Role of Syndecan-4 in the Inhibition of Articular Cartilage Degeneration in Osteoarthritis
Abstract
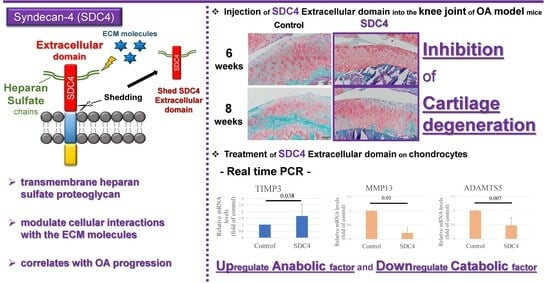
:1. Introduction
2. Materials and Methods
2.1. Experimental Animal Models
2.2. Intra-Articular Injection of SDC4 in OA Model Mice
2.3. Analysis of SDC4 Injected into Knee Joints
2.4. Histopathological Assessment
2.5. Histopathological Evaluation
2.5.1. Histological Grading of Cartilage and the Synovial Membrane
2.5.2. Immunohistochemistry
2.6. Chondrocyte Isolation and Culture
2.7. RNA Extraction and cDNA Synthesis
2.8. Real-Time PCR
2.9. Statistical Analysis
3. Results
3.1. Animal Welfare
3.2. Distribution of SDC4 Injected into Knee Joints
3.3. Histological Analysis and Grading of OA Model Mice
3.4. Immunohistochemistrical Analysis
3.5. Gene Expression in Chondrocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurtz, S.M.; Lau, E.; Ong, K.; Zhao, K.; Kelly, M.; Bozic, K.J. Future young patient demand for primary and revision joint replacement: National Projections from 2010 to 2030. Clin. Orthop. Relat. Res. 2009, 467, 2606–2612. [Google Scholar] [CrossRef]
- Lethbridge-Cejku, M.; Schiller, J.S.; Bernadel, L. Summary health statistics for U.S. adults: National Health Interview Survey, 2002. Vital Health Stat. 2004, 10, 1–151. [Google Scholar]
- Bruyère, O.; Honvo, G.; Veronese, N.; Arden, N.K.; Branco, J.; Curtis, E.M.; Al-Daghri, N.M.; Herrero-Beaumont, G.; Martel-Pelletier, J.; Pelletier, J.P.; et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin. Arthritis Rheum. 2019, 49, 337–350. [Google Scholar] [CrossRef]
- Roemer, F.W.; Guermazi, A.; Felson, D.T.; Niu, J.; Nevitt, M.C.; Crema, M.D.; Lynch, J.A.; Lewis, C.E.; Torner, J.; Zhang, Y. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: The MOST study. Ann. Rheum. Dis. 2011, 70, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Uivaraseanu, B.; Vesa, C.M.; Tit, D.M.; Abid, A.; Maghiar, O.; Maghiar, T.A.; Hozan, C.; Nechifor, A.C.; Behl, T.; Patrascu, J.M.; et al. Therapeutic approaches in the management of knee osteoarthritis (Review). Exp. Ther. Med. 2022, 23, 328. [Google Scholar] [CrossRef] [PubMed]
- Kisand, K.; Tamm, A.E.; Lintrop, M.; Tamm, A.O. New insights into the natural course of knee osteoarthritis: Early regulation of cytokines and growth factors, with emphasis on sex-dependent angiogenesis and tissue remodeling. A pilot study. Osteoarthr. Cartil. 2018, 26, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Hasegawa, M.; Iino, T.; Imanaka-Yoshida, K.; Yoshida, T.; Sudo, A. Tenascin-C Prevents Articular Cartilage Degeneration in Murine Osteoarthritis Models. Cartilage 2018, 9, 80–88. [Google Scholar] [CrossRef]
- Midwood, K.S.; Valenick, L.V.; Hsia, H.C.; Schwarzbauer, J.E. Coregulation of fibronectin signaling and matrix contraction by tenascin-C and syndecan-4. Mol. Biol. Cell 2004, 15, 5670–5677. [Google Scholar] [CrossRef] [PubMed]
- Binch, A.L.A.; Shapiro, I.M.; Risbud, M.V. Syndecan-4 in intervertebral disc and cartilage: Saint or synner? Matrix Biol. 2016, 52–54, 355–362. [Google Scholar] [CrossRef]
- Tkachenko, E.; Rhodes, J.M.; Simons, M. Syndecans: New kids on the signaling block. Circ. Res. 2005, 96, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, D.D.; Filla, M.S.; Stanley, H.M.; Rapraeger, A.C.; Olwin, B.B. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev. Biol. 2001, 239, 79–94. [Google Scholar] [CrossRef]
- Lim, S.T.; Longley, R.L.; Couchman, J.R.; Woods, A. Direct binding of syndecan-4 cytoplasmic domain to the catalytic domain of protein kinase C alpha (PKC alpha) increases focal adhesion localization of PKC alpha. J. Biol. Chem. 2003, 278, 13795–13802. [Google Scholar] [CrossRef]
- Wilcox-Adelman, S.A.; Denhez, F.; Goetinck, P.F. Syndecan-4 modulates focal adhesion kinase phosphorylation. J. Biol. Chem. 2002, 277, 32970–32977. [Google Scholar] [CrossRef]
- Saoncella, S.; Echtermeyer, F.; Denhez, F.; Nowlen, J.K.; Mosher, D.F.; Robinson, S.D.; Hynes, R.O.; Goetinck, P.F. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc. Natl. Acad. Sci. USA 1999, 96, 2805–2810. [Google Scholar] [CrossRef]
- Echtermeyer, F.; Bertrand, J.; Dreier, R.; Meinecke, I.; Neugebauer, K.; Fuerst, M.; Lee, Y.J.; Song, Y.W.; Herzog, C.; Theilmeier, G.; et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat. Med. 2009, 15, 1072–1076. [Google Scholar] [CrossRef]
- Fujita, N.; Hirose, Y.; Tran, C.M.; Chiba, K.; Miyamoto, T.; Toyama, Y.; Shapiro, I.M.; Risbud, M.V. HIF-1-PHD2 axis controls expression of syndecan 4 in nucleus pulposus cells. FASEB J. 2014, 28, 2455–2465. [Google Scholar] [CrossRef]
- Fitzgerald, M.L.; Wang, Z.; Park, P.W.; Murphy, G.; Bernfield, M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J. Cell Biol. 2000, 148, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Wang, H.; Kainulainen, V.; Fitzgerald, M.L.; Ledbetter, S.; Ornitz, D.M.; Bernfield, M. Physiological degradation converts the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of FGF-2. Nat. Med. 1998, 4, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, M.; Pinno, K.; Ehnold, L.I.; Märtens, N.; Märtson, A.; Pap, T.; Stärke, C.; Lohmann, C.H.; Bertrand, J. MMP-9 mediated Syndecan-4 shedding correlates with osteoarthritis severity. Osteoarthr. Cartil. 2021, 29, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.V.; Fitzgerald, M.L.; Bernfield, M. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J. Biol. Chem. 1997, 272, 14713–14720. [Google Scholar] [CrossRef]
- Rhodes, L.A.; Grainger, A.J.; Keenan, A.M.; Thomas, C.; Emery, P.; Conaghan, P.G. The validation of simple scoring methods for evaluating compartment-specific synovitis detected by MRI in knee osteoarthritis. Rheumatology 2005, 44, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Loeuille, D.; Rat, A.C.; Goebel, J.C.; Champigneulle, J.; Blum, A.; Netter, P.; Gillet, P.; Chary-Valckenaere, I. Magnetic resonance imaging in osteoarthritis: Which method best reflects synovial membrane inflammation? Correlations with clinical, macroscopic and microscopic features. Osteoarthr. Cartil. 2009, 17, 1186–1192. [Google Scholar] [CrossRef]
- Ayral, X.; Pickering, E.H.; Woodworth, T.G.; Mackillop, N.; Dougados, M. Synovitis: A potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis-results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr. Cartil. 2005, 13, 361–367. [Google Scholar] [CrossRef]
- Hill, C.L.; Hunter, D.J.; Niu, J.; Clancy, M.; Guermazi, A.; Genant, H.; Gale, D.; Grainger, A.; Conaghan, P.; Felson, D.T. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann. Rheum. Dis. 2007, 66, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Roemer, F.W.; Zhang, Y.; Niu, J.; Lynch, J.A.; Crema, M.D.; Marra, M.D.; Nevitt, M.C.; Felson, D.T.; Hughes, L.B.; El-Khoury, G.Y.; et al. Multicenter Osteoarthritis Study Investigators. Tibiofemoral joint osteoarthritis: Risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology 2009, 252, 772–780. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A.; Kloppenburg, M. An emerging player in knee osteoarthritis: The infrapatellar fat pad. Arthritis Res. Ther. 2013, 15, 225. [Google Scholar] [CrossRef]
- Clockaerts, S.; Bastiaansen-Jenniskens, Y.M.; Runhaar, J.; Van Osch, G.J.; Van Offel, J.F.; Verhaar, J.A.; De Clerck, L.S.; Somville, J. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: A narrative review. Osteoarthr. Cartil. 2010, 15, 876–882. [Google Scholar] [CrossRef]
- Krenn, V.; Morawietz, L.; Burmester, G.R.; Kinne, R.W.; Mueller-Ladner, U.; Muller, B.; Haupl, T. Synovitis score: Discrimination between chronic low-grade and high-grade synovitis. Histopathology 2006, 49, 358–364. [Google Scholar] [CrossRef]
- Mankin, H.J.; Dorfman, H.; Lippiello, L.; Zarins, A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Jt. Surg. Am. 1971, 53, 523–537. [Google Scholar] [CrossRef]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18, S17–S23. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Yoshida, T.; Matsumoto, K.; Katsuta, K.; Waga, S.; Sakakura, T. Differential expression of tenascin-C and tenascin-X in human astrocytomas. Acta Neuropathol. 1997, 93, 431–437. [Google Scholar] [CrossRef]
- Moreau, M.; Rialland, P.; Pelletier, J.P.; Martel-Pelletier, J.; Lajeunesse, D.; Boileau, C.; Caron, J.; Frank, D.; Lussier, B.; del Castillo, J.R.; et al. Tiludronate treatment improves structural changes and symptoms of osteoarthritis in the canine anterior cruciate ligament model. Arthritis Res. Ther. 2011, 13, R98. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Nikmanesh, M.; Cancel, L.M.; Shi, Z.D.; Tarbell, J.M. Heparan sulfate proteoglycan, integrin, and syndecan-4 are mechanosensors mediating cyclic strain-modulated endothelial gene expression in mouse embryonic stem cell-derived endothelial cells. Biotechnol. Bioeng. 2019, 116, 2730–2741. [Google Scholar] [CrossRef]
- Bernfield, M.; Götte, M.; Park, P.W.; Reizes, O.; Fitzgerald, M.L.; Lincecum, J.; Zako, M. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 1999, 68, 729–777. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; He, Z.; Shi, J.; Wang, Z.; Wu, W.; Liu, J.; Kang, H.; Li, F.; Liang, S. AMD3100 Attenuates Post-Traumatic Osteoarthritis by Maintaining Transforming Growth Factor-β1-Induced Expression of Tissue Inhibitor of Metalloproteinase-3 via the Phosphatidylinositol 3-Kinase/Akt Pathway. Front. Pharmacol. 2020, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Apte, S.S. Anti-ADAMTS5 monoclonal antibodies: Implications for aggrecanase inhibition in osteoarthritis. Biochem. J. 2016, 473, e1–e4. [Google Scholar] [CrossRef]
- Sahebjam, S.; Khokha, R.; Mort, J.S. Increased collagen and aggrecan degradation with age in the joints of Timp3(−/−) mice. Arthritis Rheum. 2007, 56, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Guns, L.A.; Monteagudo, S.; Kvasnytsia, M.; Kerckhofs, G.; Vandooren, J.; Opdenakker, G.; Lories, R.J.; Cailotto, F. Suramin increases cartilage proteoglycan accumulation in vitro and protects against joint damage triggered by papain injection in mouse knees in vivo. RMD Open 2017, 3, e000604. [Google Scholar] [CrossRef] [PubMed]
- Lim, N.H.; Kashiwagi, M.; Visse, R.; Jones, J.; Enghild, J.J.; Brew, K.; Nagase, H. Reactive-site mutants of N-TIMP-3 that selectively inhibit ADAMTS-4 and ADAMTS-5: Biological and structural implications. Biochem. J. 2010, 431, 113–122. [Google Scholar] [CrossRef]
- Zhou, K.; He, S.; Yu, H.; Pei, F.; Zhou, Z. Inhibition of syndecan-4 reduces cartilage degradation in murine models of osteoarthritis through the downregulation of HIF-2α by miR-96-5p. Lab. Investig. 2021, 101, 1060–1070. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori, Y.; Hasegawa, M.; Iino, T.; Imanaka-Yoshida, K.; Sudo, A. Role of Syndecan-4 in the Inhibition of Articular Cartilage Degeneration in Osteoarthritis. Biomedicines 2023, 11, 2257. https://doi.org/10.3390/biomedicines11082257
Hattori Y, Hasegawa M, Iino T, Imanaka-Yoshida K, Sudo A. Role of Syndecan-4 in the Inhibition of Articular Cartilage Degeneration in Osteoarthritis. Biomedicines. 2023; 11(8):2257. https://doi.org/10.3390/biomedicines11082257
Chicago/Turabian StyleHattori, Yoshio, Masahiro Hasegawa, Takahiro Iino, Kyoko Imanaka-Yoshida, and Akihiro Sudo. 2023. "Role of Syndecan-4 in the Inhibition of Articular Cartilage Degeneration in Osteoarthritis" Biomedicines 11, no. 8: 2257. https://doi.org/10.3390/biomedicines11082257
APA StyleHattori, Y., Hasegawa, M., Iino, T., Imanaka-Yoshida, K., & Sudo, A. (2023). Role of Syndecan-4 in the Inhibition of Articular Cartilage Degeneration in Osteoarthritis. Biomedicines, 11(8), 2257. https://doi.org/10.3390/biomedicines11082257