Development of Antiepileptic Drugs throughout History: From Serendipity to Artificial Intelligence
Abstract
:1. Introduction
1.1. History of Epilepsy
1.2. Definitions
1.3. Epidemiology
1.4. Epileptogenesis
2. History of Antiepileptic Drugs and Serendipity
Serendipity: Phenobarbital and Valproic Acid
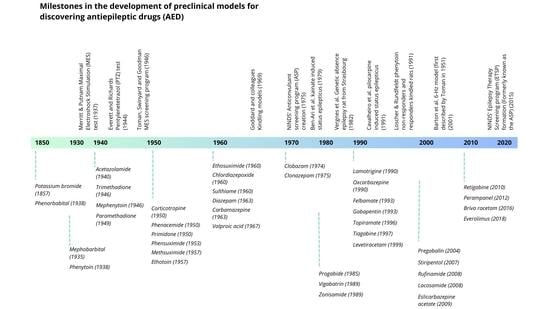
3. Preclinical Models
3.1. Maximal Electroshock Stimulation (MES) Test
3.2. Pentylenetetrazol (PTZ) Induced Test
3.3. Kindling Models
3.4. Specific Models
3.5. Contrasting Different Models

3.6. Drug Safety
4. Clinical Trials for AEDs
4.1. A New Era for the Development of Clinical Trials
4.2. Development of Second-Generation AEDs and Future Perspectives
5. Artificial Intelligence (AI) in AED Development: Challenges and Avenues
5.1. AI and Drug Design
5.2. Novel Approaches in AED Technology
6. Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bialer, M.; White, H.S. Key factors in the discovery and development of new antiepileptic drugs. Nat. Rev. Drug Discov. 2010, 9, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Brandt, C. Prevention or Modification of Epileptogenesis after Brain Insults: Experimental Approaches and Translational Research. Pharmacol. Rev. 2010, 62, 668–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker-Haliski, M.L.; Johnson, K.; Billingsley, P.; Huff, J.; Handy, L.J.; Khaleel, R.; Lu, Z.; Mau, M.J.; Pruess, T.H.; Rueda, C.; et al. Validation of a Preclinical Drug Screening Platform for Pharmacoresistant Epilepsy. Neurochem. Res. 2017, 42, 1904–1918. [Google Scholar] [CrossRef]
- Chaudhary, U.J.; Duncan, J.S.; Lemieux, L. A dialogue with historical concepts of epilepsy from the Babylonians to Hughlings Jackson: Persistent beliefs. Epilepsy Behav. 2011, 21, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Moshé, S.L. The evolution of the concepts of seizures and epilepsy: What’s in a name? Epilepsia Open 2020, 5, 22–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eadie, M.J. Epilepsy—From the Sakikku to hughlings Jackson. J. Clin. Neurosci. 1995, 2, 156–162. [Google Scholar] [CrossRef]
- Kaculini, C.M.; Tate-Looney, A.J.; Seifi, A. The History of Epilepsy: From Ancient Mystery to Modern Misconception. Cureus 2021, 13, e13953. [Google Scholar] [CrossRef]
- Fisher, R.S.; van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J. Epileptic Seizures and Epilepsy: Definitions Proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef]
- Falco-Walter, J. Epilepsy—Definition, Classification, Pathophysiology, and Epidemiology. Semin. Neurol. 2020, 40, 617–623. [Google Scholar] [CrossRef]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Fiest, K.M.; Sauro, K.M.; Wiebe, S.; Patten, S.B.; Kwon, C.-S.; Dykeman, J.; Pringsheim, T.; Lorenzetti, D.L.; Jetté, N. Prevalence and incidence of epilepsy: A Systematic Review and Meta-Analysis of International Studies. Neurology 2017, 88, 296–303. [Google Scholar] [CrossRef]
- Beghi, E.; Giussani, G.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Abraha, H.N.; Adib, M.G.; Agrawal, S.; Alahdab, F.; Awasthi, A.; et al. Global, regional, and national burden of epilepsy, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 357–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO|Epilepsy: A Public Health Imperative; WHO: Geneva, Switzerland, 2019; p. 171. [Google Scholar]
- Orozco-Hernández, J.P.; Quintero-Moreno, J.F.; Marín-Medina, D.S.; Castaño-Montoya, J.P.; Hernández-Coral, P.; Pineda, M.; Vélez, J.D.; Villada, H.C.; Martínez, J.W.; Lizcano, A. Clinical and sociodemographic profile of epilepsy in adults from a reference centre in Colombia. Neurol. (Engl. Ed.) 2019, 34, 437–444. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, M.J. Phenobarbital for the Treatment of Epilepsy in the 21st Century: A Critical Review. Epilepsia 2004, 45, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Kale, R.; Perucca, E. Revisiting phenobarbital for epilepsy: Large gaps in knowledge still exist, but we may be underestimating its clinical value. BMJ Br. Med. J. 2004, 329, 1199. [Google Scholar] [CrossRef] [PubMed]
- Nimaga, K.; Desplats, D.; Doumbo, O.; Farnarier, G. Treatment with phenobarbital and monitoring of epileptic patients in rural Mali. Bull. World Health Organ. 2002, 80, 532. [Google Scholar]
- Geronzi, U.; Lotti, F.; Grosso, S. Oxidative stress in epilepsy. Expert Rev. Neurother. 2018, 18, 427–434. [Google Scholar] [CrossRef]
- Watts, M.E.; Pocock, R.; Claudianos, C. Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease. Front. Mol. Neurosci. 2018, 11, 216. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Parsons, A.L.M.; Bucknor, E.M.V.; Castroflorio, E.; Soares, T.R.; Oliver, P.L.; Rial, D. The Interconnected Mechanisms of Oxidative Stress and Neuroinflammation in Epilepsy. Antioxidants 2022, 11, 157. [Google Scholar] [CrossRef]
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; de Curtis, M.; Perucca, P. Epilepsy. Nat. Rev. Dis. Prim. 2018, 4, 18024. [Google Scholar] [CrossRef] [PubMed]
- Patel, M. Mitochondrial dysfunction and oxidative stress: Cause and consequence of epileptic seizures. Free. Radic. Biol. Med. 2004, 37, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Guan, Q.-W.; Chen, F.-H.; Xia, Q.-X.; Yin, X.-X.; Zhou, H.-H.; Mao, X.-Y. Antioxidants Targeting Mitochondrial Oxidative Stress: Promising Neuroprotectants for Epilepsy. Oxid. Med. Cell. Longev. 2020, 2020, 6687185. [Google Scholar] [CrossRef]
- Ambrogini, P.; Torquato, P.; Bartolini, D.; Albertini, M.C.; Lattanzi, D.; Di Palma, M.; Marinelli, R.; Betti, M.; Minelli, A.; Cuppini, R.; et al. Excitotoxicity, neuroinflammation and oxidant stress as molecular bases of epileptogenesis and epilepsy-derived neurodegeneration: The role of vitamin E. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 1098–1112. [Google Scholar] [CrossRef] [PubMed]
- Mehvari, J.; Motlagh, F.G.; Najafi, M.; Ghazvini, M.R.A.; Naeini, A.A.; Zare, M. Effects of Vitamin E on seizure frequency, electroencephalogram findings, and oxidative stress status of refractory epileptic patients. Adv. Biomed. Res. 2016, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Baracaldo-Santamaría, D.; Ariza-Salamanca, D.F.; Corrales-Hernández, M.G.; Pachón-Londoño, M.J.; Hernandez-Duarte, I.; Calderon-Ospina, C.-A. Revisiting Excitotoxicity in Traumatic Brain Injury: From Bench to Bedside. Pharmaceutics 2022, 14, 152. [Google Scholar] [CrossRef]
- Schousboe, A.; Scafidi, S.; Bak, L.K.; Waagepetersen, H.S.; McKenna, M.C. Glutamate Metabolism in the Brain Focusing on Astrocytes. Adv. Neurobiol. 2014, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Swamy, M.; Yusof, W.R.W.; Sirajudeen, K.N.S.; Mustapha, Z.; Govindasamy, C. Decreased glutamine synthetase, increased citrulline–nitric oxide cycle activities, and oxidative stress in different regions of brain in epilepsy rat model. J. Physiol. Biochem. 2011, 67, 105–113. [Google Scholar] [CrossRef]
- Eid, T.; Thomas, M.J.; Spencer, D.D.; Rundén-Pran, E.; Lai, J.C.K.; Malthankar, G.V.; Kim, J.H.; Danbolt, N.C.; Ottersen, O.P.; de Lanerolle, N. Loss of glutamine synthetase in the human epileptogenic hippocampus: Possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet 2004, 363, 28–37. [Google Scholar] [CrossRef]
- Eid, T.; Williamson, A.; Lee, T.-S.W.; Petroff, O.A.; De Lanerolle, N.C. Glutamate and astrocytes-Key players in human mesial temporal lobe epilepsy? Epilepsia 2008, 49 (Suppl. 2), 42–52. [Google Scholar] [CrossRef]
- Vishnoi, S.; Raisuddin, S.; Parvez, S. Glutamate Excitotoxicity and Oxidative Stress in Epilepsy: Modulatory Role of Melatonin. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 365–374. [Google Scholar] [CrossRef]
- Valero, S.P.; García, M. Aspectos Históricos de La Epilepsia. Actividades Integradoras del Aprendizaje por Sistemas, AIAS del Sistema Nervioso; Editorial Universidad del Rosario: Bogotá, Colombia, 2017; pp. 67–76. [Google Scholar] [CrossRef]
- Palacios Sánchez, L. Abriendo La Caja Negra. Una Historia de La Neurociencia. Abriendo la caja negra. Una Hist. Neurocienc. 2020. [Google Scholar] [CrossRef]
- García-Ramos, R.; García-Pastor, A.; Masjuan, J.; Sánchez, C.; Gil, A. FEEN: Informe sociosantario FEEN sobre la epilepsia en España. Neurología 2011, 26, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Neligan, A.; Shorvon, S.D. The history of status epilepticus and its treatment. Epilepsia 2009, 50 (Suppl. 3), 56–68. [Google Scholar] [CrossRef] [PubMed]
- Yasiry, Z.; Shorvon, S.D. How phenobarbital revolutionized epilepsy therapy: The story of phenobarbital therapy in epilepsy in the last 100 years. Epilepsia 2012, 53, 26–39. [Google Scholar] [CrossRef]
- Tomson, T.; Battino, D.; Perucca, E. The remarkable story of valproic acid. Lancet Neurol. 2016, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Casper, S.T. A revisionist history of American neurology. Brain 2010, 133, 638–642. [Google Scholar] [CrossRef] [Green Version]
- Barker-Haliski, M.; White, H.S. Validated animal models for antiseizure drug (ASD) discovery: Advantages and potential pitfalls in ASD screening. Neuropharmacology 2019, 167, 107750. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Brodie, M.J.; Liew, D.; Kwan, P. Treatment Outcomes in Patients with Newly Diagnosed Epilepsy Treated with Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. JAMA Neurol. 2018, 75, 279–286. [Google Scholar] [CrossRef]
- Löscher, W. Fit for purpose application of currently existing animal models in the discovery of novel epilepsy therapies. Epilepsy Res. 2016, 126, 157–184. [Google Scholar] [CrossRef]
- Putnam, T.J.; Merritt, H.H. Experimental Determination of the Anticonvulsant Properties of Some Phenyl Derivatives. Science 1937, 85, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Barker-Haliski, M.; Harte-Hargrove, L.C.; Ravizza, T.; Smolders, I.; Xiao, B.; Brandt, C.; Löscher, W. A companion to the preclinical common data elements for pharmacologic studies in animal models of seizures and epilepsy. A Report of the TASK3 Pharmacology Working Group of the ILAE/AES Joint Translational Task Force. Epilepsia Open 2018, 3, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 2011, 20, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.; Wilcox, K.S.; White, H.S. Discovery of antiepileptic drugs. Neurotherapeutics 2007, 4, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankel, W.N.; Taylor, L.; Beyer, B.; Tempel, B.L.; White, H.S. Electroconvulsive Thresholds of Inbred Mouse Strains. Genomics 2001, 74, 306–312. [Google Scholar] [CrossRef]
- Hansen, S.L.; Sperling, B.B.; Sánchez, C. Anticonvulsant and antiepileptogenic effects of GABAA receptor ligands in pentylenetetrazole-kindled mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 105–113. [Google Scholar] [CrossRef]
- Everett, G.M.; Richards, R.K. Comparative Anticonvulsive Action of 3,5,5-trimethyloxazolidine-2,4-dione (Tridione), Dilantin and Phenobarbital. Anesthesiology 1945, 6, 448. [Google Scholar] [CrossRef]
- Goddard, G.V.; McIntyre, D.C.; Leech, C.K. A permanent change in brain function resulting from daily electrical stimulation. Exp. Neurol. 1969, 25, 295–330. [Google Scholar] [CrossRef]
- Sato, M.; Racine, R.; McIntyre, D. Kindling: Basic mechanisms and clinical validity. Electroencephalogr. Clin. Neurophysiol. 1990, 76, 459–472. [Google Scholar] [CrossRef]
- Matagne, A.; Klitgaard, H. Validation of corneally kindled mice: A sensitive screening model for partial epilepsy in man. Epilepsy Res. 1998, 31, 59–71. [Google Scholar] [CrossRef]
- Barton, M.E.; Klein, B.D.; Wolf, H.H.; White, H.S. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001, 47, 217–227. [Google Scholar] [CrossRef]
- Rho, J.M.; White, H.S. Brief history of anti-seizure drug development. Epilepsia Open 2018, 3, 114–119. [Google Scholar] [CrossRef]
- Porter, R.J.; Kupferberg, H.J. The Anticonvulsant Screening Program of the National Institute of Neurological Disorders and Stroke, NIH: History and Contributions to Clinical Care in the Twentieth Century and Beyond. Neurochem. Res. 2017, 42, 1889–1893. [Google Scholar] [CrossRef]
- Wilcox, K.S.; West, P.J.; Metcalf, C.S. The current approach of the Epilepsy Therapy Screening Program contract site for identifying improved therapies for the treatment of pharmacoresistant seizures in epilepsy. Neuropharmacology 2020, 166, 107811. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Animal Models of Seizures and Epilepsy: Past, Present, and Future Role for the Discovery of Antiseizure Drugs. Neurochem. Res. 2017, 42, 1873–1888. [Google Scholar] [CrossRef] [PubMed]
- Prior, H.; Baldrick, P.; De Haan, L.; Downes, N.; Jones, K.; Mortimer-Cassen, E.; Kimber, I. Reviewing the Utility of Two Species in General Toxicology Related to Drug Development. Int. J. Toxicol. 2018, 37, 121–124. [Google Scholar] [CrossRef]
- Hernier, A.M.; Froger-Colléaux, C.; Castagné, V. CNS safety pharmacology: A focus on cognitive functions. J. Pharmacol. Toxicol. Methods 2016, 81, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Tomson, T.; Battino, D. Teratogenic effects of antiepileptic drugs. Seizure 2008, 17, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Westall, C.A.; Wright, T.; Cortese, F.; Kumarappah, A.; Snead, O.C.; Buncic, J.R. Vigabatrin retinal toxicity in children with infantile spasms: An observational cohort study. Neurology 2014, 83, 2262–2268. [Google Scholar] [CrossRef]
- Iamsaard, S.; Sukhorum, W.; Arun, S.; Phunchago, N.; Uabundit, N.; Boonruangsri, P.; Namking, M. Valproic acid induces histologic changes and decreases androgen receptor levels of testis and epididymis in rats. Int. J. Reprod. Biomed. 2017, 15, 217. [Google Scholar] [CrossRef] [Green Version]
- Barton, A. Handbook for good clinical research practice (GCP): Guidance for implementation. J. Epidemiol. Community Health 2007, 61, 559. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, A. Evolution of Clinical Research: A History Before and Beyond James Lind. Perspect. Clin. Res. 2010, 1, 6. [Google Scholar] [PubMed]
- Merritt, H.H.; Putnam, T.J. Landmark article 17 September 1938: Sodium diphenyl hydantoinate in the treatment of convulsive disorders. By H. Houston Merritt and Tracy J. Putnam. JAMA 1984, 251, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, W.J. Putnam, Merritt, and the Discovery of Dilantin. Epilepsia 1986, 27 (Suppl. 3), S1–S20. [Google Scholar] [CrossRef]
- Coatsworth, J. Studies on the Clinical Efficacy of Marketed Antiepileptic Drugs; National Institut of Health: Bethesda, MD, USA, 1971.
- Perucca, E. What clinical trial designs have been used to test antiepileptic drugs and do we need to change them? Epileptic Disord. 2012, 14, 124–131. [Google Scholar] [CrossRef]
- Perucca, E. Antiepileptic drugs: Evolution of our knowledge and changes in drug trials. Epileptic Disord. 2019, 21, 319–329. [Google Scholar]
- Greene, J.A.; Podolsky, S.H. Reform, Regulation, and Pharmaceuticals—The Kefauver–Harris Amendments at 50. N. Engl. J. Med. 2012, 367, 1481–1483. [Google Scholar] [CrossRef] [Green Version]
- White, P.T.; Plott, D.; Norton, J. Relative Anticonvulsant Potency of Primidone; a Double Blind Comparison. Arch. Neurol. 1966, 14, 31–35. [Google Scholar] [CrossRef]
- Chung, A.; Eiland, L.S. Use of Second-Generation Antiepileptic Drugs in the Pediatric Population. Pediatr. Drugs 2008, 10, 217–254. [Google Scholar] [CrossRef]
- Shorvon, S.D. Drug treatment of epilepsy in the century of the ILAE: The second 50 years, 1959–2009. Epilepsia 2009, 50, 93–130. [Google Scholar] [CrossRef] [Green Version]
- Bialer, M.; Johannessen, S.; Kupferberg, H.; Levy, R.; Loiseau, P.; Perucca, E. Progress report on new antiepileptic drugs: A summary of the Third Eilat Conference. Epilepsy Res. 1996, 25, 299–319. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E.; Brodie, M.J.; Kwan, P.; Tomson, T. 30 years of second-generation antiseizure medications: Impact and future perspectives. Lancet Neurol. 2020, 19, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Ravizza, T.; Vezzani, A. Pharmacological targeting of brain inflammation in epilepsy: Therapeutic perspectives from experimental and clinical studies. Epilepsia Open 2018, 3, 133–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowden, H.; Munro, J. Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov. 2019, 18, 495–496. [Google Scholar] [CrossRef]
- Mallappallil, M.; Sabu, J.; Gruessner, A.; Salifu, M. A review of big data and medical research. SAGE Open Med. 2020, 8, 205031212093483. [Google Scholar] [CrossRef]
- Krenn, M.; Pollice, R.; Guo, S.Y.; Aldeghi, M.; Cervera-Lierta, A.; Friederich, P.; dos Passos Gomes, G.; Häse, F.; Jinich, A.; Nigam, A.; et al. On scientific understanding with artificial intelligence. Nat. Rev. Phys. 2022, 4, 761–769. [Google Scholar] [CrossRef]
- Schneider, P.; Walters, W.P.; Plowright, A.T.; Sieroka, N.; Listgarten, J.; Goodnow, R.A.G.; Fisher, J.; Jansen, J.M.; Duca, J.S.; Rush, T.S.; et al. Rethinking drug design in the artificial intelligence era. Nat. Rev. Drug Discov. 2020, 19, 353–364. [Google Scholar] [CrossRef]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthcar. J. 2019, 6, 94–98. [Google Scholar] [CrossRef] [Green Version]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Patel, L.; Shukla, T.; Huang, X.; Ussery, D.W.; Wang, S. Machine Learning Methods in Drug Discovery. Molecules 2020, 25, 5277. [Google Scholar] [CrossRef] [PubMed]
- Dara, S.; Dhamercherla, S.; Jadav, S.S.; Babu, C.M.; Ahsan, M.J. Machine Learning in Drug Discovery: A Review. Artif. Intell. Rev. 2021, 55, 1947–1999. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 2021, 25, 1315–1360. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.J.; Lwin, C.T.; Durrant, J.D. LigGrep: A tool for filtering docked poses to improve virtual-screening hit rates. J. Cheminform. 2020, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Ke, Y.; Lu, Y.; Du, Y.; Li, J.; Yan, H.; Zhao, H.; Zhou, Y.; Yang, Y. DLIGAND2: An improved knowledge-based energy function for protein–ligand interactions using the distance-scaled, finite, ideal-gas reference state. J. Cheminform. 2019, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Gattani, S.; Mishra, A.; Hoque, T. StackCBPred: A stacking based prediction of protein-carbohydrate binding sites from sequence. Carbohydr. Res. 2019, 486, 107857. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Yang, Y.; Gu, Q.; Zhou, H.; Du, Y.; Lu, Y.; Liao, J.; Xu, J. LSA: A local-weighted structural alignment tool for pharmaceutical virtual screening. RSC Adv. 2019, 9, 3912–3917. [Google Scholar] [CrossRef] [Green Version]
- Seifert, M.H.J. ProPose: Steered Virtual Screening by Simultaneous Protein−Ligand Docking and Ligand−Ligand Alignment. J. Chem. Inf. Model. 2005, 45, 449–460. [Google Scholar] [CrossRef]
- Schellhammer, I.; Rarey, M. TrixX: Structure-based molecule indexing for large-scale virtual screening in sublinear time. J. Comput. Aided. Mol. Des. 2007, 21, 223–238. [Google Scholar] [CrossRef]
- Rifaioglu, A.S.; Nalbat, E.; Atalay, V.; Martin, M.J.; Cetin-Atalay, R.; Doğan, T. DEEPScreen: High performance drug–target interaction prediction with convolutional neural networks using 2-D structural compound representations. Chem. Sci. 2020, 11, 2531–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Cao, D.-S.; Miao, H.-Y.; Liu, S.; Deng, B.-C.; Yun, Y.-H.; Wang, N.-N.; Lu, A.-P.; Zeng, W.-B.; Chen, A.F. ChemDes: An integrated web-based platform for molecular descriptor and fingerprint computation. J. Cheminform. 2015, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Oldenhof, M.; Arany, A.; Moreau, Y.; Simm, J. ChemGrapher: Optical Graph Recognition of Chemical Compounds by Deep Learning. J. Chem. Inf. Model. 2020, 60, 4506–4517. [Google Scholar] [CrossRef] [PubMed]
- Buyukbingol, E.; Sisman, A.; Akyildiz, M.; Alparslan, F.N.; Adejare, A. Adaptive neuro-fuzzy inference system (ANFIS): A new approach to predictive modeling in QSAR applications: A study of neuro-fuzzy modeling of PCP-based NMDA receptor antagonists. Bioorg. Med. Chem. 2007, 15, 4265–4282. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.; Navarro, C.; Cano, C.; Fajardo, W.; Blanco, A. DrugNet: Network-based drug–disease prioritization by integrating heterogeneous data. Artif. Intell. Med. 2015, 63, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Fahimian, G.; Zahiri, J.; Arab, S.S.; Sajedi, R.H. RepCOOL: Computational drug repositioning via integrating heterogeneous biological networks. J. Transl. Med. 2020, 18, 375. [Google Scholar] [CrossRef]
- Jiang, H.-J.; Huang, Y.-A.; You, Z.-H. Predicting Drug-Disease Associations via Using Gaussian Interaction Profile and Kernel-Based Autoencoder. BioMed Res. Int. 2019, 2019, 2426958. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Cui, C.; Qi, L.; Yan, H.; Zhao, X.-M. DrPOCS: Drug Repositioning Based on Projection onto Convex Sets. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018, 16, 154–162. [Google Scholar] [CrossRef]
- Sadeghi, S.S.; Keyvanpour, M. RCDR: A Recommender Based Method for Computational Drug Repurposing. In Proceedings of the 2019 5th Conference on Knowledge Based Engineering and Innovation (KBEI), Tehran, Iran, 28 February–1 March 2019; pp. 467–471. [Google Scholar] [CrossRef]
- Capuzzi, S.J.; Kim, I.S.-J.; Lam, W.I.; Thornton, T.E.; Muratov, E.N.; Pozefsky, D.; Tropsha, A. Chembench: A Publicly Accessible, Integrated Cheminformatics Portal. J. Chem. Inf. Model. 2017, 57, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. MCSM-Lig: Quantifying the Effects of Mutations on Protein-Small Molecule Affinity in Genetic Disease and Emergence of Drug Resistance. Sci. Rep. 2016, 6, 29575. [Google Scholar] [CrossRef] [Green Version]
- Kaminskas, L.M.; Pires, D.E.V.; Ascher, D.B. DendPoint: A Web Resource for Dendrimer Pharmacokinetics Investigation and Prediction. Sci. Rep. 2019, 9, 15465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, P.; Eckert, O.A.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- de la Fuente-Nunez, C. Antibiotic discovery with machine learning. Nat. Biotechnol. 2022, 40, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Butlen-Ducuing, F.; Pétavy, F.; Guizzaro, L.; Zienowicz, M.; Haas, M.; Alteri, E.; Salmonson, T.; Corruble, E. Regulatory watch: Challenges in drug development for central nervous system disorders: A European Medicines Agency perspective. Nat. Rev. Drug Discov. 2016, 15, 813–814. [Google Scholar] [CrossRef]
- Löscher, W.; Schmidt, D. Modern antiepileptic drug development has failed to deliver: Ways out of the current dilemma. Epilepsia 2011, 52, 657–678. [Google Scholar] [CrossRef]
- Bruxel, E.M.; Bruno, D.C.; do Canto, A.M.; Geraldis, J.C.; Godoi, A.B.; Martin, M.; Lopes-Cendes, I. Multi-omics in mesial temporal lobe epilepsy with hippocampal sclerosis: Clues into the underlying mechanisms leading to disease. Seizure 2021, 90, 34–50. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Q.; Ji, X.; Zhang, Y.; Li, W.; Peng, S.; Xue, F. Machine Learning Applications in Drug Repurposing. Interdiscip. Sci. 2022, 14, 15–21. [Google Scholar] [CrossRef]
- Brueggeman, L.; Sturgeon, M.L.; Martin, R.M.; Grossbach, A.J.; Nagahama, Y.; Zhang, A.; Howard, M.A.; Kawasaki, H.; Wu, S.; Cornell, R.A.; et al. Drug repositioning in epilepsy reveals novel antiseizure candidates. Ann. Clin. Transl. Neurol. 2019, 6, 295–309. [Google Scholar] [CrossRef]
- Ko, Y.; Lee, C.; Lee, Y.; Lee, J.-S. Systematic Approach for Drug Repositioning of Anti-Epileptic Drugs. Diagnostics 2019, 9, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, L.; Cai, M.; Chen, Y.; Shen, C.; Shi, L.; Guo, Y. Prediction of antiepileptic drug treatment outcomes of patients with newly diagnosed epilepsy by machine learning. Epilepsy Behav. 2019, 96, 92–97. [Google Scholar] [CrossRef]
- Chiang, S.; Rao, V.R. Choosing the Best Antiseizure Medication—Can Artificial Intelligence Help? JAMA Neurol. 2022, 79, 970–972. [Google Scholar] [CrossRef]
- Abbasi, B.; Goldenholz, D.M. Machine learning applications in epilepsy. Epilepsia 2019, 60, 2037–2047. [Google Scholar] [CrossRef]
- Day, M.; Rutkowski, J.L.; Feuerstein, G.Z. Translational Medicine—A Paradigm Shift in Modern Drug Discovery and Development: The Role of Biomarkers. Adv. Exp. Med. Biol. 2009, 655, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; de Luca, V.; Kostikova, A.; Laramie, J.; Kennedy, S.; Ferrero, E.; Siegel, R.; Fink, M.; Ahmed, S.; Millholland, J.; et al. Translational precision medicine: An industry perspective. J. Transl. Med. 2021, 19, 245. [Google Scholar] [CrossRef] [PubMed]
- Nabbout, R.; Kuchenbuch, M. Impact of predictive, preventive and precision medicine strategies in epilepsy. Nat. Rev. Neurol. 2020, 16, 674–688. [Google Scholar] [CrossRef]
- Beltrán-Corbellini, Á.; Aledo-Serrano, Á.; Møller, R.S.; Pérez-Palma, E.; García-Morales, I.; Toledano, R.; Gil-Nagel, A. Epilepsy Genetics and Precision Medicine in Adults: A New Landscape for Developmental and Epileptic Encephalopathies. Front. Neurol. 2022, 13, 777115. [Google Scholar] [CrossRef] [PubMed]
- Brogi, S.; Calderone, V. Artificial Intelligence in Translational Medicine. Int. J. Transl. Med. 2021, 1, 223–285. [Google Scholar] [CrossRef]


| Animal Model | Species | Seizure Phenotype | Human Correlate | Clinical Validation | Predictive of AED Toxicity | AEDs Showing Efficacy | Mechanism of Action Identified |
|---|---|---|---|---|---|---|---|
| Maximal electroshock stimulation (MES) | Mice/rats | Tonic extension seizure | Generalized tonic–clonic seizures. | Identification and development of phenytoin. | No |
|
|
| Subcutaneous pentylenetetrazol (scPTZ) | Mice/rats | Minimal clonic seizure | Generalized myoclonic seizures and spike–wave seizures. | Discovery of trimethadione, phensuximide, and ethosuximide. | No |
|
|
| Kindling models | Rats | Limbic seizures | Partial seizures. | Only model to correctly identify antiseizure activity of levetiracetam. | Yes |
|
|
| Tool and Software | Method | Features | Performance Metric | Objective |
|---|---|---|---|---|
| LS-align: Algorithm evaluating ligand structural alignment [87]. | Machine learning | Generates atom-level structural alignments of ligand molecules. | AUC | Structure–ligand identification |
| Input: query structure, template structure, initial alignment. Output: final alignment, final alignment score. | ||||
| LigGrep: Tool identifying docked poses in specified receptor/ligand interactions [88]. | Machine learning | Prioritizes candidate small molecule ligands using computer docking. | AUROC and pAUROC * | |
| Input: docked-compound files for drug target receptor and candidate ligands. Output: names of candidate compounds with poses that satisfy all user-defined filters. | ||||
| AutoGrow4 Generates novel drug-like molecules and optimizes pre-existing ligands [88]. | Genetic algorithm | Using a genetic algorithm draws on a population of seed molecules to create a new population of potential ligands. Ranks the candidates by calculated fitness. | Docking score (NNScore1, NNScore2), ligand efficiency, diversity score. | |
| Input: first generation of independent seed pools formed from high-scoring compounds and diverse compounds. Output: last generation of selected seeds ranked by fitness scores. | ||||
| DLIGAND2 [89] | Distance scaled | Predicts protein–ligand binding affinity based on a distance-scaled, finite, ideal gas reference (DFIRE) state. | AUC, EF | |
| Input: residue-specific types for protein atoms and a large protein structural dataset for training. Output: binding affinity prediction using either native or docking-predicted complex structures. | ||||
| StackCBPred [90] | Machine learning | Predicts structural properties of amino acids to effectively train a stacking-based machine learning method for the accurate prediction of protein–carbohydrate binding sites. | AUC ROC ACC F1 score | |
| Input: protein sequence. Output: predictors of protein–carbohydrate binding sites. | ||||
| LSA [91] | Machine learning | Computes the similarity of two molecular structures by considering the contributions of both overall similarity and local substructure match. | AUC | |
| Input: three-dimensional molecular structures with substructure focus; computing the similarity score based on superimposing. Output: similarity of two molecular structures by considering the contributions of both overall similarity and local substructure match. | ||||
| ProPose [92] | Incremental construction algorithm | The combination of ligand- and receptor-based methods steers the virtual screening by ranking molecules according to the similarity of their interaction pattern with known ligands. | N/A | |
| Result: energy torsion angle for incremental molecule construction within an active site of a selected receptor. | ||||
| TrixX [93] | Machine learning | Structure-based molecule indexing for large-scale virtual screening in sublinear time is among the fastest virtual screening tools currently available. | Enrichment behavior | |
| Input: compounds, parameters, receptors. Output: compound placement score. | ||||
| DEEPScreen [94] | Convolutional neural networks | High-performance drug target interaction prediction. Used in the fields of drug discovery and repurposing for in silico screening of chemogenomic space. | F1 score, MCC | |
| Input: 2D images of compounds Output: binary classification | ||||
| QSAR modeling | Structure and biological activity relation | |||
| ChemDes [95] | Pybel, CDK, RDKit, BlueDesc, Chemopy, PaDEL, and jCompoundMapper | An integrated web-based platform for the calculation of molecular descriptors and fingerprint computation. | AUC | |
| Input: molecules. Output: QSAR, virtual screening, ranking, ADME/T prediction. | ||||
| ChemGrapher [96] | Deep learning | Optical graph recognition of chemical compounds. Produces all information necessary to relate each component of the resulting graph to the source image. | AUC | |
| Input: image. Output: molecular graph structure. | ||||
| ANFIS [97] | Neuro-fuzzy modeling and principal component analysis | Evaluates physicochemical descriptors of certain chemical compounds for their appropriate biological activities in terms of QSAR models with the aid of an artificial neural network (ANN) approach combined with the principle of fuzzy logic. | AUC | |
| Input: fuzzy linear regression. Output: adaptive neuro-fuzzy inference (ANFIS). | ||||
| DrugNet [98] | Machine learning | Simultaneous integration of information about diseases, drugs, and targets can lead to a significant improvement in drug repositioning. | AUC | |
| Input: drugs. Output: repositioning of drugs with ranked lists for a given disease. | ||||
| RepCOOL [99] | Random forest classifier | The potency of the proposed method is in detecting true drug–disease relationships. | AUC and ROC | |
| Input: extracting primary data. Output: suggested new drug. | ||||
| GIPAE [100] | Gaussian interaction profile kernel and autoencoder | Computational drug repositioning is designed to identify new indications for existing drugs. The batch normalization layer and the full-connected layer are introduced to reduce training complexity. | AUC and ROC, F1 score | |
| Input: drug and disease Gaussian interaction. Output: drug and disease association prediction. | ||||
| DrPOCS [101] | Machine learning | Predicts potential associations between drugs and diseases with matrix completion. | AUC, F1 score | |
| Input: drug, disease. Output: association prediction. | ||||
| RCDR [102] | Collaborative filtering model | Prioritizes candidate drugs for diseases. | AUC and ROC | |
| Input: drug set, disease set, drug–disease association. Output: predicted association matrix. | ||||
| Chembench [103] | Quantitative structure–activity relationship (QSAR) modeling methods | Tools and services for computer-assisted drug design and computational toxicology. | Correct classification rate, accuracy, negative predictive value, positive predictive value | Physicochemical properties |
| Input: standardized chemical compounds. Output: dataset visualization, modeling, model validation, virtual screening. | ||||
| mCSM-lig [104] | Machine learning models, Platinum database | Effective in predicting a range of chemotherapeutic, antiviral, and antibiotic resistance mutations, providing useful insights for genotypic screening and guiding drug development. | AUC and ROC, precision, accuracy | |
| Input: mutation in protein–ligand complexes. Output: mCSM—lig signature. | ||||
| DendPoint [105] | Machine learning and principal component analysis | Used to guide dendrimer construct design and refinement before embarking on more time-consuming and expensive in vivo testing. | AUC and ROC | |
| Input: pharmacokinetic parameters. Output: predictive values (half-life, clearance, %Dose urine, %Dose liver). | ||||
| ProTOX-II [106] | Molecular similarity, fragment propensities, and machine learning | Webserver for the prediction of toxicity of chemicals. Predicts acute toxicity, hepatotoxicity, cytotoxicity, carcinogenicity, mutagenicity, and immunotoxicity. | AUC, balanced accuracy, Kappa index | Mode of action and toxicity |
| Input: SMILES string, drawing of the chemical structure, compound name (pubchem). Output: median lethal dose, toxicity class, average similarity with three most similar toxic compounds. | ||||
| ADMETlab [107] | Designed based on the Django framework in Python | Early drug-likeness evaluation, rapid ADMET virtual screening or filtering, and prioritization of chemical structures. | ACC, SP, SE, AUC, and ROC | |
| Input: SMILES string, drawing of the chemical structure. Output: ADMET profile. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrales-Hernández, M.G.; Villarroel-Hagemann, S.K.; Mendoza-Rodelo, I.E.; Palacios-Sánchez, L.; Gaviria-Carrillo, M.; Buitrago-Ricaurte, N.; Espinosa-Lugo, S.; Calderon-Ospina, C.-A.; Rodríguez-Quintana, J.H. Development of Antiepileptic Drugs throughout History: From Serendipity to Artificial Intelligence. Biomedicines 2023, 11, 1632. https://doi.org/10.3390/biomedicines11061632
Corrales-Hernández MG, Villarroel-Hagemann SK, Mendoza-Rodelo IE, Palacios-Sánchez L, Gaviria-Carrillo M, Buitrago-Ricaurte N, Espinosa-Lugo S, Calderon-Ospina C-A, Rodríguez-Quintana JH. Development of Antiepileptic Drugs throughout History: From Serendipity to Artificial Intelligence. Biomedicines. 2023; 11(6):1632. https://doi.org/10.3390/biomedicines11061632
Chicago/Turabian StyleCorrales-Hernández, María Gabriela, Sebastián Kurt Villarroel-Hagemann, Isabella Esther Mendoza-Rodelo, Leonardo Palacios-Sánchez, Mariana Gaviria-Carrillo, Natalia Buitrago-Ricaurte, Santiago Espinosa-Lugo, Carlos-Alberto Calderon-Ospina, and Jesús Hernán Rodríguez-Quintana. 2023. "Development of Antiepileptic Drugs throughout History: From Serendipity to Artificial Intelligence" Biomedicines 11, no. 6: 1632. https://doi.org/10.3390/biomedicines11061632
APA StyleCorrales-Hernández, M. G., Villarroel-Hagemann, S. K., Mendoza-Rodelo, I. E., Palacios-Sánchez, L., Gaviria-Carrillo, M., Buitrago-Ricaurte, N., Espinosa-Lugo, S., Calderon-Ospina, C. -A., & Rodríguez-Quintana, J. H. (2023). Development of Antiepileptic Drugs throughout History: From Serendipity to Artificial Intelligence. Biomedicines, 11(6), 1632. https://doi.org/10.3390/biomedicines11061632