Evaluation and Application of Silk Fibroin Based Biomaterials to Promote Cartilage Regeneration in Osteoarthritis Therapy
Abstract
:1. Introduction
2. Biological Properties of SF
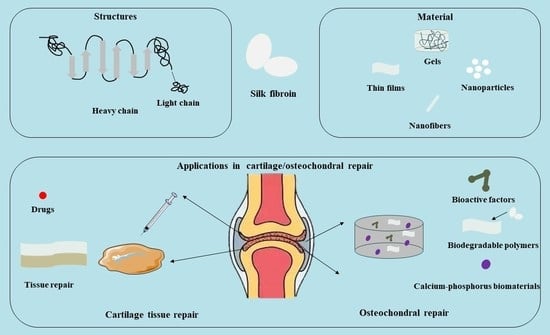
2.1. Structure of SF
2.2. Properties of SF
2.3. Preparation Method of SF-Based Biomaterials
2.4. The Main Types of SF-Based Biomaterials
3. Osteoarthritic Articular Cartilage Model
4. The Role of SF in Bone/Cartilage Damage
4.1. The Role of SF in Bone Tissue
4.1.1. Bioactive Factor-Based Biomaterials
4.1.2. Biodegradable Polymer-Based Biomaterials
4.1.3. Calcium- and Phosphorus-Based Biomaterials
4.2. The Role of SF in Cartilage Tissue
4.2.1. Application of SFs in Cartilage Tissue Engineering
4.2.2. SF in the Treatment of Patients with OA
4.2.3. Application of SFs in Drug Delivery
4.3. Prospects for SF in Clinical Applications
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiao, K.; Xu, L.; Tang, J.; Wang, Q.; Lim, K.S.; Hooper, G.; Woodfield, T.B.F.; Liu, G.; Tian, K.; Zhang, W.; et al. The advances in nanomedicine for bone and cartilage repair. J. Nanobiotechnol. 2022, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Tuckermann, J.; Adams, R.H. The endothelium-bone axis in development, homeostasis and bone and joint disease. Nat. Rev. Rheumatol. 2021, 17, 608–620. [Google Scholar] [CrossRef]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American association of clinical endocrinologists/american college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2020 update. Endocr. Pract. 2020, 26, 564–570. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Dagher, H.; Sharara, S.; Ajjour, S.; Chamoun, N.; Cauley, J.; Mahfoud, Z.; Boudreau, R.; El Hajj Fuleihan, G. Systematic review of major osteoporotic fracture to hip fracture incidence rate ratios worldwide: Implications for Fracture Risk Assessment Tool (FRAX)-derived estimates. J. Bone Miner. Res. 2021, 36, 1942–1956. [Google Scholar] [CrossRef] [PubMed]
- Akizuki, S.; Yasukawa, Y.; Takizawa, T. Does arthroscopic abrasion arthroplasty promote cartilage regeneration in osteoarthritic knees with eburnation? A prospective study of high tibial osteotomy with abrasion arthroplasty versus high tibial osteotomy alone. Arthroscopy 1997, 13, 9–17. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 88, 2023–2038. [Google Scholar] [CrossRef]
- Vina, E.R.; Kwoh, C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis. Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [Green Version]
- Medical Advisory Secretariat. Arthroscopic lavage and debridement for osteoarthritis of the knee: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2005, 5, 1–37. [Google Scholar]
- Xia, B.; Chen, D.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue. Int. 2014, 95, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Vonk, L.A.; Roël, G.; Hernigou, J.; Kaps, C.; Hernigou, P. Role of Matrix-Associated Autologous Chondrocyte Implantation with Spheroids in the Treatment of Large Chondral Defects in the Knee: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7149. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Zhang, J.; Yuan, Y.; Wang, J. Exosomes: A Novel Therapeutic Agent for Cartilage and Bone Tissue Regeneration. Dose Response 2019, 17, 1559325819892702. [Google Scholar]
- Kolambkar, Y.M.; Boerckel, J.D.; Dupont, K.M.; Bajin, M.; Huebsch, N.; Mooney, D.J.; Hutmacher, D.W.; Guldberg, R.E. Spatiotemporal delivery of bone morphogenetic protein enhances functional repair of segmental bone defects. Bone 2011, 49, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Davoudi, Z.; Xing, X.; Yu, X.; Cheng, X.; Li, Z.; Deng, H.; Wang, Q. Advanced SF Biomaterials for Cartilage Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 2704–2715. [Google Scholar]
- Clark, R.A.; Ghosh, K.; Tonnesen, M.G. Tissue engineering for cutaneous wounds. J. Investig. Dermatol. 2007, 127, 1018–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, Y.; Jana, S. Strategies for development of decellularized heart valve scaffolds for tissue engineering. Biomaterials 2022, 288, 121675. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Ma, J.; Li, W.; Zhang, X.; Gao, X. Microfiber Fabricated via Microfluidic Spinning toward Tissue Engineering Applications. Macromol. Biosci. 2023, 23, e2200429. [Google Scholar] [CrossRef]
- Petre, D.G.; Leeuwenburgh, S.C.G. The Use of Fibers in Bone Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 141–159. [Google Scholar]
- Li, G.; Sun, S. Silk Fibroin-Based Biomaterials for Tissue Engineering Applications. Molecules 2022, 27, 2757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Shao, H.; Hu, X. Hybrid Silk Fibers Dry-Spun from Regenerated Silk Fibroin/Graphene Oxide Aqueous Solutions. ACS Appl. Mater. Interfaces 2016, 8, 3349–3358. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Muthana, M.; Mukherjee, J.; Falconer, R.J.; Biggs, C.A.; Zhao, X. Magnetic-Silk Core-Shell Nanoparticles as Potential Carriers for Targeted Delivery of Curcumin into Human Breast Cancer Cells. ACS Biomater. Sci. Eng. 2017, 3, 1027–1038. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta. Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Ling, S.; Li, C.; Omenetto, F.G.; Kaplan, D.L. Silkworm silk-based materials and devices generated using bio-nanotechnology. Chem. Soc. Rev. 2018, 47, 6486–6504. [Google Scholar] [CrossRef]
- Dong, Q.; Cai, J.; Wang, H.; Chen, S.; Liu, Y.; Yao, J.; Shao, Z.; Chen, X. Artificial ligament made from silk protein/Laponite hybrid fibers. Acta Biomater. 2020, 106, 102–113. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Y.; Dai, W. Silk fibroin-based biomaterials for musculoskeletal tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 456–469. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Q.; Cheng, N.; Tao, X.; Zhang, Z.; Sun, X.; Zhang, Q. Collagen/silk fibroin composite scaffold incorporated with PLGA microsphere for cartilage repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 705–711. [Google Scholar] [CrossRef]
- Sheng, R.; Chen, J.; Wang, H.; Luo, Y.; Liu, J.; Chen, Z.; Mo, Q.; Chi, J.; Ling, C.; Tan, X.; et al. Nanosilicate-Reinforced Silk Fibroin Hydrogel for Endogenous Regeneration of Both Cartilage and Subchondral Bone. Adv. Healthc. Mater. 2022, 11, e2200602. [Google Scholar] [CrossRef]
- Jeyakumar, V.; Amraish, N.; Niculescu-Morsza, E.; Bauer, C.; Pahr, D.; Nehrer, S. Decellularized Cartilage Extracellular Matrix Incorporated Silk Fibroin Hybrid Scaffolds for Endochondral Ossification Mediated Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 4055. [Google Scholar] [CrossRef]
- Zhou, Z.; Cui, J.; Wu, S.; Geng, Z.; Su, J. Silk fibroin-based biomaterials for cartilage/osteochondral repair. Theranostics 2022, 12, 5103–5124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ling, C.; Zhang, A.; Liu, H.; Jiang, Y.; Li, X.; Sheng, R.; Yao, Q.; Chen, J. An all-silk-derived functional nanosphere matrix for sequential biomolecule delivery and in situ osteochondral regeneration. Bioact. Mater. 2020, 5, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Shahbazi, M.A.; Montes, S.; Hosseini, S.H.; Eskandari, M.R.; Zaunschirm, S.; Verwanger, T.; Mathur, S.; Milow, B.; Krammer, B.; et al. Mechanically Strong Silica-Silk Fibroin Bioaerogel: A Hybrid Scaffold with Ordered Honeycomb Micromorphology and Multiscale Porosity for Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 17256–17269. [Google Scholar] [PubMed]
- Zheng, A.; Cao, L.; Liu, Y.; Wu, J.; Zeng, D.; Hu, L.; Zhang, X.; Jiang, X. Biocompatible silk/calcium silicate/sodium alginate composite scaffolds for bone tissue engineering. Carbohydr. Polym. 2018, 199, 244–255. [Google Scholar] [CrossRef]
- Chen, F.; Porter, D.; Vollrath, F. Structure and physical properties of silkworm cocoons. J. R. Soc. Interface 2012, 9, 2299–2308. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sheng, R.; Chen, J.; Wang, H.; Zhu, Y.; Cao, Z.; Zhao, X.; Wang, Z.; Liu, C.; Chen, Z.; et al. Silk Fibroin and Sericin Differentially Potentiate the Paracrine and Regenerative Functions of Stem Cells through Multiomics Analysis. Adv. Mater. 2023, 13, e2210517. [Google Scholar]
- Wang, C.; Xia, K.; Zhang, Y.; Kaplan, D.L. Silk-Based Advanced Materials for Soft Electronics. Acc. Chem. Res. 2019, 52, 2916–2927. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ma, Y.; Xia, Y.Y.; Shen, W.D.; Mao, J.P.; Zha, X.M.; Shirai, K.; Kiguchi, K. Synthesis of silk fibroin-insulin bioconjugates and their characterization and activities in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 79, 275–283. [Google Scholar]
- Carrascoza Mayen, J.F.; Lupan, A.; Cosar, C.; Kun, A.Z.; Silaghi-Dumitrescu, R. On the roles of the alanine and serine in the β-sheet structure of fibroin. Biophys. Chem. 2015, 197, 10–17. [Google Scholar]
- Zhao, Y.; Zhu, Z.S.; Guan, J.; Wu, S.J. Processing, mechanical properties and bio-applications of silk fibroin-based high-strength hydrogels. Acta Biomater. 2021, 125, 57–71. [Google Scholar] [CrossRef]
- Takei, F.; Kikuchi, Y.; Kikuchi, A.; Mizuno, S.; Shimura, K. Further evidence for importance of the subunit combination of silk fibroin in its efficient secretion from the posterior silk gland cells. J. Cell Biol. 1987, 105, 175–180. [Google Scholar]
- Tanaka, K.; Mori, K.; Mizuno, S. Immunological identification of the major disulfide-linked light component of silk fibroin. J. Biochem. 1993, 114, 1–4. [Google Scholar]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.Z.; Confalonieri, F.; Medina, N.; Zivanovic, Y.; Esnault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic Acids Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Kajiyama, N.; Ishikura, K.; Waga, S.; Kikuchi, A.; Ohtomo, K.; Takagi, T.; Mizuno, S. Determination of the site of disulfide linkage between heavy and light chains of silk fibroin produced by Bombyx mori. Biochim. Biophys. Acta 1999, 1432, 92–103. [Google Scholar] [PubMed]
- Hui, C.C.; Matsuno, K.; Suzuki, Y. Fibroin gene promoter contains a cluster of homeodomain binding sites that interact with three silk gland factors. J. Mol. Biol. 1990, 213, 651–670. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Mori, K.; Suzuki, S.; Yamaguchi, K.; Mizuno, S. Structure of the Bombyx mori fibroin light-chain-encoding gene: Upstream sequence elements common to the light and heavy chain. Gene 1992, 110, 151–158. [Google Scholar] [PubMed]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Drug and Gene Delivery. Pharmaceutics 2019, 11, 494. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.Y.; Kim, I.S.; Zhang, K.Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins 2001, 44, 119–122. [Google Scholar] [CrossRef]
- Boi, M.; Marchiori, G.; Berni, M.; Gambardella, A.; Salamanna, F.; Visani, A.; Bianchi, M.; Fini, M.; Filardo, G. Nanoindentation: An advanced procedure to investigate osteochondral engineered tissues. J. Mech. Behav. Biomed. Mater. 2019, 96, 79–87. [Google Scholar] [CrossRef]
- Lu, Q.; Zhu, H.; Zhang, C.; Zhang, F.; Zhang, B.; Kaplan, D.L. Silk self-assembly mechanisms and control from thermodynamics to kinetics. Biomacromolecules 2012, 13, 826–832. [Google Scholar]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [Green Version]
- Kratky, O.; Schauenstein, E.; Sekora, A. An Unstable Lattice in Silk Fibroin. Nature 1950, 165, 319–320. [Google Scholar]
- Cebe, P.; Partlow, B.P.; Kaplan, D.L.; Wurm, A.; Zhuravlev, E.; Schick, C. Silk I and Silk II studied by fast scanning calorimetry. Acta Biomater. 2017, 55, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, Y.; Zhang, W.; Qi, P.; Ren, J.; Pei, Y.; Ling, S. Formation, Structure, and Mechanical Performance of Silk Nanofibrils Produced by Heat-Induced Self-Assembly. Macromol. Rapid Commun. 2021, 42, e2000435. [Google Scholar] [PubMed]
- Chen, W.; Xu, Y.; Li, H.; Dai, Y.; Zhou, G.; Zhou, Z.; Xia, H.; Liu, H. Tanshinone IIA Delivery Silk Fibroin Scaffolds Significantly Enhance Articular Cartilage Defect Repairing via Promoting Cartilage Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 21470–21480. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kojima, K.; Tamada, Y. Higher Gene Expression Related to Wound Healing by Fibroblasts on Silk Fibroin Biomaterial than on Collagen. Molecules 2020, 25, 1939. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Huang, W.; Xiong, K.; Ruan, S.; Yuan, C.; Mo, G.; Tian, R.; Zhou, S.; She, R.; Ye, P.; et al. Osteochondral repair using scaffolds with gradient pore sizes constructed with silk fibroin, chitosan, and nano-hydroxyapatite. Int. J. Nanomed. 2019, 14, 2011–2027. [Google Scholar]
- Koullali, B.; Zhang, Y.; Peterson, A.; Raia, N.; Kaplan, D.L.; House, M.D. Cervical Augmentation with an Injectable Silk-Based Gel: Biocompatibility in a Rat Model of Pregnancy. Reprod. Sci. 2020, 27, 1215–1221. [Google Scholar]
- Fountain, J.N.; Hawker, M.J.; Hartle, L.; Wu, J.; Montanari, V.; Sahoo, J.K.; Davis, L.M.; Kaplan, D.L.; Kumar, K. Towards Non-stick Silk: Tuning the Hydrophobicity of Silk Fibroin Protein. Chembiochem 2022, 23, e202200429. [Google Scholar]
- Sun, S.; Ding, C.; Liu, X.; Zhao, Y.; Zhang, J.; Ding, Q.; Zhang, Y.; Zhang, Y.; Hao, M.; Zheng, Y.; et al. Silk protein/polyvinylpyrrolidone nanofiber membranes loaded with puerarin accelerate wound healing in mice by reducing the inflammatory response. Biomater. Adv. 2022, 135, 212734. [Google Scholar]
- Liang, Y.; Tang, B.; Sharma, A.; Perera, D.; Allardyce, B.J.; Ghosh, S.; Schniepp, H.C.; Rajkhowa, R. Silk Protein Paper with In Situ Synthesized Silver Nanoparticles. Macromol. Biosci. 2021, 21, e2000357. [Google Scholar] [CrossRef] [PubMed]
- Griswold, E.; Cappello, J.; Ghandehari, H. Silk-elastinlike protein-based hydrogels for drug delivery and embolization. Adv. Drug Deliv. Rev. 2022, 191, 114579. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, H.; Ajiteru, O.; Sultan, M.T.; Lee, Y.J.; Lee, J.S.; Lee, O.J.; Lee, H.; Park, H.S.; Choi, K.Y.; et al. 3D bioprinted silk fibroin hydrogels for tissue engineering. Nat. Protoc. 2021, 16, 5484–5532. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Trucco, D.; Sharma, A.; Manferdini, C.; Gabusi, E.; Petretta, M.; Desando, G.; Ricotti, L.; Chakraborty, J.; Ghosh, S.; Lisignoli, G. Modeling and Fabrication of Silk Fibroin-Gelatin-Based Constructs Using Extrusion-Based Three-Dimensional Bioprinting. ACS Biomater. Sci. Eng. 2021, 7, 3306–3320. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Hua, Y.; Zeng, J.; Liu, W.; Wang, D.; Zhou, G.; Liu, X.; Jiang, H. Bioprinting and regeneration of auricular cartilage using a bioactive bioink based on microporous photocrosslinkable acellular cartilage matrix. Bioact. Mater. 2022, 16, 66–81. [Google Scholar]
- Li, Z.; Zhang, X.; Yuan, T.; Zhang, Y.; Luo, C.; Zhang, J.; Liu, Y.; Fan, W. Addition of Platelet-Rich Plasma to Silk Fibroin Hydrogel Bioprinting for Cartilage Regeneration. Tissue Eng. Part A 2020, 26, 886–895. [Google Scholar] [CrossRef]
- Deng, C.; Yang, J.; He, H.; Ma, Z.; Wang, W.; Zhang, Y.; Li, T.; He, C.; Wang, J. 3D bio-printed biphasic scaffolds with dual modification of silk fibroin for the integrated repair of osteochondral defects. Biomater. Sci. 2021, 9, 4891–4903. [Google Scholar] [CrossRef]
- Liu, J.; Fang, Q.; Yu, X.; Wan, Y.; Xiao, B. Chitosan-Based Nanofibrous Membrane Unit with Gradient Compositional and Structural Features for Mimicking Calcified Layer in Osteochondral Matrix. Int. J. Mol. Sci. 2018, 19, 2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Zuo, B.; Fan, Z.; Xie, Z.; Lu, Q.; Zhang, X.; Kaplan, D.L. Mechanisms and control of silk-based electrospinning. Biomacromolecules 2012, 13, 798–804. [Google Scholar] [CrossRef] [Green Version]
- Kankala, R.K.; Zhu, K.; Li, J.; Wang, C.S.; Wang, S.B.; Chen, A.Z. Fabrication of arbitrary 3D components in cardiac surgery: From macro-, micro- to nanoscale. Biofabrication 2017, 9, 032002. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Burdick, J.A. Engineering synthetic hydrogel microenvironments to instruct stem cells. Curr. Opin. Biotechnol. 2013, 24, 841–846. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Thorrez, L.; Shansky, J.; Wang, L.; Fast, L.; VandenDriessche, T.; Chuah, M.; Mooney, D.; Vandenburgh, H. Growth, differentiation, transplantation and survival of human skeletal myofibers on biodegradable scaffolds. Biomaterials 2008, 29, 75–84. [Google Scholar]
- Ran, J.; Fei, Y.; Wang, C.; Ruan, D.; Hu, Y.; Zheng, Z.; Chen, X.; Yin, Z.; Tang, C.; Chen, Y.; et al. An Off-the-Shelf Tissue Engineered Cartilage Composed of Optimally Sized Pellets of Cartilage Progenitor/Stem Cells. ACS Biomater. Sci. Eng. 2021, 7, 881–892. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhao, J.; Liu, C.G.; Song, X.J.; Yang, D.Y.; Zhu, K.; Wang, S.B.; Zhang, Y.S.; Chen, A.Z. Highly Porous Microcarriers for Minimally Invasive In Situ Skeletal Muscle Cell Delivery. Small 2019, 15, e1901397. [Google Scholar] [CrossRef]
- Wang, X.; Yucel, T.; Lu, Q.; Hu, X.; Kaplan, D.L. Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials 2010, 31, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Hua, X.; Shu, L.; Li, J. Multiscale modelling for investigating the long-term time-dependent biphasic behaviour of the articular cartilage in the natural hip joint. Biomech. Model. Mechanobiol. 2022, 21, 1145–1155. [Google Scholar] [CrossRef]
- Boyde, A. The Bone Cartilage Interface and Osteoarthritis. Calcif. Tissue Int. 2021, 109, 303–328. [Google Scholar]
- Karns, M.R.; Epperson, R.T.; Baran, S.; Nielsen, M.B.; Taylor, N.B.; Burks, R.T. Revisiting the Anterior Glenoid: An Analysis of the Calcified Cartilage Layer, Capsulolabral Complex, and Glenoid Bone Density. Arthroscopy 2018, 34, 2309–2318. [Google Scholar] [CrossRef]
- Schreiner, A.J.; Stoker, A.M.; Bozynski, C.C.; Kuroki, K.; Stannard, J.P.; Cook, J.L. Clinical Application of the Basic Science of Articular Cartilage Pathology and Treatment. J. Knee Surg. 2020, 33, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Gill, S.E.; Massena de Camin, S.; Wilson, D.; McWilliams, D.F.; Walsh, D.A. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann. Rheum. Dis. 2007, 66, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Mostakhdemin, M.; Nand, A.; Ramezani, M. Articular and Artificial Cartilage, Characteristics, Properties and Testing Approaches—A Review. Polymers 2021, 13, 2000. [Google Scholar] [CrossRef]
- Deng, B.; Wang, F.; Yin, L.; Chen, C.; Guo, L.; Chen, H.; Gong, X.; Li, Y.; Yang, L. Quantitative study on morphology of calcified cartilage zone in OARSI 0∼4 cartilage from osteoarthritic knees. Curr. Res. Transl. Med. 2016, 64, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ealla, K.K.R.; Veeraraghavan, V.P.; Ravula, N.R.; Durga, C.S.; Ramani, P.; Sahu, V.; Poola, P.K.; Patil, S.; Panta, P. Silk Hydrogel for Tissue Engineering: A Review. J. Contemp. Dent. Pract. 2022, 23, 467–477. [Google Scholar] [PubMed]
- Hoemann, C.D.; Lafantaisie-Favreau, C.H.; Lascau-Coman, V.; Chen, G.; Guzmán-Morales, J. The cartilage-bone interface. J. Knee Surg. 2012, 25, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef]
- Geyer, M.; Schönfeld, C. Novel Insights into the Pathogenesis of Osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 98–107. [Google Scholar] [CrossRef]
- Moo, E.K.; Tanska, P.; Federico, S.; Al-Saffar, Y.; Herzog, W.; Korhonen, R.K. Collagen fibres determine the crack morphology in articular cartilage. Acta Biomater. 2021, 126, 301–314. [Google Scholar] [PubMed]
- Michalek, A.J.; Kuxhaus, L.; Jaremczuk, D.; Zaino, N.L. Proteoglycans contribute locally to swelling, but globally to compressive mechanics, in intact cervine medial meniscus. J. Biomech. 2018, 74, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Wolschrijn, C.F.; Weijs, W.A. Development of the subchondral bone layer of the medial coronoid process of the canine ulna. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2005, 284, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Vanhouten, J.; Macica, C.M. An atypical degenerative osteoarthropathy in Hyp mice is characterized by a loss in the mineralized zone of articular cartilage. Calcif. Tissue Int. 2011, 89, 151–162. [Google Scholar]
- Qian, J.J.; Xu, Q.; Xu, W.M.; Cai, R.; Huang, G.C. Expression of VEGF-A Signaling Pathway in Cartilage of ACLT-induced Osteoarthritis Mouse Model. J. Orthop. Surg. Res. 2021, 16, 379. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Alvarez-Garcia, O.; Mokuda, S.; Nagira, K.; Olmer, M.; Gamini, R.; Miyata, K.; Akasaki, Y.; Su, A.I.; Asahara, H.; et al. FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis. Sci. Transl. Med. 2018, 10, eaan0746. [Google Scholar] [CrossRef] [Green Version]
- Thonar, E.J.; Manicourt, D.M.; Williams, J.; Lenz, M.E.; Sweet, M.B.; Schnitzer, T.J.; Otten, L.; Glant, T.; Kuettner, K.E. Circulating keratan sulfate: A marker of cartilage proteoglycan catabolism in osteoarthritis. J. Rheumatol. Suppl. 1991, 27, 24–26. [Google Scholar]
- Slovacek, H.; Khanna, R.; Poredos, P.; Poredos, P.; Jezovnik, M.; Hoppensteadt, D.; Fareed, J.; Hopkinson, W. Interrelationship of MMP-9, Proteoglycan-4, and Inflammation in Osteoarthritis Patients Undergoing Total Hip Arthroplasty. Clin. Appl. Thromb. Hemost. 2021, 27, 1076029621995569. [Google Scholar] [CrossRef]
- Wu, T.; Chen, Y.; Liu, W.; Tong, K.L.; Suen, C.W.; Huang, S.; Hou, H.; She, G.; Zhang, H.; Zheng, X.; et al. Ginsenoside Rb1/TGF-β1 loaded biodegradable silk fibroin-gelatin porous scaffolds for inflammation inhibition and cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110757. [Google Scholar]
- Zhang, W.; Zhang, Y.; Zhang, A.; Ling, C.; Sheng, R.; Li, X.; Yao, Q.; Chen, J. Enzymatically crosslinked silk-nanosilicate reinforced hydrogel with dual-lineage bioactivity for osteochondral tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112215. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, L.; Li, Y.; Lou, X. Osteoblast-derived extracellular matrix coated PLLA/silk fibroin composite nanofibers promote osteogenic differentiation of bone mesenchymal stem cells. J. Biomed. Mater. Res. A 2022, 110, 525–534. [Google Scholar] [CrossRef]
- Geão, C.; Costa-Pinto, A.R.; Cunha-Reis, C.; Ribeiro, V.P.; Vieira, S.; Oliveira, J.M.; Reis, R.L.; Oliveira, A.L. Thermal annealed silk fibroin membranes for periodontal guided tissue regeneration. Journal of materials science. J. Mater. Sci. Mater. Med. 2019, 30, 27. [Google Scholar] [PubMed]
- Pignolo, R.J.; Wang, H.; Kaplan, F.S. Fibrodysplasia Ossificans Progressiva (FOP): A Segmental Progeroid Syndrome. Front. Endocrinol. 2020, 10, 908. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.P.; Pina, S.; Costa, J.B.; Cengiz, I.F.; García-Fernández, L.; Fernández-Gutiérrez, M.D.M.; Paiva, O.C.; Oliveira, A.L.; San-Román, J.; Oliveira, J.M.; et al. Enzymatically Cross-Linked Silk Fibroin-Based Hierarchical Scaffolds for Osteochondral Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 3781–3799. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; You, J.; Qin, Q.; Liu, M.; Yang, Y.; Jia, K.; Zhang, Y.; Zhou, Y. A Comprehensive Review on Silk Fibroin as a Persuasive Biomaterial for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 2660. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, D.K.; Song, J.E.; Oliveira, J.M.; Reis, R.L.; Khang, G. Silk Fibroin-Based Scaffold for Bone Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1077, 371–387. [Google Scholar] [PubMed]
- Kasoju, N.; Bora, U. Silk fibroin in tissue engineering. Adv. Healthc. Mater. 2012, 1, 393–412. [Google Scholar] [CrossRef]
- Cai, Y.; Guo, J.; Chen, C.; Yao, C.; Chung, S.M.; Yao, J.; Lee, I.S.; Kong, X. Silk fibroin membrane used for guided bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 148–154. [Google Scholar] [CrossRef]
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110116. [Google Scholar] [CrossRef]
- Fitzpatrick, V.; Martín-Moldes, Z.; Deck, A.; Torres-Sanchez, R.; Valat, A.; Cairns, D.; Li, C.; Kaplan, D.L. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials 2021, 276, 120995. [Google Scholar] [CrossRef] [PubMed]
- Jouan, Y.; Bouchemla, Z.; Bardèche-Trystram, B.; Sana, J.; Andrique, C.; Ea, H.K.; Richette, P.; Latourte, A.; Cohen-Solal, M.; Hay, E. Lin28a induces SOX9 and chondrocyte reprogramming via HMGA2 and blunts cartilage loss in mice. Sci. Adv. 2022, 8, eabn3106. [Google Scholar] [PubMed]
- Meinel, L.; Hofmann, S.; Betz, O.; Fajardo, R.; Merkle, H.P.; Langer, R.; Evans, C.H.; Vunjak-Novakovic, G.; Kaplan, D.L. Osteogenesis by human mesenchymal stem cells cultured on silk biomaterials: Comparison of adenovirus mediated gene transfer and protein delivery of BMP-2. Biomaterials 2006, 27, 4993–5002. [Google Scholar] [PubMed]
- Meinel, L.; Fajardo, R.; Hofmann, S.; Langer, R.; Chen, J.; Snyder, B.; Vunjak-Novakovic, G.; Kaplan, D. Silk implants for the healing of critical size bone defects. Bone 2005, 37, 688–698. [Google Scholar] [CrossRef]
- He, P.; Sahoo, S.; Ng, K.S.; Chen, K.; Toh, S.L.; Goh, J.C. Enhanced osteoinductivity and osteoconductivity through hydroxyapatite coating of silk-based tissue-engineered ligament scaffold. J. Biomed. Mater. Res. A 2013, 101, 555–566. [Google Scholar] [CrossRef]
- Ghanaati, S.; Unger, R.E.; Webber, M.J.; Barbeck, M.; Orth, C.; Kirkpatrick, J.A.; Booms, P.; Motta, A.; Migliaresi, C.; Sader, R.A.; et al. Scaffold vascularization in vivo driven by primary human osteoblasts in concert with host inflammatory cells. Biomaterials 2011, 32, 8150–8160. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Ai, J.; Hadjati, J.; Azami, M. Bio-hybrid silk fibroin/calcium phosphate/PLGA nanocomposite scaffold to control the delivery of vascular endothelial growth factor. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 35, 401–410. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, C.; Ye, D.; Xu, L.; Zhang, X.; Wu, Q.; Zhang, X.; Kaplan, D.L.; Jiang, X. Porous silk scaffolds for delivery of growth factors and stem cells to enhance bone regeneration. PLoS ONE 2014, 9, e102371. [Google Scholar]
- Xu, C.; Sun, Y.; Jansen, J.A.; Li, M.; Wei, L.; Wu, Y.; Liu, Y. Calcium phosphate ceramics and synergistic bioactive agents for osteogenesis in implant dentistry. Tissue Eng. Part C Methods 2023, 29, 197–215. [Google Scholar] [CrossRef]
- Lv, L.; Cheng, W.; Wang, S.; Lin, S.; Dang, J.; Ran, Z.; Zhu, H.; Xu, W.; Huang, Z.; Xu, P.; et al. Poly(β-amino ester) Dual-Drug-Loaded Hydrogels with Antibacterial and Osteogenic Properties for Bone Repair. ACS Biomater. Sci. Eng. 2023, 9, 1976–1990. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, L.; Chen, J.; Li, Y.; Wen, W.; Lu, L.; Li, L.; Li, H.; Liu, M.; Zhou, C.; et al. Bone ECM-like 3D Printing Scaffold with Liquid Crystalline and Viscoelastic Microenvironment for Bone Regeneration. ACS Nano 2022, 16, 21020–21035. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, Y.; Gu, Y.; Xu, Y.; Liu, Y.; Li, B.; Chen, L. Sequential and sustained release of SDF-1 and BMP-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Biomaterials 2016, 106, 205–216. [Google Scholar] [CrossRef]
- Trubiani, O.; Marconi, G.D.; Pierdomenico, S.D.; Piattelli, A.; Diomede, F.; Pizzicannella, J. Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair. Int. J. Mol. Sci. 2019, 20, 4987. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.H.; Hur, S.S.; Lobionda, S.; Chaycham, S.; Oh, J.S.; Lee, Y.K.; Hwang, Y. Heparin-mimicking polymer-based hydrogel matrix regulates macrophage polarization by controlling cell adhesion. Biochem. Biophys. Res. Commun. 2023, 642, 154–161. [Google Scholar] [CrossRef]
- Kojima, N.; Matsuo, T.; Sakai, Y. Rapid hepatic cell attachment onto biodegradable polymer surfaces without toxicity using an avidin-biotin binding system. Biomaterials 2006, 27, 4904–4910. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S.; Sinha, V.R. Polymer based microspheres of aceclofenac as sustained release parenterals for prolonged anti-inflammatory effect. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 492–500. [Google Scholar] [CrossRef]
- Tanetsugu, Y.; Tagami, T.; Terukina, T.; Ogawa, T.; Ohta, M.; Ozeki, T. Development of a Sustainable Release System for a Ranibizumab Biosimilar Using Poly(lactic-co-glycolic acid) Biodegradable Polymer-Based Microparticles as a Platform. Biol. Pharm. Bull. 2017, 40, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Gomez, L.; García-González, C.A.; Wang, J.; Yang, F.; Aznar-Cervantes, S.; Cenis, J.L.; Reyes, R.; Delgado, A.; Évora, C.; Concheiro, A.; et al. Biodegradable PCL/fibroin/hydroxyapatite porous scaffolds prepared by supercritical foaming for bone regeneration. Int. J. Pharm. 2017, 527, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; He, J.; He, F.L.; Liu, Y.L.; Liu, Y.Y.; Ye, Y.J.; Deng, X.; Yin, D.C. Silk fibroin/chitosan thin film promotes osteogenic and adipogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J. Biomater. Appl. 2018, 32, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Feng, B.W.; He, S.R.; Liu, Y.M.; Chen, L.; Chen, Y.L.; Yao, Z.Y.; Jian, M.Q. Preparation and evaluation of a silk fibroin-polycaprolactone biodegradable biomimetic tracheal scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1292–1305. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, Ł.; Szudzik, M.; Rybka, M.; Konop, M. Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing. Biomolecules 2022, 12, 1852. [Google Scholar] [CrossRef] [PubMed]
- Pudkon, W.; Laomeephol, C.; Damrongsakkul, S.; Kanokpanont, S.; Ratanavaraporn, J. Comparative Study of Silk Fibroin-Based Hydrogels and Their Potential as Material for 3-Dimensional (3D) Printing. Molecules 2021, 26, 3887. [Google Scholar] [CrossRef]
- Hong, M.H.; Lee, J.H.; Jung, H.S.; Shin, H.; Shin, H. Biomineralization of bone tissue: Calcium phosphate-based inorganics in collagen fibrillar organic matrices. Biomater. Res. 2022, 26, 42. [Google Scholar] [CrossRef]
- Khurshid, Z.; Alfarhan, M.F.; Mazher, J.; Bayan, Y.; Cooper, P.R.; Dias, G.J.; Adanir, N.; Ratnayake, J. Extraction of Hydroxyapatite from Camel Bone for Bone Tissue Engineering Application. Molecules 2022, 27, 7946. [Google Scholar] [CrossRef]
- Pazarçeviren, A.E.; Tezcaner, A.; Keskin, D.; Kolukısa, S.T.; Sürdem, S.; Evis, Z. Boron-doped Biphasic Hydroxyapatite/β-Tricalcium Phosphate for Bone Tissue Engineering. Biol. Trace Elem. Res. 2021, 199, 968–980. [Google Scholar] [CrossRef]
- Jing, T.; Yi, L.; Xu, L.; Chen, C.; Liu, F. The incorporation of β-tricalcium phosphate nanoparticles within silk fibroin composite scaffolds for enhanced bone regeneration: An in vitro and in vivo study. J. Biomater. Appl. 2022, 36, 1567–1578. [Google Scholar] [CrossRef]
- Jia, X.; Zhou, J.; Ning, J.; Li, M.; Yao, Y.; Wang, X.; Jian, Y.; Zhao, K. The polycaprolactone/silk fibroin/carbonate hydroxyapatite electrospun scaffold promotes bone reconstruction by regulating the polarization of macrophages. Regen. Biomater. 2022, 9, rbac035. [Google Scholar] [CrossRef]
- Hong, H.; Seo, Y.B.; Kim, D.Y.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Sultan, M.T.; Lee, O.J.; Kim, S.H.; et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 2020, 232, 119679. [Google Scholar] [CrossRef]
- Bai, B.; Hou, M.; Hao, J.; Liu, Y.; Ji, G.; Zhou, G. Research progress in seed cells for cartilage tissue engineering. Regen. Med. 2022, 17, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Stefanik, J.J.; Guermazi, A.; Roemer, F.W.; Peat, G.; Niu, J.; Segal, N.A.; Lewis, C.E.; Nevitt, M.; Felson, D.T. Changes in patellofemoral and tibiofemoral joint cartilage damage and bone marrow lesions over 7 years: The Multicenter Osteoarthritis Study. Osteoarthr. Cartil. 2016, 24, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Kulchar, R.J.; Denzer, B.R.; Chavre, B.M.; Takegami, M.; Patterson, J. A Review of the Use of Microparticles for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 10292. [Google Scholar] [CrossRef]
- Huang, K.; Li, Q.; Li, Y.; Yao, Z.; Luo, D.; Rao, P.; Xiao, J. Cartilage Tissue Regeneration: The Roles of Cells, Stimulating Factors and Scaffolds. Curr. Stem. Cell Res. Ther. 2018, 13, 547–567. [Google Scholar] [CrossRef] [PubMed]
- Rainbow, R.S.; Kwon, H.; Foote, A.T.; Preda, R.C.; Kaplan, D.L.; Zeng, L. Muscle cell-derived factors inhibit inflammatory stimuli-induced damage in hMSC-derived chondrocytes. Osteoarthr. Cartil. 2013, 21, 990–998. [Google Scholar] [CrossRef] [Green Version]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zheng, Z.; Chen, K.; Li, Y.; Wang, X.; Li, G. A heparin-functionalized woven stent graft for endovascular exclusion. Colloids Surf. B Biointerfaces 2019, 180, 118–126. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Liu, J.; Fang, Y.; Guo, W.; Liu, Y.; Li, X.; Li, G.; Wang, X.; Zheng, Z.; et al. Intraarticularly injectable silk hydrogel microspheres with enhanced mechanical and structural stability to attenuate osteoarthritis. Biomaterials 2022, 286, 121611. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Wang, S.; Fu, X.; Zhao, T.; Chen, X.; Shao, Z. Physically Cross-Linked Silk Fibroin-Based Tough Hydrogel Electrolyte with Exceptional Water Retention and Freezing Tolerance. ACS Appl. Mater. Interfaces 2020, 12, 25353–25362. [Google Scholar] [CrossRef]
- Saha, S.; Kundu, B.; Kirkham, J.; Wood, D.; Kundu, S.C.; Yang, X.B. Osteochondral tissue engineering in vivo: A comparative study using layered silk fibroin scaffolds from mulberry and nonmulberry silkworms. PLoS ONE 2013, 8, e80004. [Google Scholar] [CrossRef]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef]
- Wang, X.; Wenk, E.; Hu, X.; Castro, G.R.; Meinel, L.; Wang, X.; Li, C.; Merkle, H.; Kaplan, D.L. Silk coatings on PLGA and alginate microspheres for protein delivery. Biomaterials 2007, 28, 4161–4169. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Song, X.; Weng, C.; Wang, X.; Gu, L.; Gong, X.; Wei, Q.; Duan, X.; Yang, L.; Chen, C. Silk fibroin/carboxymethyl chitosan hydrogel with tunable biomechanical properties has application potential as cartilage scaffold. Int. J. Biol. Macromol. 2019, 137, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Z.; Zheng, L.; Zhang, X.; Liu, P.; Yang, T.; Han, B. Electrospun fibrous silk fibroin/poly(L-lactic acid) scaffold for cartilage tissue engineering. Tissue Eng. Regen. Med. 2016, 13, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Ratanavaraporn, J.; Fauzi, M.B. Comprehensive Review of Hybrid Collagen and Silk Fibroin for Cutaneous Wound Healing. Materials 2020, 13, 3097. [Google Scholar] [CrossRef] [PubMed]
- Sharafat-Vaziri, A.; Khorasani, S.; Darzi, M.; Saffarian, Z.; Alizadeh, Z.; Tahmasebi, M.N.; Kazemnejad, S. Safety and efficacy of engineered tissue composed of silk fibroin/collagen and autologous chondrocytes in two patients with cartilage defects: A pilot clinical trial study. Knee 2020, 27, 1300–1309. [Google Scholar] [CrossRef]
- Jaipaew, J.; Wangkulangkul, P.; Meesane, J.; Raungrut, P.; Puttawibul, P. Mimicked cartilage scaffolds of silk fibroin/hyaluronic acid with stem cells for osteoarthritis surgery: Morphological, mechanical, and physical clues. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 173–182. [Google Scholar] [CrossRef]
- Wani, S.U.D.; Veerabhadrappa, G.H. Silk Fibroin Based Drug Delivery Applications: Promises and Challenges. Curr. Drug Targets 2018, 19, 1177–1190. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Ding, M.; Song, G.; Wu, M.; Kang, Z.; Wang, J. Sequence-structure characterization of recombinant polypeptides derived from silk fibroin heavy chain. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110831. [Google Scholar] [CrossRef]
- Bargel, H.; Trossmann, V.T.; Sommer, C.; Scheibel, T. Bioselectivity of silk protein-based materials and their bio-inspired applications. Beilstein J. Nanotechnol. 2022, 13, 902–921. [Google Scholar] [CrossRef]
- Ratanavaraporn, J.; Soontornvipart, K.; Shuangshoti, S.; Shuangshoti, S.; Damrongsakkul, S. Localized delivery of curcumin from injectable gelatin/Thai silk fibroin microspheres for anti-inflammatory treatment of osteoarthritis in a rat model. Inflammopharmacology 2017, 25, 211–221. [Google Scholar] [CrossRef]
- Seib, F.P.; Pritchard, E.M.; Kaplan, D.L. Self-assembling doxorubicin silk hydrogels for the focal treatment of primary breast cancer. Adv. Funct. Mater. 2013, 23, 58–65. [Google Scholar] [CrossRef]
- Qu, J.; Liu, Y.; Yu, Y.; Li, J.; Luo, J.; Li, M. Silk fibroin nanoparticles prepared by electrospray as controlled release carriers of cisplatin. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bano, S.; Ghosh, A.S.; Mandal, M.; Kim, H.W.; Dey, T.; Kundu, S.C. Silk fibroin nanoparticles support in vitro sustained antibiotic release and osteogenesis on titanium surface. Nanomedicine 2016, 12, 1193–1204. [Google Scholar] [CrossRef]
- Hassani Besheli, N.; Mottaghitalab, F.; Eslami, M.; Gholami, M.; Kundu, S.C.; Kaplan, D.L.; Farokhi, M. Sustainable Release of Vancomycin from Silk Fibroin Nanoparticles for Treating Severe Bone Infection in Rat Tibia Osteomyelitis Model. ACS Appl. Mater. Interfaces 2017, 9, 5128–5138. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.; Jiang, J.; Shi, Z.; Mao, Y.; Qin, N.; Tao, T.H. Silk Microneedle Patch Capable of On-Demand Multidrug Delivery to the Brain for Glioblastoma Treatment. Adv. Mater. 2022, 34, e2106606. [Google Scholar] [CrossRef]
- Lehmann, T.; Vaughn, A.E.; Seal, S.; Liechty, K.W.; Zgheib, C. Silk Fibroin-Based Therapeutics for Impaired Wound Healing. Pharmaceutics 2022, 14, 651. [Google Scholar] [CrossRef] [PubMed]
- Di Buduo, C.A.; Laurent, P.A.; Zaninetti, C.; Lordier, L.; Soprano, P.M.; Ntai, A.; Barozzi, S.; La Spada, A.; Biunno, I.; Raslova, H.; et al. Miniaturized 3D bone marrow tissue model to assess response to Thrombopoietin-receptor agonists in patients. Elife 2021, 10, e58775. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, S.; Huang, X.; Chen, X.; Shan, H.; Chen, X.; Tao, L.; Zhang, M. Silk Fibroin Hydrogels Could Be Therapeutic Biomaterials for Neurological Diseases. Oxidative Med. Cell. Longev. 2022, 2022, 2076680. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Sapru, S.; Samadikuchaksaraei, A.; Reis, R.L.; Kaplan, D.L.; Kundu, S.C. Silk fibroin for skin injury repair: Where do things stand? Adv. Drug Deliv. Rev. 2020, 153, 28–53. [Google Scholar] [CrossRef]
- Crakes, K.R.; Herrera, C.; Morgan, J.L.; Olstad, K.; Hessell, A.J.; Ziprin, P.; LiWang, P.J.; Dandekar, S. Efficacy of silk fibroin biomaterial vehicle for in vivo mucosal delivery of Griffithsin and protection against HIV and SHIV infection ex vivo. J. Int. AIDS Soc. 2020, 23, e25628. [Google Scholar] [CrossRef]
- Lam, Y.T.; Tan, R.P.; Michael, P.L.; Lau, K.; Yang, N.; Rnjak-Kovacina, J.; Wise, S.G. Bioengineering silk into blood vessels. Biochem. Soc. Trans. 2021, 49, 2271–2286. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Fu, X.; Wang, L.; Xue, Y.; Zhou, L.; Qiao, S.; An, J.; Xia, T. Self-Assemble Silk Fibroin Microcapsules for Cartilage Regeneration through Gene Delivery and Immune Regulation. Small 2023, e2302799. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lawrence, B.D.; Liu, A.; Schwab, I.R.; Oliveira, L.A.; Rosenblatt, M.I. Silk fibroin as a biomaterial substrate for corneal epithelial cell sheet generation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4130–4138. [Google Scholar] [CrossRef] [Green Version]
- Jeong, L.; Kim, M.H.; Jung, J.Y.; Min, B.M.; Park, W.H. Effect of silk fibroin nanofibers containing silver sulfadiazine on wound healing. Int. J. Nanomed. 2014, 9, 5277–5287. [Google Scholar]
- Nakayama, K.; Chinen, S.; Teshima, J.; Tamada, Y.; Hirabayashi, M.; Hochi, S. Silk fibroin sheet multilayer suitable for vitrification of in vitro-matured bovine oocytes. Theriogenology 2020, 145, 109–114. [Google Scholar] [CrossRef]
- Perrone, G.S.; Leisk, G.G.; Lo, T.J.; Moreau, J.E.; Haas, D.S.; Papenburg, B.J.; Golden, E.B.; Partlow, B.P.; Fox, S.E.; Ibrahim, A.M.; et al. The use of silk-based devices for fracture fixation. Nat. Commun. 2014, 5, 3385. [Google Scholar] [CrossRef] [Green Version]
- Yuan, T.; Li, Z.; Zhang, Y.; Shen, K.; Zhang, X.; Xie, R.; Liu, F.; Fan, W. Injectable Ultrasonication-Induced Silk Fibroin Hydrogel for Cartilage Repair and Regeneration. Tissue Eng. Part A 2021, 27, 1213–1224. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, B.B. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef]
- Sultan, M.T.; Hong, H.; Lee, O.J.; Ajiteru, O.; Lee, Y.J.; Lee, J.S.; Lee, H.; Kim, S.H.; Park, C.H. Silk Fibroin-Based Biomaterials for Hemostatic Applications. Biomolecules 2022, 12, 660. [Google Scholar] [CrossRef]
- Huang, J.; Qin, J.; Zhang, P.; Chen, X.; You, X.; Zhang, F.; Zuo, B.; Yao, M. Facile preparation of a strong chitosan-silk biocomposite film. Carbohydr. Polym. 2020, 229, 115515. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Fan, Z.; Huang, X.; Lu, Q.; Xu, W.; Kaplan, D.L. Silk-Hydroxyapatite Nanoscale Scaffolds with Programmable Growth Factor Delivery for Bone Repair. ACS Appl. Mater. Interfaces 2016, 8, 24463–24470. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, X.; Geng, Z.; Su, J. The horizon of bone organoid: A perspective on construction and application. Bioact. Mater. 2022, 18, 15–25. [Google Scholar] [CrossRef]
- Warnecke, D.; Stein, S.; Haffner-Luntzer, M.; de Roy, L.; Skaer, N.; Walker, R.; Kessler, O.; Ignatius, A.; Dürselen, L. Biomechanical, structural and biological characterisation of a new silk fibroin scaffold for meniscal repair. J. Mech. Behav. Biomed. Mater. 2018, 86, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Rajput, M.; Mondal, P.; Yadav, P.; Chatterjee, K. Light-based 3D bioprinting of bone tissue scaffolds with tunable mechanical properties and architecture from photocurable silk fibroin. Int. J. Biol. Macromol. 2022, 202, 644–656. [Google Scholar] [CrossRef] [PubMed]







| Application Areas | Mechanism of Action | Application Results | References |
|---|---|---|---|
| Bone regeneration and repair | Promotes osteoblast proliferation and differentiation, bone matrix production, and epiphyseal migration | Promotes the speed of fracture healing, enhances fracture stability, and promotes bone defect repair | [31,69] |
| Cartilage regeneration and repair | Promotes proliferation and differentiation of chondroblasts and synthesis of collagen and cartilage matrix | Promotes healing of cartilage defects and improves cartilage tissue structure and functional recovery | [99,100] |
| Bone implant repair | Provides an extracellular matrix scaffold to improve the biocompatibility and adhesion of bone implants | Enhances the bonding of the bone implant to the surrounding tissue and promotes stability and growth of the bone implant | [101] |
| Oral periodontal restoration | Promotes the growth of dental bone attachment tissue and soft tissue repair | Improves the effectiveness of periodontitis treatment and promotes oral wound healing | [102] |
| Other applications | Various tissue engineering repairs, angiogenesis, immunomodulation, etc. | SF has potential for a wide range of applications in tissue engineering and regenerative medicine | [44] |
| Application Areas | SF in Drug Delivery | References |
|---|---|---|
| Oncology treatment | To deliver drugs to tumor tissue, SFs are used as carriers to improve the stability and bioavailability of drugs | [166] |
| Wound healing | SFs promote cell migration, proliferation, and repair during wound healing and can be used to prepare drug-delivery systems to promote wound healing | [167] |
| Treatment of blood disorders | SFs provide reliable carriers for the transport and release of drugs for the treatment of blood disorders, such as anticoagulants and anti-platelet agents | [168] |
| Treatment of neurological disorders | SFs can be used to deliver drugs for the treatment of neurological disorders, such as neuroprotective agents and anti-epileptic drugs, to promote the protection and repair of nerve cells | [169] |
| Skin beauty and treatment | SFs are widely used in cosmetic skin products and delivery systems for therapeutic drugs to improve skin texture, promote wound healing, reduce scar formation, etc. | [170] |
| Infectious disease control | SFs are used as carriers for drug delivery systems to deliver antiviral, antibacterial, and antifungal drugs, improving their efficacy and bioaccessibility | [171] |
| Treatment of orthopedic diseases | SFs are used to deliver drugs for treating orthopedic diseases, such as bone growth factors and anti-inflammatory drugs, and to promote the growth and repair of bone cells | [106,107] |
| Cardiovascular disease treatment | SFs are used as carriers in drug delivery systems for the delivery of drugs treating cardiovascular disease, such as anti-hypertensives and anti-heart failure drugs, to alleviate the symptoms of cardiovascular disease | [172] |
| Immune disease treatment | SFs can be used in drug delivery systems to deliver drugs for treating immune diseases, such as anti-inflammatory drugs and immunomodulators, to regulate the function of the immune system and treat diseases | [102] |
| Dental treatment | SFs are widely used in dental therapeutic drug delivery systems for the delivery of antibacterial drugs, natural anti-inflammatory agents, and bone growth factors to promote dental restoration and healing | [173] |
| Company Name | Region | SF Products |
|---|---|---|
| Sofregen Inc. | US | SERI surgical stents |
| Injectable fillers | ||
| Vaxess Technologies Inc. | US | Drug and vaccine delivery |
| Evolved by nature | US | Skincare products, textile coatings, topical ophthalmic treatments, etc. |
| Cocoon Biotech Inc. | US | Drug delivery systems (hydrogels, osteoarticular microspheres, etc.) |
| Kraig Biocraft Laboratories Inc. | US | Special textiles |
| Oxford Biomaterials Ltd. | UK | Artificial blood vessels |
| Orthox Ltd. | UK | Meniscal repair stents, tissue stents |
| Suzhou Semtex Biotechnology Co. | China | Injectable gels, stents, and dressings |
| Suzhou Suhao Biomaterials Technology Co. | China | Trauma dressings |
| Zhejiang Xingyue Biotechnology Co. | China | Raw materials such as SF gels, microspheres, solutions, and sponges |
| SF skin rejuvenation mask |
| Product Type | Trademarks | Uses | Time to Market |
|---|---|---|---|
| SERI Surgical Stent | Allergan | Full body contouring, brachioplasty, abdominoplasty, breast fixation, breast reconstruction, etc. | 2013 |
| Silk Voice Injection | Sofregen | Vocal cord dielectricity and vocal cord insufficiency | United States, 2018 |
| Silk-substituted isoserine wound dressing | Soho Biotechnology Co. | Wound healing | China, 2012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Wei, L.; Xu, Z.; Qin, L.; Yang, J.; Zou, Y.; Zhao, C.; Chen, L.; Hu, N. Evaluation and Application of Silk Fibroin Based Biomaterials to Promote Cartilage Regeneration in Osteoarthritis Therapy. Biomedicines 2023, 11, 2244. https://doi.org/10.3390/biomedicines11082244
Su X, Wei L, Xu Z, Qin L, Yang J, Zou Y, Zhao C, Chen L, Hu N. Evaluation and Application of Silk Fibroin Based Biomaterials to Promote Cartilage Regeneration in Osteoarthritis Therapy. Biomedicines. 2023; 11(8):2244. https://doi.org/10.3390/biomedicines11082244
Chicago/Turabian StyleSu, Xudong, Li Wei, Zhenghao Xu, Leilei Qin, Jianye Yang, Yinshuang Zou, Chen Zhao, Li Chen, and Ning Hu. 2023. "Evaluation and Application of Silk Fibroin Based Biomaterials to Promote Cartilage Regeneration in Osteoarthritis Therapy" Biomedicines 11, no. 8: 2244. https://doi.org/10.3390/biomedicines11082244
APA StyleSu, X., Wei, L., Xu, Z., Qin, L., Yang, J., Zou, Y., Zhao, C., Chen, L., & Hu, N. (2023). Evaluation and Application of Silk Fibroin Based Biomaterials to Promote Cartilage Regeneration in Osteoarthritis Therapy. Biomedicines, 11(8), 2244. https://doi.org/10.3390/biomedicines11082244