | Plasma Protein Binding Rate, PPB Rate | Clearance, CL | Half-Life Period, T1/2 | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Elderly Patients | Adults | Elderly Patients | Adults | Elderly Patients | Adults | Elderly Patients | Adults | ||
| Linezolid | 0.61 ± 0.08 L/kg, (mean age 70.1 ± 3.4 years, men) 0.54 ± 0.13 L/kg, (mean age 69.9 ± 3.4 years, women) | 0.77 ± 0.25 L/kg, (mean age 29.6 ± 7.1 years, men) 0.54 ± 0.17 L/kg, (mean age 29.5 ± 6.0 years, women) | NA | 31% | CLPO 1.63 ± 0.44 mL/min/kg CLR 0.31 ± 0.06 mL/min/kg CLNR 1.31 ± 0.42 mL/min/kg, (mean age 70.1 ± 3.4 years, men) CLPO 1.30 ± 0.42 mL/min/kg CLR 0.36 ± 0.10 mL/min/kg CLNR 0.94 ± 0.47 mL/min/kg, (mean age 69.9 ± 3.4 years, women) | CLPO 1.67 ± 0.27 mL/min/kg CLR 0.44 ± 0.07 mL/min/kg CLNR 1.23 ± 0.25 mL/min/kg, (mean age 29.6 ± 7.1 years, men) CLPO 1.34 ± 0.33 mL/min/kg CLR 0.43 ± 0.09 mL/min/kg CLNR 0.91 ± 0.26 mL/min/kg, (mean age 29.5 ± 6.0 years, women) | 4.6 ± 1.3 h, (mean age 70.1 ± 3.4 years, men) 5.3 ± 2.2 h (mean age 69.9 ± 3.4 years, women) | 5.3 ± 1.7 h, (mean age 29.6 ± 7.1 years, men) 4.8 ± 1.5 h, (mean age 29.5 ± 6.0 years, women) | [301,302] |
| Tedizolid | mean age 71.9 ± 5.08 years: 91.6 ± 28.2 L | age 18 to 48 years: 95.7 ± 23.5 L | NA | 70–90% | mean age 71.9 ± 5.08 years: 5.2 ± 1.6 L/h | age 18 to 48 years: 6.08 ± 1.08 L/h | mean age 71.9 ± 5.08 years: 12.3 ± 1.3 h | age 18 to 48 years: 11 h | [303,304] |
| Drug | Volume of Distribution, Vd | Plasma Protein Binding Rate, PPB Rate | Clearance, CL | Half-Life Period, T1/2 | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Elderly Patients | Adults | Elderly Patients | Adults | Elderly Patients | Adults | Elderly Patients | Adults | ||
| Tigecycline | mean Vss 367 ± 96 L (mean age 65–75 years, women) mean Vss 499 ± 78 L (mean age 65–75 years, men) mean Vss 377 ± 123 L (mean age > 75 years, women) 401 ± 58 L (mean age > 75 years, men) | Vss 355 ± 95 L (mean age < 50 years, women) 554 ± 158 L (mean age < 50 years, men) | NA | 71–89% | 20.4 ± 4.7 L/h (mean age 65–75 years, women) 23.8 ± 4.3 L/h (mean age 65–75 years, women) 19.6 ± 3.6 L/h (mean age > 75 years, women) 18.7 ± 3.0 L/h (mean age 65–75 years, men) | <50 years: 20.6 ± 4.8 L/h (women) 28.5 ± 11.8 L/h (men) | 16.5 ± 4.1 h (mean age 65–75 years, women) 19.5 ± 3.1 h (mean age 65–75 years, men) 21.2 ± 12.5 h (mean age > 75 years, women) 19.0 ± 5.0 h (mean age > 75 years (men) | 17.1 ± 8.4 h (mean age < 50 years, women) 22.3 ± 15.3 h (mean age < 50 years, men) | [305,306] |
| Drug | Volume of Distribution, Vd | Plasma Protein Binding Rate, PPB Rate | Clearance, CL | Half-Life Period, T1/2 | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Elderly Patients | Adults | Elderly Patients | Adults | Elderly Patients | Adults | Elderly Patients | Adults | ||
| Levofloxacin | Vc 52.95 ± 21.57 L (mean age 81.2 ± 5.08 years) | Vc 106 ± 12 L | NA | 24–38% | 2.53 ± 1.46 L/h (mean age 81.2 ± 5.08 years) | 186 ± 5 mL/min | 1.47 ± 0.65 h (mean age 81.2 ± 5.08 years) | 6.91 ± 0.83 h | [261,307,308] |
| Moxifloxacin | 2.24 L/kg (age range 69 to 81 years, men) 2.12 L/kg (age range 68 to 80 years, women) | 2.60 L/kg (age range 22 to 44 years) | NA | 40–50% | 10.38 L/h (age range 69 to 81 years, men) 8.05 L/h (age range 68 to 80 years, women) | 10.61 L/h (age range 22 to 44 years) | 12.42 h (age range 69 to 81 years, men) 11.47 h (age range 68 to 80 years, women) | 13.16 h (age range 22 to 44 years) | [309] |
| Ciprofloxacin | mean Vc 49.8 L mean Vp 63.3 L (mean age 70 ± 9 years) | 2.00–3.04 L/kg | 20–40% | mean CL 17.8 L/h (mean age 70 ± 9 years) | 9.62 mL/min/kg | mean half-life 6.7 ± 4.1 h (mean age 70 ± 9 years) | 4 h | [310,311] | |
| Drug | Volume of Distribution, Vd | Plasma Protein Binding Rate, PPB Rate | Clearance, CL | Half-Life Period, T1/2 | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Elderly Patients | Adults | Elderly Patients | Adults | Elderly Patients | Adults | Elderly Patients | Adults | ||
| Amikacin | 18.0 ± 3.4 L (mean age 80.6 ± 7.3 years) 0.47 ± 0.14 L/kg (mean age 73.6 ± 9.1 years) | 0.27 ± 0.06 L/kg | NA | ≤10% | 2.25 ± 0.78 L/h (mean age 80.6 ± 7.3 years) [309] 64.7 ± 42.7 mL/min (mean age 73.6 ± 9.1 years) | 1.32 ± 0.55 mL/min/kg | 5.8 ± 2.5 h (mean age 73.6 ± 9.1 years) | 2.3 ± 0.44 h | [312,313,314,315] |
| Gentamicin | 14.8 ± 1.4 L (mean age 80.4 ± 6.4 years, frail patients) 15.2 ± 2.2 L (mean age 80.4 ± 6.4 years, non-frail) 0.37 L/kg (age 70 to 96 years) | 0.35 L/kg | NA | <20% | 46.6 ± 10.7 mL/min (mean age 80.4 ± 6.4 years frail patients) 58.2 ± 12.4 mL/min (mean age 80.4 ± 6.4 years, non-frail) 1.0 mL/min/kg (age 70 to 96 years) | 1.67 mL/min/kg | 4.1 h (age 70 to 96 years) | 2.5 h | [316,317] |
| Drug | Volume of Distribution, Vd | Plasma Protein Binding Rate, PPB Rate | Clearance, CL | Half-Life Period, T1/2 | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Elderly Patients | Adults | Elderly Patients | Adults | Elderly Patients | Adults | Elderly Patients | Adults | ||
| Polymyxin B | Vc 8.17 ± 0.67 L Vp 21.21 ± 8.28 L (age > 65 years) 0.490 ± 0.142 L/kg (age 63 to 73 years) | Vc 0.0929 L/kg Vp 0.330 L/kg | NA | 92–99% | 1.98 ± 0.67 L/h (age > 65 years) 0.028 ± 0.007 L/kg/h (age 63 to 73 years) | 2.5 L/h | 12.5 ± 3.11 h (age 63 to 73 years) | 9–11.5 h | [318,319,320,321] |
| Drug | Regimen for Patients with Different Renal Function | PK/PD Target in the Elderly | Regimen for Patients with Hepatic Impairment | Safety | References |
|---|---|---|---|---|---|
| Penicillin group | |||||
| Ampicillin/Sulbactam | mean age > 65 years: 2 g of ampicillin/ 1 g of sulbactam every 8 h (normal renal function) | 75–100 % T > MIC (MIC90 = 1 mg/L) | NA | Transient low-level elevations of ALT or AST in serum indicating transient liver damage | [322,323] |
| mean age > 75 years: 1 g of ampicillin/ 0.5 g of sulbactam every 6 h (10 ≤ CLCR < 50 mL/min) | 40% T > MIC (MIC = 8 μg/mL) | ||||
| Piperacillin/Tazobactam | mean age 85 (82–87) years: 4.5 g every 24 h (CLCR 0–19 mL/min/1.73 m2) | fCss/MIC ≥ 1 MIC ≤ 8 mg/L | 4.5 g every 4–6 h (loading dose) 4.5 g every 6 h (maintenance dose) | Plasma concentration ≥ 157.2 μg/mL—risk of neurotoxicity | [324,325,326] |
| mean age 85 (82–87) years: 9 g every 24 h (CLCR 20–39 mL/min/1.73 m2) | |||||
| mean age 85 (82–87) years: 11.25 g every 24 h (CLCR 40–59 mL/min/1.73 m2) | |||||
| mean age 85 (82–87) years: 13.5 g every 24 h (CLCR 60–79 mL/min/1.73 m2) | |||||
| Cephalosporins | |||||
| Cefepime | frail patients: 1 g every 12 h (CLCR = 30 mL/min) | fT > 50% MIC (susceptible strains) | 1–2 g every 8–12 h (loading dose) 1–2 g every 8–12 h (maintenance dose) | Plasma concentration ≥ 38.1 mg/L—risk of neurotoxicity | [325,327,328] |
| frail patients: 1 g every 8 h (CLCR 30–60 mL/min) | |||||
| frail patients: 2 g every 8 h (normal renal function) | fT > 80% MIC (susceptible strains) | ||||
| Ceftriaxone | mean age > 65 years: 1 g every 48 h (eGFRcys 10 mL/min/1.73 m2) [326] | unbound fraction of ceftriaxone >MIC (MIC = 0.5–1 mg/L) [326] | 1–2 g every 12 h (loading dose) 1–2 g every 12 h (maintenance dose) [322] | Plasma concentration ≥ 22 mg/L—risk of neurotoxicity and ceftriaxone-induced encephalopathy [327] | [329,330] |
| mean age > 65 years: 2 g every 48 h (eGFRCR-cys 40 mL/min/1.73 m2) [326] | |||||
| Ceftazidime/avibactam | age 66 years (clinical case): 0.94 g every 12 h (CLCR 30–40 mL/min) | 100% fT > 4 × MIC for ceftazidime 99% fT > 4 mg/L for avibactam (MIC = 1.5/4 mg/L) | 2.5 g every 8 h (loading dose) 2.5 g every 8 h (maintenance dose) | Concentration in cerebrospinal fluid ≥ 9.4 mg/L—risk of neurotoxicity | [325,331,332] |
| Ceftobiprole | CLCR < 50 mL/min: 0.5 g as a 2-h intravenous infusion every 12 h | 30–40% T > MIC MIC = 2 mg/L | NA | NA | [333] |
| CLCR < 30 mL/min: 0.25 g as a 2-h intravenous infusion every 12 h | |||||
| Carbapenems | |||||
| Doripenem | mean age > 60 years, mean CLCR = 53.0 mL/min: 0.5 g every 8 h [258] | 40% fT > MIC (MIC = 2 μg/mL) [258] | NA | NA | [258] |
| Ertapenem | mean age 73.1 ± 4.8 years: 1 g every 24 h (normal renal function) | AUC0-24 746.1 ± 79.4 μg·h/mL at 1 day AUC0-24 681.9 ± 47.0 μg·h/mL at 7 day | 1 g every 12 h (loading dose) 1 g every 12 h (maintenance dose) | Plasma concentration > 79.2 µg/mL—risk of neurotoxicity | [325,334,335] |
| Meropenem | mean age > 65 years, CLCR ≤ 50 mL/min: 1 g every 8 h; | 40% fT> MIC (MIC≤ 2–8 mg/L) | 2 g every 8 h (loading dose) 1 g every 8 h (maintenance dose) | Plasma concentration ≥ 64.2 μg/mL—risk of neurotoxicity Cmin ≥ 44.45 μg/mL—risk of nephrotoxicity | [259,336] |
| mean age > 65 years, CLCR > 100 mL/min: 2 g every 8 h | 40% T > MIC (MIC > 8 mg/L) | ||||
| Biapenem | mean age > 65 years: 0.3 g every 8 h | 40% T > MIC (MIC = 2 μg/mL) | NA | NA | [337] |
| Drug | Regimen for Patients with Different Renal Function | PK/PD Target in the Elderly | Regimen for Patients with Hepatic Impairment | Safety | References |
|---|---|---|---|---|---|
| Amikacin | mean age > 70 years: 1.8 g every 72 h (CLCR = 40–50 mL/min) 1.8 g every 48 h (CLCR = 60–90 mL/min) | Cmax > MIC (MIC ≤ 8 mg/L) | NA | Cmin > 4 μg/mL—risk of nephrotoxicity | [312] |
| Gentamicin | Geriatric population, CLCR > 60 mL/min: 3 mg/kg every 24 h | Cmax > MIC (MIC = 1 μg/mL) | NA | Cmin > 2 μg/mL—risk of nephrotoxicity | [338] |
| Drug | Regimen for Patients with Different Renal Function | PK/PD Target in the Elderly | Regimen for Patients with Hepatic Impairment | Safety | References |
|---|---|---|---|---|---|
| Glycopeptides | |||||
| Vancomycin | mean age ≥ 65 years: 1.0 g every 8 (CLCR > 50 mL/min) 1.0 g every 12 h (CLCR ≤ 50 mL/min) | Cmin, ss > MIC | NA | Cmin > 20 mg/L—risk of nephrotoxicity | [290] |
| Lipopeptides | |||||
| Daptomycin | eGFRcys = 20 mL/min: age 65 years: 600 mg (loading dose) 350 mg (maintenance dose) every 24 h age 75 years: 550 mg (loading dose) 300 mg (maintenance dose) every 24 h age 85 years: 500 mg (loading dose) 250 mg (maintenance dose) every 24 h age 95 years: 450 mg (loading dose) 200 mg (maintenance dose) every 24 h | (fAUCss)/MIC ≥ 66.6 | NA | Risk of toxic reactions at Cmin > 24 mg/L and Cmax > 60 mg/L | [339,340] |
| Drug | Regimen for Patients with Different Renal Function | PK/PD Target in the Elderly | Regimen for Patients with Hepatic Impairment | Safety | References |
|---|---|---|---|---|---|
| Levofloxacin | mean age 81 years: CLCR 0–19 mL/min: 125 mg every 48 h (MIC = 0.125 mg/L) 250 mg every 48 h (MIC = 0.25 mg/L) 500 mg every 48 h (MIC = 0.5 mg/L) CLCR 20–39 mL/min: 500 mg every 48 h (MIC = 0.125 mg/L) 500 mg every 48 h (MIC = 0.25 mg/L) 750 mg every 48 h (MIC = 0.5 mg/L) CLCR 40–59 mL/min: 500 mg every 48 h (MIC = 0.125 mg/L) 500 mg every 48 h (MIC = 0.25 mg/L) 500 mg every 24 h (MIC = 0.5 mg/L) CLCR 60–79 mL/min: 500 mg every 48 h (MIC = 0.125 mg/L) 750 mg every 48 h (MIC = 0.25 mg/L) 750 mg every 24 h (MIC = 0.5 mg/L) CLCR > 80 mL/min: 750 mg every 48 h (MIC = 0.125 mg/L) 750 mg every 24 h (MIC = 0.25 mg/L) 500 mg every 12 h (MIC = 0.5 mg/L) | AUC0-24/MIC ratio (≥95.7) | NA | NA | [261] |
| Moxifloxacin | No age adjustment 400 mg every 24 h per os | AUC0-24ss 46.67 µg·h/mL | NA | NA | [341] |
| Drug | Regimen for Patients with Different Renal Function | PK/PD Target in the Elderly | Regimen for Patients with Hepatic Impairment | Safety | References |
|---|---|---|---|---|---|
| Tedizolid | No age adjustment 200 mg every 24 h | fAUC/MIC (MIC ≤0.5 μg/mL) | NA | NA | [303] |
| Polymyxin B | Median age 68 years (IQR: 63–73), median CRCL 89 (IQR: 68–106) mL/min, bloodstream infection caused by carbapenem-resistant Klebsiella pneumoniae: 1.25 mg/kg every 12 h | AUC0-24ss/MIC ≥ 54.4 | NA | Risks of nephrotoxicity (manifesations may vary from proteinuria to acute kidney injury) and neurotoxicity | [319,342] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butranova, O.I.; Ushkalova, E.A.; Zyryanov, S.K.; Chenkurov, M.S.; Baybulatova, E.A. Pharmacokinetics of Antibacterial Agents in the Elderly: The Body of Evidence. Biomedicines 2023, 11, 1633. https://doi.org/10.3390/biomedicines11061633
Butranova OI, Ushkalova EA, Zyryanov SK, Chenkurov MS, Baybulatova EA. Pharmacokinetics of Antibacterial Agents in the Elderly: The Body of Evidence. Biomedicines. 2023; 11(6):1633. https://doi.org/10.3390/biomedicines11061633
Chicago/Turabian StyleButranova, Olga I., Elena A. Ushkalova, Sergey K. Zyryanov, Mikhail S. Chenkurov, and Elena A. Baybulatova. 2023. "Pharmacokinetics of Antibacterial Agents in the Elderly: The Body of Evidence" Biomedicines 11, no. 6: 1633. https://doi.org/10.3390/biomedicines11061633
APA StyleButranova, O. I., Ushkalova, E. A., Zyryanov, S. K., Chenkurov, M. S., & Baybulatova, E. A. (2023). Pharmacokinetics of Antibacterial Agents in the Elderly: The Body of Evidence. Biomedicines, 11(6), 1633. https://doi.org/10.3390/biomedicines11061633