Role of Citrullus colocynthis and Psidium guajava Mediated Green Synthesized Silver Nanoparticles in Disease Resistance against Aeromonas hydrophila Challenge in Labeo rohita
Abstract
:1. Introduction
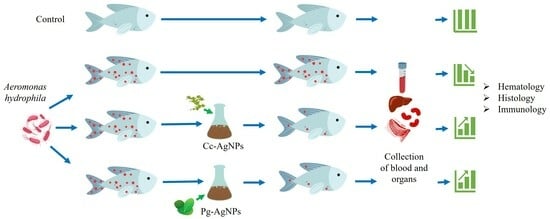
2. Materials and Methods
2.1. Fish Studies
2.2. AgNPs Synthesis
2.3. Characterization of AgNPs
2.4. A. Hydrophila Infection Challenge Assay
2.4.1. Cc-AgNPs Treatment
2.4.2. Pg-AgNPs Treatment
2.5. Sample Collection
2.6. Hematological Evaluation
2.7. Histopathological Evaluation
2.8. Oxidative and Immune Stress Responses
2.9. Statistical Analysis
3. Results
3.1. Analysis of Cc-AgNPs and Pg-AgNPs
3.1.1. XRD Analysis
3.1.2. UV-Vis
3.1.3. FTIR
3.1.4. SEM
3.2. Behavioral Changes
3.3. Hematological Analysis
3.4. Histological Studies
3.4.1. Gills
3.4.2. Liver
3.4.3. Kidney
3.5. Antioxidant Analysis
3.5.1. SOD
3.5.2. CAT
3.5.3. MDA
3.6. Cytokines Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.S.; Qureshi, N.A.; Jabeen, F.; Asghar, M.S.; Shakeel, M.; Fakhar-E-Alam, M. Eco-Friendly Synthesis of Silver Nanoparticles Through Economical Methods and Assessment of Toxicity Through Oxidative Stress Analysis in the Labeo Rohita. Biol. Trace Elem. Res. 2017, 176, 416–428. [Google Scholar] [CrossRef]
- Fahimirad, S.; Ajalloueian, F.; Ghorbanpour, M. Synthesis and therapeutic potential of silver nanomaterials derived from plant extracts. Ecotoxicol. Environ. Saf. 2019, 168, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Borase, H.P.; Salunke, B.K.; Salunkhe, R.B.; Patil, C.D.; Hallsworth, J.E.; Kim, B.S.; Patil, S.V. Plant extract: A promising biomatrix for ecofriendly, controlled synthesis of silver nanoparticles. Appl. Biochem. Biotechnol. 2014, 173, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Krishnasamy Sekar, R.; Arunachalam, R.; Anbazhagan, M.; Palaniyappan, S.; Veeran, S.; Sridhar, A.; Ramasamy, T. Accumulation, Chronicity, and Induction of Oxidative Stress Regulating Genes Through Allium cepa L. Functionalized Silver Nanoparticles in Freshwater Common Carp (Cyprinus carpio). Biol. Trace Elem. Res. 2022, 201, 904–925. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Ferdowsy, H.; Kashem, M.; Foysal, M. Tail and fin rot disease of Indian major carp and climbing perch in Bangladesh. J. Biol. Sci. 2010, 10, 800–804. [Google Scholar] [CrossRef]
- Khalil, M.; Perveen, S.; Arslan-Amin, H.; Anwar, I.; Butt, M. The effectiveness of silver nanoparticles for the treatment of abdominal dropsy in Labeo rohita. J. Aquac. Res. Dev. 2020, 11, 9. [Google Scholar]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Dahl, J.A.; Maddux, B.L.; Hutchison, J.E. Toward greener nanosynthesis. Chem. Rev. 2007, 107, 2228–2269. [Google Scholar] [CrossRef]
- Daniel, S.; Sironmani, T.A.; Dinakaran, S. Nano formulations as curative and protective agent for fish diseases: Studies on red spot and white spot diseases of ornamental gold fish Carassius auratus. Int. J. Fish. Aqua. Stud. 2016, 4, 255–261. [Google Scholar]
- Gowramma, B.; Keerthi, U.; Rafi, M.; Muralidhara Rao, D. Biogenic silver nanoparticles production and characterization from native stain of Corynebacterium species and its antimicrobial activity. 3 Biotech 2015, 5, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Panda, K.K.; Achary, V.M.M.; Krishnaveni, R.; Padhi, B.K.; Sarangi, S.N.; Sahu, S.N.; Panda, B.B. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol. In Vitro 2011, 25, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Bose, D.; Chatterjee, S. Biogenic synthesis of silver nanoparticles using guava (Psidium guajava) leaf extract and its antibacterial activity against Pseudomonas aeruginosa. Appl. Nanosci. 2016, 6, 895–901. [Google Scholar] [CrossRef]
- Nguyen, D.-K.; Hung, N.Q.; Dinh, V.-P. Antibacterial properties of silver nanoparticles greenly synthesized using guava fruit extract as a reducing agent and stabilizer. Appl. Nanosci. 2022, 13, 3709–3720. [Google Scholar] [CrossRef]
- Rasool, S.; Raza, M.A.; Manzoor, F.; Kanwal, Z.; Riaz, S.; Iqbal, M.J.; Naseem, S. Biosynthesis, characterization and anti-dengue vector activity of silver nanoparticles prepared from Azadirachta indica and Citrullus colocynthis. R. Soc. Open Sci. 2020, 7, 200540. [Google Scholar] [CrossRef]
- Nagaraja, S.; Ahmed, S.S.; Bharathi, D.R.; Goudanavar, P.; Fattepur, S.; Meravanige, G.; Shariff, A.; Shiroorkar, P.N.; Habeebuddin, M.; Telsang, M. Green Synthesis and Characterization of Silver Nanoparticles of Psidium guajava Leaf Extract and Evaluation for Its Antidiabetic Activity. Molecules 2022, 27, 4336. [Google Scholar] [CrossRef]
- Mani, R.; Vijayakumar, P.; Dhas, T.S.; Velu, K.; Inbakandan, D.; Thamaraiselvi, C.; Greff, B.; Chandrasekaran, M.; Almutairi, S.M.; Alharbi, F.S. Synthesis of biogenic silver nanoparticles using butter fruit pulp extract and evaluation of their antibacterial activity against Providencia vermicola in Rohu. J. King Saud Univ. Sci. 2022, 34, 101814. [Google Scholar] [CrossRef]
- Popoola, O.M.; Behera, B.K.; Kumar, V. Dietary silver nanoparticles as immunostimulant on rohu (Labeo rohita): Effects on the growth, cellular ultrastructure, immune-gene expression, and survival against Aeromonas hydrophila. Fish Shellfish Immunol. 2023, 4, 100080. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Vickram, S.; Saranya, V.; Ali, H.; Alarifi, S.; Modigunta, J.K.R.; Anbarasu, K.; Lakshmipathy, R.; Rohini, K. Seaweed polysaccharide mediated synthesis of silver nanoparticles and its enhanced disease resistance in Oreochromis mossambicus. J. King Saud Univ. Sci. 2022, 34, 101771. [Google Scholar] [CrossRef]
- Rodríguez-León, E.; Iñiguez-Palomares, R.; Navarro, R.E.; Herrera-Urbina, R.; Tánori, J.; Iñiguez-Palomares, C.; Maldonado, A. Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res. Lett. 2013, 8, 318. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Mohamed, A.; El-Naggar, N.E.-A.; Mahrous, H.; Nasr, G.M.; Abdella, A.; Ahmed, R.H.; Irmak, S.; Elsayed, M.S.; Selim, S. Antioxidant and antibacterial activities of silver nanoparticles biosynthesized by Moringa Oleifera through response surface methodology. J. Nanomater. 2022, 2022, 9984308. [Google Scholar] [CrossRef]
- Moodley, J.S.; Krishna, S.B.N.; Pillay, K.; Sershen; Govender, P. Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015011. [Google Scholar] [CrossRef]
- Shah, S.; Gaikwad, S.; Nagar, S.; Kulshrestha, S.; Vaidya, V.; Nawani, N.; Pawar, S. Biofilm inhibition and anti-quorum sensing activity of phytosynthesized silver nanoparticles against the nosocomial pathogen Pseudomonas aeruginosa. Biofouling 2019, 35, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Yasmin, A.; Townley, H.E. An evaluation of the activity of biologically synthesized silver nanoparticles against bacteria, fungi and mammalian cell lines. Colloids Surf. B Biointerfaces 2020, 194, 111156. [Google Scholar] [CrossRef]
- Botcha, S.; Prattipati, S.D. Callus extract mediated green synthesis of silver nanoparticles, their characterization and cytotoxicity evaluation against MDA-MB-231 and PC-3 cells. BioNanoScience 2020, 10, 11–22. [Google Scholar] [CrossRef]
- Kanwal, Z.; Raza, M.A.; Manzoor, F.; Riaz, S.; Jabeen, G.; Fatima, S.; Naseem, S. A comparative assessment of nanotoxicity induced by metal (silver, nickel) and metal oxide (cobalt, chromium) nanoparticles in Labeo rohita. Nanomaterials 2019, 9, 309. [Google Scholar] [CrossRef]
- Haidari, H.; Bright, R.; Garg, S.; Vasilev, K.; Cowin, A.J.; Kopecki, Z. Eradication of mature bacterial biofilms with concurrent improvement in chronic wound healing using silver nanoparticle hydrogel treatment. Biomedicines 2021, 9, 1182. [Google Scholar] [CrossRef]
- Kanwal, Z.; Raza, M.A.; Manzoor, F.; Arshad, M.; Rashid, F.; Riaz, S.; Pervaiz, S.; Naseem, S. In vivo anti-proliferative activity of silver nanoparticles against Pseudomonas aeruginosa in freshwater Labeo rohita. Appl. Nanosci. 2019, 9, 2039–2049. [Google Scholar] [CrossRef]
- Aguirre, D.P.R.; Loyola, E.F.; Norma, M.; Sifuentes, L.R.; Moreno, A.R.; Marszalek, J.E. Comparative antibacterial potential of silver nanoparticles prepared via chemical and biological synthesis. Arab. J. Chem. 2020, 13, 8662–8670. [Google Scholar] [CrossRef]
- Gade, A.; Bonde, P.; Ingle, A.; Marcato, P.; Durán, N.; Rai, M. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J. Biobased Mater. Bioenergy 2008, 2, 243–247. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Alzohairy, M.A.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Raees, K. Biosynthesis of silver nanoparticles from oropharyngeal Candida glabrata isolates and their antimicrobial activity against clinical strains of bacteria and fungi. Nanomaterials 2018, 8, 586. [Google Scholar] [CrossRef]
- Tharani, M.; Rajeshkumar, S.; Al-Ghanim, K.A.; Nicoletti, M.; Sachivkina, N.; Govindarajan, M. Terminalia chebula-Assisted Silver Nanoparticles: Biological Potential, Synthesis, Characterization, and Ecotoxicity. Biomedicines 2023, 11, 1472. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmoodi, M.; Munir, A.; Zahra, S.A.; Shahbaz, A.; Shaukat, M.; Kanwal, S.; Uddin, S. Phytogenic synthesis of nickel oxide nanoparticles (NiO) using fresh leaves extract of Rhamnus triquetra (wall.) and investigation of its multiple in vitro biological potentials. Biomedicines 2020, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Banerjee, P.P.; Sadhu, A.; Sengupta, A.; Chatterjee, S.; Sarkar, S.; Barman, S.; Chattopadhyay, A.; Bhattacharya, S.; Mondal, N.C. Silver nanoparticles as antibacterial and anticancer materials against human breast, cervical and oral cancer cells. J. Nanosci. Nanotechnol. 2017, 17, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Devi, G.P.; Ahmed, K.B.A.; Varsha, M.S.; Shrijha, B.; Lal, K.S.; Anbazhagan, V.; Thiagarajan, R. Sulfidation of silver nanoparticle reduces its toxicity in zebrafish. Aquat. Toxicol. 2015, 158, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hu, X.; Wang, R.; Yuan, J.; Yin, D. Fullerene inhibits benzo (a) pyrene Efflux from Cyprinus carpio hepatocytes by affecting cell membrane fluidity and P-glycoprotein expression. Aquat. Toxicol. 2016, 174, 36–45. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi Chem. Soc. 2015, 19, 311–317. [Google Scholar] [CrossRef]
- Torres, M.A.; Testa, C.P.; Gáspari, C.; Masutti, M.B.; Panitz, C.M.N.; Curi-Pedrosa, R.; de Almeida, E.A.; Di Mascio, P.; Wilhelm Filho, D. Oxidative stress in the mussel Mytella guyanensis from polluted mangroves on Santa Catarina Island, Brazil. Mar. Pollut. Bull. 2002, 44, 923–932. [Google Scholar] [CrossRef]
- Lee, B.; Duong, C.N.; Cho, J.; Lee, J.; Kim, K.; Seo, Y.; Kim, P.; Choi, K.; Yoon, J. Toxicity of citrate-capped silver nanoparticles in common carp (Cyprinus carpio). J. Biotechnol. Biomed. 2012, 2012, 262670. [Google Scholar] [CrossRef] [PubMed]
- Vutukuru, S.S.; Chintada, S.; Radha Madhavi, K.; Venkateswara Rao, J.; Anjaneyulu, Y. Acute effects of copper on superoxide dismutase, catalase and lipid peroxidation in the freshwater teleost fish, Esomus danricus. Fish Physiol. Biochem. 2006, 32, 221–229. [Google Scholar] [CrossRef]
- Fard, M.T.; Arulselvan, P.; Karthivashan, G.; Adam, S.K.; Fakurazi, S. Bioactive extract from Moringa oleifera inhibits the pro-inflammatory mediators in lipopolysaccharide stimulated macrophages. Pharmacogn. Mag. 2015, 11, S556–S563. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef] [PubMed]
- Mahanty, A.; Mishra, S.; Bosu, R.; Maurya, U.; Netam, S.P.; Sarkar, B. Phytoextracts-synthesized silver nanoparticles inhibit bacterial fish pathogen Aeromonas hydrophila. Indian J. Microbiol. 2013, 53, 438–446. [Google Scholar] [CrossRef] [PubMed]












| Groups | Swimming | Feed Intake | Fin Movement | Hovering Behavior |
|---|---|---|---|---|
| Control | + | + | + | + |
| A. hyd | − | − | +− | − |
| A. hyd + Amp (75 µg/L) | − | + | +− | + |
| A. hyd + Cc-AgNPs (25 µg/L) | + | +− | + | + |
| A. hyd + Cc-AgNPs (50 µg/L) | + | + | + | + |
| A. hyd + Cc-AgNPs (75 µg/L) | +− | +− | +− | + |
| A. hyd + Pg-AgNPs (25 µg/L) | + | +− | + | + |
| A. hyd + Pg-AgNPs (50 µg/L) | + | + | − | +− |
| A. hyd + Pg-AgNPs (75 µg/L) | + | + | +− | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafeez, R.; Kanwal, Z.; Raza, M.A.; Rasool, S.; Riaz, S.; Naseem, S.; Rabani, S.; Haider, I.; Ahmad, N.; Alomar, S.Y. Role of Citrullus colocynthis and Psidium guajava Mediated Green Synthesized Silver Nanoparticles in Disease Resistance against Aeromonas hydrophila Challenge in Labeo rohita. Biomedicines 2023, 11, 2349. https://doi.org/10.3390/biomedicines11092349
Hafeez R, Kanwal Z, Raza MA, Rasool S, Riaz S, Naseem S, Rabani S, Haider I, Ahmad N, Alomar SY. Role of Citrullus colocynthis and Psidium guajava Mediated Green Synthesized Silver Nanoparticles in Disease Resistance against Aeromonas hydrophila Challenge in Labeo rohita. Biomedicines. 2023; 11(9):2349. https://doi.org/10.3390/biomedicines11092349
Chicago/Turabian StyleHafeez, Ramsha, Zakia Kanwal, Muhammad Akram Raza, Shafqat Rasool, Saira Riaz, Shahzad Naseem, Shifa Rabani, Imran Haider, Naushad Ahmad, and Suliman Yousef Alomar. 2023. "Role of Citrullus colocynthis and Psidium guajava Mediated Green Synthesized Silver Nanoparticles in Disease Resistance against Aeromonas hydrophila Challenge in Labeo rohita" Biomedicines 11, no. 9: 2349. https://doi.org/10.3390/biomedicines11092349
APA StyleHafeez, R., Kanwal, Z., Raza, M. A., Rasool, S., Riaz, S., Naseem, S., Rabani, S., Haider, I., Ahmad, N., & Alomar, S. Y. (2023). Role of Citrullus colocynthis and Psidium guajava Mediated Green Synthesized Silver Nanoparticles in Disease Resistance against Aeromonas hydrophila Challenge in Labeo rohita. Biomedicines, 11(9), 2349. https://doi.org/10.3390/biomedicines11092349