In Vitro Activity of Eravacycline against Carbapenemase-Producing Gram-Negative Bacilli Clinical Isolates in Central Poland
Abstract
:1. Introduction
2. Materials and Methods
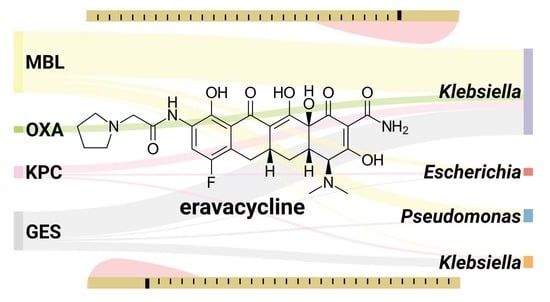
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhanel, G.G.; Cheung, D.; Adam, H.; Zelenitsky, S.; Golden, A.; Schweizer, F.; Gorityala, B.; Lagacé-Wiens, P.R.; Walkty, A.; Gin, A.S.; et al. Review of Eravacycline, a Novel Fluorocycline Antibacterial Agent. Drugs 2016, 76, 567–588. [Google Scholar] [CrossRef]
- XERAVA (Eravacycline) for Injection, for Intravenous Use—Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211109lbl.pdf (accessed on 6 May 2023).
- EUCAST Breakpoint Tables for Interpretation of MICs and Zone Diameters, Ver. 13.0. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_13.0_Breakpoint_Tables.pdf (accessed on 26 April 2023).
- Brauncajs, M.; Bielec, F.; Macieja, A.; Pastuszak-Lewandoska, D. Carbapenem-Resistant Gram-Negative Fermenting and Non-Fermenting Rods Isolated from Hospital Patients in Poland-What Are They Susceptible to? Biomedicines 2022, 10, 3049. [Google Scholar] [CrossRef]
- Connors, K.P.; Housman, S.T.; Pope, J.S.; Russomanno, J.; Salerno, E.; Shore, E.; Redican, S.; Nicolau, D.P. Phase I, open-label, safety and pharmacokinetic study to assess bronchopulmonary disposition of intravenous eravacycline in healthy men and women. Antimicrob. Agents Chemother. 2014, 58, 2113–2118. [Google Scholar] [CrossRef] [Green Version]
- Seifert, H.; Stefanik, D.; Sutcliffe, J.A.; Higgins, P.G. In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int. J. Antimicrob. Agents 2018, 51, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Baxter, M.R.; Adam, H.J.; Sutcliffe, J.; Karlowsky, J.A. In vitro activity of eravacycline against 2213 Gram-negative and 2424 Gram-positive bacterial pathogens isolated in Canadian hospital laboratories: CANWARD surveillance study 2014-2015. Diagn. Microbiol. Infect. Dis. 2018, 91, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M.; Mushtaq, S.; Warner, M.; Woodford, N. In Vitro Activity of Eravacycline against Carbapenem-Resistant Enterobacteriaceae and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 3840–3844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, L.J. Eravacycline: A Review in Complicated Intra-Abdominal Infections. Drugs 2019, 79, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalhoff, A. Global fluoroquinolone resistance epidemiology and implictions for clinical use. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 976273. [Google Scholar] [CrossRef] [Green Version]
- Okuniewicz, R.; Moos, Ł.; Brzoza, Z. Small Intestinal Bacterial Overgrowth Syndrome. Postepy Mikrobiol. 2021, 60, 203–210. [Google Scholar] [CrossRef]
- Tang, K.; Zhao, H. Quinolone Antibiotics: Resistance and Therapy. Infect. Drug Resist. 2023, 16, 811–820. [Google Scholar] [CrossRef]
- Efficacy and Safety Study of Eravacycline Compared with Levofloxacin in Complicated Urinary Tract Infections. Available online: https://clinicaltrials.gov/ct2/show/record/NCT01978938 (accessed on 26 April 2023).
- Grossman, T.H.; O’Brien, W.; Kerstein, K.O.; Sutcliffe, J.A. Eravacycline (TP-434) is active in vitro against biofilms formed by uropathogenic Escherichia coli. Antimicrob. Agents Chemother. 2015, 59, 2446–2449. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Jin, S.; Chen, L.; Li, J.; Zhang, X.; Zhou, H.; Li, X.; Huang, H. Antibacterial Activity of Eravacycline Against Carbapenem-Resistant Gram-Negative Isolates in China: An in vitro Study. Infect. Drug Resist. 2023, 16, 2271–2279. [Google Scholar] [CrossRef]
- Morrissey, I.; Olesky, M.; Hawser, S.; Lob, S.H.; Karlowsky, J.A.; Corey, G.R.; Bassetti, M.; Fyfe, C. In Vitro Activity of Eravacycline against Gram-Negative Bacilli Isolated in Clinical Laboratories Worldwide from 2013 to 2017. Antimicrob. Agents Chemother. 2020, 64, e01699-19. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, M.; Olafisoye, O.; Cortes, C.; Urban, C.; Landman, D.; Quale, J. Activity of eravacycline against Enterobacteriaceae and Acinetobacter baumannii, including multidrug-resistant isolates, from New York City. Antimicrob. Agents Chemother. 2015, 59, 1802–1805. [Google Scholar] [CrossRef] [Green Version]
- Sutcliffe, J.A.; O’Brien, W.; Fyfe, C.; Grossman, T.H. Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob. Agents Chemother. 2013, 57, 5548–5558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lin, X.; Bush, K. In vitro susceptibility of β-lactamase-producing carbapenem-resistant Enterobacteriaceae (CRE) to eravacycline. J. Antibiot. 2016, 69, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Gilchrist, M.; Heard, K.; Hamilton, R.; Sneddon, J. Treating infections caused by carbapenemase-producing Enterobacterales (CPE): A pragmatic approach to antimicrobial stewardship on behalf of the UKCPA Pharmacy Infection Network (PIN). JAC Antimicrob. Resist. 2020, 2, dlaa075. [Google Scholar] [CrossRef]
- Walsh, T.R.; Toleman, M.A.; Poirel, L.; Nordmann, P. Metallo-beta-lactamases: The quiet before the storm? Clin. Microbiol. Rev. 2005, 18, 306–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, S.E.; Livermore, D.M.; Hooper, D.C.; Hope, W.W. Metallo-β-Lactamases: Structure, Function, Epidemiology, Treatment Options, and the Development Pipeline. Antimicrob. Agents Chemother. 2020, 64, e00397-20. [Google Scholar] [CrossRef]
- McCreary, E.K.; Heil, E.L.; Tamma, P.D. New Perspectives on Antimicrobial Agents: Cefiderocol. Antimicrob. Agents Chemother. 2021, 65, e0217120. [Google Scholar] [CrossRef]
- Syed, Y.Y. Cefiderocol: A Review in Serious Gram-Negative Bacterial Infections. Drugs 2021, 81, 1559–1571. [Google Scholar] [CrossRef] [PubMed]



| Organism/Organisms’ Group Name | Breakpoint (S≤; R>) [mg/L] |
|---|---|
| Escherichia coli | 0.5 |
| Staphylococcus aureus | 0.25 |
| Enterococcus spp.; Viridans group streptococci | 0.125 |
| other Enterobacterales; other Staphylococcus spp.; other Streptococcus spp.; Acinetobacter spp.; Haemophilus influenzae; Neisseria spp.; Moraxella catarrhalis | Insufficient evidence that the organism or group is a good target for therapy with the agent. |
| Pseudomonas spp. | No breakpoints. Susceptibility testing is not recommended. |
| Resistance Mechanism | N | MIC50 [mg/L] | MIC90 [mg/L] | MIC Range [mg/L] |
|---|---|---|---|---|
| CIM | 26 | 0.75 | 32 | 0.047→32 |
| MBL | 58 | 0.38 | 32 | 0.047→32 |
| OXA-48 | 6 | 0.5 | 3 | 0.19–3 |
| KPC | 11 | 0.38 | 1 | 0.125–2 |
| GES | 35 | 0.38 | 2 | 0.047→32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brauncajs, M.; Bielec, F.; Macieja, A.; Pastuszak-Lewandoska, D. In Vitro Activity of Eravacycline against Carbapenemase-Producing Gram-Negative Bacilli Clinical Isolates in Central Poland. Biomedicines 2023, 11, 1784. https://doi.org/10.3390/biomedicines11071784
Brauncajs M, Bielec F, Macieja A, Pastuszak-Lewandoska D. In Vitro Activity of Eravacycline against Carbapenemase-Producing Gram-Negative Bacilli Clinical Isolates in Central Poland. Biomedicines. 2023; 11(7):1784. https://doi.org/10.3390/biomedicines11071784
Chicago/Turabian StyleBrauncajs, Małgorzata, Filip Bielec, Anna Macieja, and Dorota Pastuszak-Lewandoska. 2023. "In Vitro Activity of Eravacycline against Carbapenemase-Producing Gram-Negative Bacilli Clinical Isolates in Central Poland" Biomedicines 11, no. 7: 1784. https://doi.org/10.3390/biomedicines11071784
APA StyleBrauncajs, M., Bielec, F., Macieja, A., & Pastuszak-Lewandoska, D. (2023). In Vitro Activity of Eravacycline against Carbapenemase-Producing Gram-Negative Bacilli Clinical Isolates in Central Poland. Biomedicines, 11(7), 1784. https://doi.org/10.3390/biomedicines11071784