Free Fatty Acid Receptors 2 and 3 as Microbial Metabolite Sensors to Shape Host Health: Pharmacophysiological View
Abstract
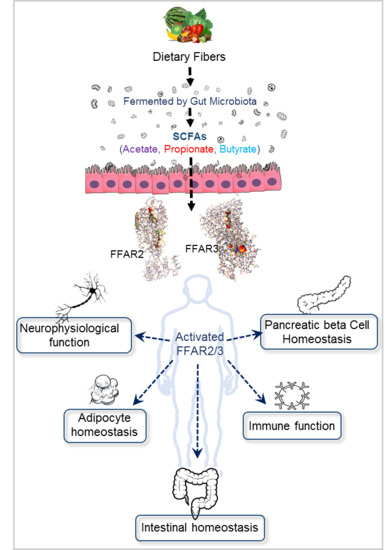
:1. Introduction
2. Experimental Section
2.1. Expression of FFAR2 and FFAR3 in Different Species and Tissues/Cells
2.1.1. FFAR2 Expression
2.1.2. FFAR3 Expression
3. Structures of FFAR2 and FFAR3
3.1. FFAR2
3.2. FFAR3
3.3. Comparative Structural Analyses of FFAR2 and FFAR3
4. Interactions of SCFAs with FFAR2/3
4.1. FFAR2 Interaction with SCFA
4.2. FFAR3 Interaction with SCFA
5. Interaction of Synthetic Ligands with FFAR2/3
5.1. FFAR2 Interaction with Synthetic Ligands
5.2. FFAR3 Interaction with Synthetic Ligands
6. Gut Microbiome Produces FFAR2/3 Ligands-SCFAs
7. Biological Functions Regulated by FFAR2/3 Signaling
7.1. FFAR2/3 in Immune Regulation
7.1.1. FFAR2 in Immune Regulation
7.1.2. FFAR3 in Immune Regulation
7.2. FFAR2/3 in Gut Hormonal Synthesis
7.2.1. FFAR2 in Gut Hormone Synthesis and Secretion
7.2.2. FFAR3 in Gut Hormone Synthesis
7.3. FFAR2/3 in Intestinal Epithelial Integrity and Inflammation
7.3.1. FFAR2 in Intestinal Epithelial Integrity and Inflammation
7.3.2. FFAR3 in Intestinal Epithelial Integrity and Inflammation
7.4. FFAR2/3 in Neurophysiology
7.4.1. FFAR2 in Neurophysiology
7.4.2. FFAR3 in Neurophysiology
7.5. FFAR2/3 in Adipogenesis and Lipolysis
7.5.1. FFAR2 in Adipogenesis and Lipolysis
| S.No. | Tissue/Organ | Research Findings | Ref. |
|---|---|---|---|
| Human | |||
| 1 | Intestinal L- cells | -Secrete GLP-1 and PYY in response to glucose | [3,11,76] |
| 2 | Intestinal I- cells | -Secrete Cholecystokinin (CCK) in response to glucose. | [3] |
| 3 | Intestinal K- cells | -Secrete glucose-dependent insulinotropic peptide (GIP) in response to glucose. | [3] |
| 4 | Colon | -No effect of propionate response on Intestinal Gluconeogenesis (IGN) genes (G6PC, PCK1, MUT) expression with either FFAR2 agonist such as tiglic acid (TA) or FFAR3 agonist i.e,1-methylcyclopropanecarboxylic acid (MA). -IGN gene expression increases by butyrate mediated through cAMP pathway but not via Gi- nor Gq pathway. -Neither Gi- nor Gq-sensitive inhibitors (PTX and U73122) able to reduce the IGN gene expression induced by butyrate. | [149] |
| 5 | Monocyte | -Human monocyte FFAR3 reduces cytokine expression in response to acetate. -The receptor modulates p38-MAPK signaling in response to acetate and FFAR3 agonist (AR420626). | [22] |
| 6 | Adipocytes | -FFAR3 expressed in the human multipotent adipose tissue-derived stem cells (hMADS). -Acetate is responsible for the antilipolytic response luminal and systemic level. -Rosiglitazone increases the expression of FFAR3. -FFAR3 stimulation develop anti-inflammatory action targeting TNFα and IL-1β. -Treating with Gi-sensitive PTX inhibitors prevents antilipolytic response develop by acetate. -Colonic or systemic acetate modulation helps in improving the insulin resistance in human adipocytes via FFAR3 mediated attenuation of hormone-sensitive lipase (HSL) phosphorylation. | [15,202] |
| 7 | Enteric Neurons | -FFAR3 agonist, AR420626 response at colon mucusa showed monophasic reductions in short-circuit currents (Isc) and sensitive to neurotoxin tetrodotoxin (TTX). -At submucosal and myenteric neuronal plexus, the FFAR3 is colocalized with Vasoactive intestinal polypeptide (VIP). -FFAR3 antagonist AR399519 inhibits FFAR3 agonism activity in entire colonic region. | [3,165] |
| Mouse/Rodent | |||
| 1 | Pancreatic α- and β-cells | -FFAR3 is transcribed from the promoter of the GPR40. -The expression is mediated via an internal ribosomal entry site (IRES) located in the intergenic region of a bicistronic mRNA. -Helps in proper understanding in the identification of therapeutic target. | [16,28,73,203] |
| 2 | Primary Pancretic Islet | -FFAR3 expression in murine pancreatic islet -Leads to reduction of insulin secretion by coupling to Gi-type G Proteins in type-2 diabetic condition. -Locally to islet as well as in systemic circulation acetate concentration increases. -So, in type-2 diabetic condition FFAR3 antagonist may increase insulin secretion | [16,28,168] |
| 3 | Primary Pancreatic Islet | -Infusion of Acetate, propionate and butyrate has no profound effect on insulin and glucagon secretion regardless of glucose level. -Whereas, FFAR3 agonist Compound 4 (N-(2,5-dichlorophenyl)-4-(furan2-yl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxamide) has significant effect in increasing the somatostatin and insulin secretion but showed no effect on glucagon synthesis. | [168] |
| 4 | Sympathetic Nervous System (SNS) | -Expressed in the rodent SNS especially at Superior cervical ganglia (SCG) and Celiac-mesenteric Ganglia (CSMG). -Induced variable ICa2+ modulation activity by sodium propionate in the FFAR3+/+ mice. -Moreover, along with acetate and propionate, ketogenic metabolites β-hydroxybutyrate (BHB) produced voltage dependent reduction of N-type Ca2+ channel in SNS. -FFAR3-expressing neurons from reporter mice expressed decrease in Cav2.2-FFAR3 inhibitory coupling variability. -FFAR3 is expressed primarily in neurons with a vasoconstrictor phenotype. | [101,102,104] |
| 5 | Superior cervical ganglia (SCG) | -Propionate enhances the norepinephrine (NE) release from primary-cultured mice SCG. -Pretreatment with Gi/o pathway sensitive-PTX; Gβγ inhibitor-Gallin; PLC inhibitor U73122 and MEK inhibitor U0126 significantly reduces NE secretion indicating the involvement of Gi/o, Gβγ, PLCβ3 pathway in hormonal secretory function. -Treatment with Gαi/o inhibitor NF023 shows no inhibition of NE release, so FFAR3 response is independent of Gαi/o pathway. -Further, treatment with siRNA against either PLCβ3 or ERK1/2 decreases the expression NE protein by more than 80%. -So, SCFA receptor FFAR3 is coupled with Gi/o protein, to release NE via Gβγ-PLCβ3- ERK1/2-synapsin 2 pathway. | [103] |
| 6 | Intestine | -IGN induction is mediated by propionate through gut-brain axis. -Dietary propionate leads to c-Fos (neuronal activation marker) activation in the hypothalamic region which receives neuronal signal from both parabrachial nucleus (PBN) and dorsal vagal complex (DVC), mostly paraventricular nucleus (PVN), the lateral hypothalamus (LH) and the arcuate nucleus (ARC) of hypothalamus. | [149] |
| 7 | Intestinal Enteroendocrine Cells | -Acetate, propionate and butyrate administration in mice protect against diet-induced obesity and insulin resistance. -Propionate and butyrate but not acetate induce gut hormones and reduces food intake. -Butyrate had minor effect in stimulation of GLP-1 through FFAR3. -FFAR3 KO mice shows normal body weight and glucose homeostasis, indicating some additional mediators are involves in these mechanism. -FFAR3 KO mice shows impair GLP-1 synthesis with altered in mRNA expression of Glucagon, PYY and active GLP-1 peptide. | [11,121,148,204] |
| 8 | Monocytes | -Mice monocyte shows increase in IL-1α, IL-1β and GM-CSF cytokine expression in response to acetate. -Even in FFAR2/3 KO mouse monocyte displays elevate cytokine response on treatment with SCFAs. -So, SCFA does not act through FFAR2 to modulate mice monocyte inflammatory responses. | [22] |
| 9 | Neutrophil | -FFAR3 pathway is associated with airway neutrophil response subjected to influenza infection verified in FFAR3 KO mice. | [114] |
| 10 | Bone marrow | -FFAR3 KO mice produce less monocytes and interstitial macrophages from the bone marrow in response to butyrate | [114] |
| 11 | Ileum and Colon | -Moreover, dietary (Flaxseed) fibers restructured the gut microbiota with proliferation of the genera Bifidobacterium and Akkermansia reduces fat mass and show improve tolerance to intraperitoneal and oral glucose via FFAR3. -Microbiota is associate with increase SCFA production acting through FFAR3 signaling. -Through selective FFAR3-agonist, AR420626 showed greatest efficacy of FFAR3 at distal regions of intestine to protect mice from diet induced obesity by preventing a reduction in energy expenditure induced by an HFD. | [148,165,198] |
| 12 | Colonic Mucosa | -FFAR2 express in the colonic mucosa -Withdrawal of ceftriaxone antibiotic leads to reduction in SCFA concentration and increase number of conditionally pathogenic Enterobacteria, E. coli, Clostridium, Staphylococcus spp. and hemolytic bacteria in colonic gut. -FFAR2 immune regulation mechanism get hampered with increase in cytokine concentration in colonic mucosa. -Increased histopathology condition of colitis with goblet cell dysfunction, colonic dilatation and wall thickening, ultimate leads to IBD. | [78] |
| 13 | Duodenum L- cells | -FFAR3 is colocalized with GLP1 and expressed in L cells. -SCFAs (mostly acetate) activate FFAR2 and FFAR3 followed by 5-HT and GLP-2 release. | [171] |
| 14 | Enteric Neurons | -FFAR3 agonism (by AR420626) at descending colon mucusa was inhibited by neurogenic sensitive tetrodotoxin (TTX). -FFAR3 agonist activity is sensitive to acetylcholinergic (ACh) neurotransmission in rat colon mucosa. -ACh muscarinic antagonist atropine, nicotinic sensitive hexamethonium, FFAR3 antagonist AR399519, GLP1 antagonist Ex(3-39) or calcitonin gene related peptide (CGRP) blocker BIBN4096 abolished FFAR3 agonism activity in mouse colon region. | [3,165,197] |
| 15 | Stomach | -By qrtPCR and immunohistochemistry showed the expression of FFAR3 in villi and microvilli of gastric brush cells of mice stomach. | [3,7,172] |
| 16 | Enteric mucosal and submucosal cholinergic neurons of rat | -Suppresses carbachol (CCh)- or luminal propionate-induced Cl- secretion influenced by TTX, hexamethonium and MQC through nicotinic ACh receptor activation. -SCFA-FFAR3 pathway responsible for anti-secretory function inhibited through cholinergic neural reflexes. -Pretreatment with serosal PTX along with MQC application restored the CCh response indicating the FFAR3 anti-secretory effect is mediated through Gi/o pathway in rat proximal colon. | [197] |
| 17 | Adipocytes | -A mixture of SCFA reduces plasma FFA in DIO mice along with beige adipogenesis marker. -Increase in adipose tissues with reduction in colon size. -Reduction in Firmicutes: Bacteroidetes ratio. -Reduces body weight by increasing mitochondrial biogenesis and reducing chronic inflammation. | [19,199] |
| 18 | Lungs | -Expressed in the mice lungs. -Propionate minimize allergy airway inflammation in mice lungs mediated through FFAR3. | [6] |
| 19 | Duodenal I-cells | -The receptor senses the circulating SCFA in plasma to modulate I-cell functions. -But unlike the LCFA, SCFAs are not involved in the cholecystokinin synthesis from duodenal I-cells. | [205] |
7.5.2. FFAR3 in Adipogenesis and Lipolysis
7.6. FFAR2/3 in Regulating Pancreatic Beta-Cells Proliferation and Functions
7.6.1. FFAR2 in Regulating Pancreatic Beta-Cell Proliferation and Functions
7.6.2. FFAR3 in Regulating Pancreatic Beta-Cell Proliferation and Functions
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SCFAs | Short-chain fatty acids |
| FFAR2 | Free fatty acid receptor 2 |
| FFAR3 | Free fatty acid receptor 3 |
| GPCRs | G-coupled protein receptors |
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes |
| IBD | Inflammatory bowel disease |
| TM | Transmembrane |
| PBMCs | Peripheral blood mononuclear cells |
| PMNs | Polymorphonuclear cells |
| DCs | Dendritic cells |
| WAT | White adipose tissue |
| BAT | Brown adipose tissue |
| bMEC | Bovine mammary epithelial cell line |
| SNS | Sympathetic nervous system |
| CNS | Central nervous system |
| CRC | Colorectal cancer |
| SCG | Superior cervical ganglia |
| CSMG | Celiac sympathetic-mesenteric ganglia |
| GI | Gastrointestinal |
| AAs | Amino acids |
| Tyr | Tyrosine |
| Ile | Isoleucine |
| Arg | Arginine |
| Glu | Glutamate |
| EL | Extracellular loop |
| SCAs | Small carboxylic acids |
| Trp | Tryptophan |
| Gln | Glutamine |
| His | Histidine |
| Thr | Threonine |
| Phe | Phenylalanine |
| Leu | Leucine |
| SAR | Structure activity relationship |
| Å | Angstrom |
| SASA | Solvent accessible surface area |
| H | Hydrogen |
| PYY | Peptide YY |
| Cmp1 | Compound 1 |
| CFMB/Phenylacetamide 1 | [(S)-2-(4-chlorophenyl)-3,3-dimethyl- N-(5-phenylthiazol-2-yl)butamide |
| AMG-7703 | (2S)-2-(4-chlorophenyl)-3-methyl-N-(1,3-thiazol-2-yl)butanamide |
| Val | Valine |
| 4-CMTB | 2-(4-chlorophenyl)-3-methyl-N-(thiazole-2-yl)butanamide |
| 2CTAP | 4-((4-(2-chlorophenyl)thiazole-2-yl)amino)-4oxo-3-phenylbutanoic acid |
| BTI-A-404 | [4-[4-(dimethylamino)phenyl]-N-(3,5-dimethylphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydro-5-pyrimidinecarboxamide] |
| BTI-A-292 | [4-[4-(dimethylamino) phenyl]-N-(4,5-dimethylphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydro-5-pyrimidinecarboxamide] |
| CATPB | (S)-3-(2-(3-chlorophenyl)acetamido)-4-(4-(trifluoromethyl)phenyl)butanoic acid |
| GLPG0974 | 4-[[(2R)-1-(1-benzothiophene-3-carbonyl)-2-methylazetidine-2-carbonyl]-[(3-chlorophenyl)methyl]amino]butanoic acid |
| Compound 1 | 3-benzyl-4-(cyclopropyl-(4-(2,5-dichlorophenyl)thiazol-2-yl)amino)-4-oxobutanoic acid |
| Phenylacetamide 2/Compound 44 | (S)-2-(4-chlorophenyl)-N-(5-fluorothiazol-2-yl)-3-methylbutanamide |
| Phenylacetamide 58 | (S)-2-(4-chlorophenyl)-3,3-dimethyl-N-(5-phenylthiazol-2-yl)butanamide |
| 1-MCPC | 1-methylcyclopropane carboxylate; AR420626: N-(2,5-dichlorophenyl)-4-(furan-2-yl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxamide |
| CF3-MQC | N-(2-methylphenyl)-4-[5-(2-trifluoromethoxy-phenyl)-furan-2-yl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxamide |
| MQC | N-[2-methylphenyl]-[4-furan-3-yl]-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxamide |
| DSS | Dextran sodium sulphate |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| Ig | Immunoglobulin |
| CRAMP | Cathelicidin related antimicrobial peptide |
| NFκB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| AMPK-α | 5’ adenosine monophosphate-activated protein kinase |
| PI3K | Phosphatidylinositol 3-kinase |
| Akt | Protein Kinase B |
| ERK | Extracellular signal-regulated kinases |
| INFγ | Interferon gamma |
| PTX | Pertussis toxin |
| JNK | c-Jun N-terminal kinase |
| iNOS | Induced nitric oxide synthase |
| HUVEC | Human umbilical vein endothelial cells |
| HDACi | Histone deacetylases inhibitor |
| Treg | Regulatory T-cells |
| WT | Wild type |
| mTOR | The mammalian target of rapamycin |
| eNOS | Exhaled nitric oxide |
| TNF-α | Tumor Necrosis Factor alpha |
| GIT | Gastrointestinal tract |
| GLP-1 | Glucagon-like peptide 1 |
| POMC | Pro-opiomelanocortin |
| NPY | Neuropeptide Y |
| AgRP | Agouti-related peptide |
| qRTPCR | Quantitative reverse transcription polymerase chain reaction |
| 5-HT | Hydroxytryptamine |
| 5-HCO3- | Bicarbonate |
| cAMP | Cyclic adenosine monophosphate |
| FACS | Fluorescence-activated cell sorting |
| mRFP | Monomeric red fluorescent protein |
| GIP | Gastric inhibitory polypeptide |
| RYGB | Roux-en-Y gastric bypass |
| M-cells | Myeloid cells |
| IECs | Intestinal epithelial cells |
| 5-HT | 5-Hydroxytryptamine |
| IL | Interleukin |
| Tjp1 | Tight junction protein 1 |
| Ocln | Occludin |
| Cldn | Claudin |
| Muc | Mucin |
| AMPK | AMP-activated protein kinase |
| HIF | Hypoxia-inducible factor |
| DSS | Dextran sodium sulphate |
| TNBS | Trinitrobenzoic sulphonic acid |
| PNS | Peripheral nervous system |
| BBB | Blood brain barrier |
| TH | Tyrosine hydroxylase |
| PLC | Phospholipase C |
| nAChR | Nicotinic acetylcholine receptor |
| Ca | Calcium |
| IP | Intraperitoneal |
| DIO | Diet induced obese |
| HFD | High fat diet |
| siRNA | Small interfering ribonucleic acid |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| hMADS | Human multipotent adipose tissue-derived stem |
| NC | Normal chow |
| OGTT | Oral glucose tolerance test |
| IM-BAT | Immortalized brown adipocyte cell line |
| GSIS | Glucose-stimulated insulin secretion |
| DSS | Dextran sodium sulphate |
| DAI | Daily activity index |
References
- Nagpal, R.; Wang, S.; Ahmadi, S.; Hayes, J.; Gagliano, J.; Subashchandrabose, S.; Kitzman, D.W.; Becton, T.; Read, R.; Yadav, H. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci. Rep. 2018, 8, 12649. [Google Scholar] [CrossRef] [Green Version]
- Nohr, M.K.; Pedersen, M.H.; Gille, A.; Egerod, K.L.; Engelstoft, M.S.; Husted, A.S.; Sichlau, R.M.; Grunddal, K.V.; Poulsen, S.S.; Han, S.; et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 2013, 154, 3552–3564. [Google Scholar] [CrossRef]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. PNAS USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, H.; Masujima, Y.; Ushiroda, C.; Mizushima, R.; Taira, S.; Ohue-Kitano, R.; Kimura, I. Dietary short-chain fatty acid intake improves the hepatic metabolic condition via FFAR3. Sci. Rep. 2019, 9, 16574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Eberle, J.A.; Widmayer, P.; Breer, H. Receptors for short-chain fatty acids in brush cells at the “gastric groove”. Front. Physiol. 2014, 5, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza, W.N.; Douangpanya, J.; Mu, S.; Jaeckel, P.; Zhang, M.; Maxwell, J.R.; Rottman, J.B.; Labitzke, K.; Willee, A.; Beckmann, H.; et al. Differing roles for short chain fatty acids and GPR43 agonism in the regulation of intestinal barrier function and immune responses. PLoS ONE 2017, 12, e0180190. [Google Scholar] [CrossRef] [Green Version]
- Erny, D.; de Angelis Hrabe, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Montalvany-Antonucci, C.C.; Duffles, L.F.; de Arruda, J.A.A.; Zicker, M.C.; de Oliveira, S.; Macari, S.; Garlet, G.P.; Madeira, M.F.M.; Fukada, S.Y.; Andrade, I., Jr.; et al. Short-chain fatty acids and FFAR2 as suppressors of bone resorption. Bone 2019, 125, 112–121. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Friedrichs, P.; Sauerwein, H.; Huber, K.; Locher, L.F.; Rehage, J.; Meyer, U.; Danicke, S.; Kuhla, B.; Mielenz, M. Expression of metabolic sensing receptors in adipose tissues of periparturient dairy cows with differing extent of negative energy balance. Animal 2016, 10, 623–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Su, H.; Zhou, Z.; Yao, W. Identification of the porcine G protein-coupled receptor 41 and 43 genes and their expression pattern in different tissues and development stages. PLoS ONE 2014, 9, e97342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivan, J.; Major, E.; Sipos, A.; Kovacs, K.; Horvath, D.; Tamas, I.; Bay, P.; Dombradi, V.; Lontay, B. The short-chain fatty acid propionate inhibits adipogenic differentiation of human chorion-derived mesenchymal stem cells through the free fatty acid receptor 2. Stem Cells Dev. 2017, 26, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Jocken, J.W.E.; Gonzalez Hernandez, M.A.; Hoebers, N.T.H.; van der Beek, C.M.; Essers, Y.P.G.; Blaak, E.E.; Canfora, E.E. Short-chain fatty acids differentially affect intracellular lipolysis in a human white adipocyte model. Front. Endocrinol. (Lausanne) 2017, 8, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veprik, A.; Laufer, D.; Weiss, S.; Rubins, N.; Walker, M.D. GPR41 modulates insulin secretion and gene expression in pancreatic beta-cells and modifies metabolic homeostasis in fed and fasting states. Faseb. J. 2016, 30, 3860–3869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Muller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M.; et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G900–G910. [Google Scholar] [CrossRef]
- Bindels, L.B.; Porporato, P.; Dewulf, E.M.; Verrax, J.; Neyrinck, A.M.; Martin, J.C.; Scott, K.P.; Buc Calderon, P.; Feron, O.; Muccioli, G.G.; et al. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br. J. Cancer 2012, 107, 1337–1344. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef] [Green Version]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. PNAS USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef] [Green Version]
- Bindels, L.B.; Porporato, P.E.; Ducastel, S.; Sboarina, M.; Neyrinck, A.M.; Dewulf, E.M.; Feron, O.; Lestavel, S.; Cani, P.D.; Staels, B.; et al. Ffar2 expression regulates leukaemic cell growth in vivo. Br. J. Cancer 2017, 117, 1336–1340. [Google Scholar] [CrossRef] [Green Version]
- Ang, Z.; Er, J.Z.; Tan, N.S.; Lu, J.; Liou, Y.C.; Grosse, J.; Ding, J.L. Human and mouse monocytes display distinct signalling and cytokine profiles upon stimulation with FFAR2/FFAR3 short-chain fatty acid receptor agonists. Sci. Rep. 2016, 6, 34145. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). PNAS USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, L.; Viardot, A.; Tsakmaki, A.; Stolarczyk, E.; Howard, J.K.; Cani, P.D.; Everard, A.; Sleeth, M.L.; Psichas, A.; Anastasovskaj, J.; et al. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol. Metab. 2017, 6, 48–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, S.R.; Priyadarshini, M.; Fuller, M.H.; Bhardwaj, T.; Brodsky, M.R.; Angueira, A.R.; Mosser, R.E.; Carboneau, B.A.; Tersey, S.A.; Mancebo, H.; et al. Loss of free fatty acid receptor 2 leads to impaired islet mass and beta cell survival. Sci. Rep. 2016, 6, 28159. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshini, M.; Villa, S.R.; Fuller, M.; Wicksteed, B.; Mackay, C.R.; Alquier, T.; Poitout, V.; Mancebo, H.; Mirmira, R.G.; Gilchrist, A.; et al. An acetate-specific GPCR, FFAR2, regulates insulin secretion. Mol. Endocrinol. 2015, 29, 1055–1066. [Google Scholar] [CrossRef] [Green Version]
- Remely, M.; Aumueller, E.; Merold, C.; Dworzak, S.; Hippe, B.; Zanner, J.; Pointner, A.; Brath, H.; Haslberger, A.G. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene 2014, 537, 85–92. [Google Scholar] [CrossRef]
- Tang, C.; Ahmed, K.; Gille, A.; Lu, S.; Grone, H.J.; Tunaru, S.; Offermanns, S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat. Med. 2015, 21, 173–177. [Google Scholar] [CrossRef]
- Shi, G.; Sun, C.; Gu, W.; Yang, M.; Zhang, X.; Zhai, N.; Lu, Y.; Zhang, Z.; Shou, P.; Zhang, Z.; et al. Free fatty acid receptor 2, a candidate target for type 1 diabetes, induces cell apoptosis through ERK signaling. J. Mol. Endocrinol. 2014, 53, 367–380. [Google Scholar] [CrossRef] [Green Version]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.; Li, X.; Weiszmann, J.; Wang, P.; Baribault, H.; Chen, J.L.; Tian, H.; Li, Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 2008, 149, 4519–4526. [Google Scholar] [CrossRef] [Green Version]
- McNelis, J.C.; Lee, Y.S.; Mayoral, R.; van der Kant, R.; Johnson, A.M.; Wollam, J.; Olefsky, J.M. GPR43 potentiates beta-cell function in obesity. Diabetes 2015, 64, 3203–3217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjursell, M.; Admyre, T.; Goransson, M.; Marley, A.E.; Smith, D.M.; Oscarsson, J.; Bohlooly, Y.M. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E211–E220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Chen, Y.; Jiang, H.; Robbins, G.T.; Nie, D. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int. J. Cancer 2011, 128, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhu, W.; Gong, J.; Zuo, L.; Zhao, J.; Sun, J.; Li, N.; Li, J. Dietary fiber intake is associated with increased colonic mucosal GPR43+ polymorphonuclear infiltration in active Crohn’s disease. Nutrients 2015, 7, 5327–5346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natarajan, N.; Hori, D.; Flavahan, S.; Steppan, J.; Flavahan, N.A.; Berkowitz, D.E.; Pluznick, J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016, 48, 826–834. [Google Scholar] [CrossRef]
- Ruan, J.; Meng, H.; Wang, X.; Chen, W.; Tian, X.; Meng, F. Low expression of FFAR2 in peripheral white blood cells may be a genetic marker for early diagnosis of Acute myocardial infarction. Cardiol. Res. Pract. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Vieira, A.T.; Macia, L.; Galvao, I.; Martins, F.S.; Canesso, M.C.; Amaral, F.A.; Garcia, C.C.; Maslowski, K.M.; De Leon, E.; Shim, D.; et al. A Role for gut microbiota and the metabolite-sensing receptor GPR43 in a murine model of gout. Arthritis Rheumatol. 2015, 67, 1646–1656. [Google Scholar] [CrossRef]
- Halnes, I.; Baines, K.J.; Berthon, B.S.; MacDonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Soluble fibre meal challenge reduces airway inflammation and expression of GPR43 and GPR41 in asthma. Nutrients 2017, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Eckalbar, W.L.; Erle, D.J. Singling out Th2 cells in eosinophilic esophagitis. J. Clin. Investig. 2019, 129, 1830–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masui, R.; Sasaki, M.; Funaki, Y.; Ogasawara, N.; Mizuno, M.; Iida, A.; Izawa, S.; Kondo, Y.; Ito, Y.; Tamura, Y.; et al. G protein-coupled receptor 43 moderates gut inflammation through cytokine regulation from mononuclear cells. Inflamm. Bowel Dis. 2013, 19, 2848–2856. [Google Scholar] [CrossRef] [PubMed]
- Sina, C.; Gavrilova, O.; Forster, M.; Till, A.; Derer, S.; Hildebrand, F.; Raabe, B.; Chalaris, A.; Scheller, J.; Rehmann, A.; et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 2009, 183, 7514–7522. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikhonova, I.G. Application of GPCR Structures for Modelling of Free Fatty Acid Receptors. Handb. Exp. Pharmacol. 2017, 236, 57–77. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Jiang, L.; Wang, J.; Zhang, J.; Kong, F.; Li, Q.; Yan, Y.; Huang, S.; Zhao, Y.; Liang, L.; et al. The G protein-coupled receptor FFAR2 promotes internalization during Influenza A Virus entry. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [Green Version]
- Ohira, H.; Fujioka, Y.; Katagiri, C.; Mamoto, R.; Aoyama-Ishikawa, M.; Amako, K.; Izumi, Y.; Nishiumi, S.; Yoshida, M.; Usami, M.; et al. Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J. Atheroscler. Thromb. 2013, 20, 425–442. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshini, M.; Layden, B.T. FFAR3 modulates insulin secretion and global gene expression in mouse islets. Islets 2015, 7, e1045182. [Google Scholar] [CrossRef] [Green Version]
- Hudson, B.D.; Tikhonova, I.G.; Pandey, S.K.; Ulven, T.; Milligan, G. Extracellular ionic locks determine variation in constitutive activity and ligand potency between species orthologs of the free fatty acid receptors FFA2 and FFA3. J. Biol. Chem. 2012, 287, 41195–41209. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.; Smith, N.J.; Christiansen, E.; Tikhonova, I.G.; Grundmann, M.; Hudson, B.D.; Ward, R.J.; Drewke, C.; Milligan, G.; Kostenis, E.; et al. Selective orthosteric free fatty acid receptor 2 (FFA2) agonists: Identification of the structural and chemical requirements for selective activation of FFA2 versus FFA3. J. Biol. Chem. 2011, 286, 10628–10640. [Google Scholar] [CrossRef] [Green Version]
- Tikhonova, I.G.; Poerio, E. Free fatty acid receptors: Structural models and elucidation of ligand binding interactions. BMC Struct. Biol. 2015, 15, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, N.E.; Kotarsky, K.; Owman, C.; Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003, 303, 1047–1052. [Google Scholar] [CrossRef]
- Cox, M.A.; Jackson, J.; Stanton, M.; Rojas-Triana, A.; Bober, L.; Laverty, M.; Yang, X.; Zhu, F.; Liu, J.; Wang, S.; et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J. Gastroenterol. 2009, 15, 5549–5557. [Google Scholar] [CrossRef]
- Carr, Z.J.; Van De Louw, A.; Fehr, G.; Li, J.D.; Kunselman, A.; Ruiz-Velasco, V. Increased whole blood FFA2/GPR43 receptor expression is associated with increased 30-day survival in patients with sepsis. BMC Res. Notes 2018, 11, 41. [Google Scholar] [CrossRef]
- Liu, D.; Costanzo, A.; Evans, M.D.M.; Archer, N.S.; Nowson, C.; Duesing, K.; Keast, R. Expression of the candidate fat taste receptors in human fungiform papillae and the association with fat taste function. Br. J. Nutr. 2018, 120, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Kirkling, M.E.; Cytlak, U.; Lau, C.M.; Lewis, K.L.; Resteu, A.; Khodadadi-Jamayran, A.; Siebel, C.W.; Salmon, H.; Merad, M.; Tsirigos, A.; et al. Notch signaling facilitates in vitro generation of cross-presenting classical dendritic cells. Cell Rep. 2018, 23, 3658–3672.e3656. [Google Scholar] [CrossRef]
- Pingitore, A.; Gonzalez-Abuin, N.; Ruz-Maldonado, I.; Huang, G.C.; Frost, G.; Persaud, S.J. Short chain fatty acids stimulate insulin secretion and reduce apoptosis in mouse and human islets in vitro: Role of free fatty acid receptor 2. Diabetes Obes. Metab. 2019, 21, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Pingitore, A.; Chambers, E.S.; Hill, T.; Maldonado, I.R.; Liu, B.; Bewick, G.; Morrison, D.J.; Preston, T.; Wallis, G.A.; Tedford, C.; et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes. Metab. 2017, 19, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Voltolini, C.; Battersby, S.; Etherington, S.L.; Petraglia, F.; Norman, J.E.; Jabbour, H.N. A novel antiinflammatory role for the short-chain fatty acids in human labor. Endocrinology 2012, 153, 395–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaki, S.; Tazoe, H.; Hayashi, H.; Kashiwabara, H.; Tooyama, K.; Suzuki, Y.; Kuwahara, A. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J. Mol. Histol. 2008, 39, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hoyles, L.; Snelling, T.; Umlai, U.K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome-host systems interactions: Protective effects of propionate upon the blood-brain barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonezawa, T.; Kobayashi, Y.; Obara, Y. Short-chain fatty acids induce acute phosphorylation of the p38 mitogen-activated protein kinase/heat shock protein 27 pathway via GPR43 in the MCF-7 human breast cancer cell line. Cell. Signal. 2007, 19, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Tazoe, H.; Otomo, Y.; Kaji, I.; Tanaka, R.; Karaki, S.I.; Kuwahara, A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J. Physiol. Pharmacol. 2008, 59 (Suppl. 2), 251–262. [Google Scholar]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, W.L., Jr. Short chain fatty acids and their receptors: New metabolic targets. J. Lab. Clin. Med. 2013, 161, 131–140. [Google Scholar] [CrossRef]
- Li, L.; Hua, Y.; Ren, J. Short-chain fatty acid propionate alleviates Akt2 knockout-induced myocardial contractile dysfunction. Exp. Diabetes Res. 2012, 2012, 851717. [Google Scholar] [CrossRef] [Green Version]
- Senga, T.; Iwamoto, S.; Yoshida, T.; Yokota, T.; Adachi, K.; Azuma, E.; Hamaguchi, M.; Iwamoto, T. LSSIG is a novel murine leukocyte-specific GPCR that is induced by the activation of STAT3. Blood 2003, 101, 1185–1187. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Agus, A.; Denizot, J.; Thevenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef] [Green Version]
- Den Besten, G.; Gerding, A.; van Dijk, T.H.; Ciapaite, J.; Bleeker, A.; van Eunen, K.; Havinga, R.; Groen, A.K.; Reijngoud, D.J.; Bakker, B.M. Protection against the metabolic syndrome by guar gum-derived short-chain fatty acids depends on peroxisome proliferator-activated receptor gamma and glucagon-like peptide-1. PLoS ONE 2015, 10, e0136364. [Google Scholar] [CrossRef] [PubMed]
- Bahar Halpern, K.; Veprik, A.; Rubins, N.; Naaman, O.; Walker, M.D. GPR41 gene expression is mediated by internal ribosome entry site (IRES)-dependent translation of bicistronic mRNA encoding GPR40 and GPR41 proteins. J. Biol. Chem. 2012, 287, 20154–20163. [Google Scholar] [CrossRef] [Green Version]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.; Smith, D.M.; Arch, J.R. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.C.; Feng, D.D.; Chen, C.; Lee, H.G.; et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaki, S.; Mitsui, R.; Hayashi, H.; Kato, I.; Sugiya, H.; Iwanaga, T.; Furness, J.B.; Kuwahara, A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006, 324, 353–360. [Google Scholar] [CrossRef]
- Dass, N.B.; John, A.K.; Bassil, A.K.; Crumbley, C.W.; Shehee, W.R.; Maurio, F.P.; Moore, G.B.; Taylor, C.M.; Sanger, G.J. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol. Motil. 2007, 19, 66–74. [Google Scholar] [CrossRef]
- Holota, Y.; Dovbynchuk, T.; Kaji, I.; Vareniuk, I.; Dzyubenko, N.; Chervinska, T.; Zakordonets, L.; Stetska, V.; Ostapchenko, L.; Serhiychuk, T.; et al. The long-term consequences of antibiotic therapy: Role of colonic short-chain fatty acids (SCFA) system and intestinal barrier integrity. PLoS ONE 2019, 14, e0220642. [Google Scholar] [CrossRef]
- Wang, A.; Gu, Z.; Heid, B.; Akers, R.M.; Jiang, H. Identification and characterization of the bovine G protein-coupled receptor GPR41 and GPR43 genes. J. Dairy Sci. 2009, 92, 2696–2705. [Google Scholar] [CrossRef] [Green Version]
- Aguinaga Casanas, M.A.; Schaff, C.T.; Albrecht, E.; Hammon, H.M.; Kuhla, B.; Rontgen, M.; Nurnberg, G.; Mielenz, M. Short communication: Free fatty acid receptors FFAR1 and FFAR2 during the peripartal period in liver of dairy cows grouped by their postpartum plasma beta-hydroxybutyrate concentrations. J. Dairy Sci. 2017, 100, 3287–3292. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.L.G.; Freitas, J.A.; Micai, B.; Azevedo, R.A.; Greco, L.F.; Santos, J.E.P. Effect of supplemental yeast culture and dietary starch content on rumen fermentation and digestion in dairy cows. J. Dairy Sci. 2018, 101, 201–221. [Google Scholar] [CrossRef]
- Hosseini, A.; Behrendt, C.; Regenhard, P.; Sauerwein, H.; Mielenz, M. Differential effects of propionate or beta-hydroxybutyrate on genes related to energy balance and insulin sensitivity in bovine white adipose tissue explants from a subcutaneous and a visceral depot. J. Anim. Physiol. Anim. Nutr. 2012, 96, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Chung, K.Y.; Johnson, B.J.; Go, G.W.; Kim, K.H.; Choi, C.W.; Smith, S.B. Co-culture of bovine muscle satellite cells with preadipocytes increases PPARgamma and C/EBPbeta gene expression in differentiated myoblasts and increases GPR43 gene expression in adipocytes. J. Nutr. Biochem. 2013, 24, 539–543. [Google Scholar] [CrossRef]
- Lemor, A.; Hosseini, A.; Sauerwein, H.; Mielenz, M. Transition period-related changes in the abundance of the mRNAs of adiponectin and its receptors, of visfatin, and of fatty acid binding receptors in adipose tissue of high-yielding dairy cows. Domest. Anim. Endocrinol. 2009, 37, 37–44. [Google Scholar] [CrossRef]
- Yonezawa, T.; Haga, S.; Kobayashi, Y.; Katoh, K.; Obara, Y. Short-chain fatty acid signaling pathways in bovine mammary epithelial cells. Regul. Pept. 2009, 153, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yao, W.; Jiang, H. Short-chain fatty acids enhance adipocyte differentiation in the stromal vascular fraction of porcine adipose tissue. J. Nutr. 2014, 144, 1887–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Ajuwon, K.M. Mechanism of Butyrate Stimulation of Triglyceride Storage and Adipokine Expression during Adipogenic Differentiation of Porcine Stromovascular Cells. PLoS ONE 2015, 10, e0145940. [Google Scholar] [CrossRef] [Green Version]
- Haenen, D.; Zhang, J.; da Silva Souza, C.; Bosch, G.; van der Meer, I.M.; van Arkel, J.; van den Borne, J.J.; Perez Gutierrez, O.; Smidt, H.; Kemp, B.; et al. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J. Nutr. 2013, 143, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Gossner, A.; Wilkie, H.; Joshi, A.; Hopkins, J. Exploring the abomasal lymph node transcriptome for genes associated with resistance to the sheep nematode Teladorsagia circumcincta. Vet. Res. 2013, 44, 68. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.Y.; Liu, L.; Gao, Q.; Sui, X.Y.; Li, F.C. Cloning, molecular characterization, and spatial and developmental expression analysis of GPR41 and GPR43 genes in New Zealand rabbits. Animal 2017, 11, 1798–1806. [Google Scholar] [CrossRef]
- Meslin, C.; Desert, C.; Callebaut, I.; Djari, A.; Klopp, C.; Pitel, F.; Leroux, S.; Martin, P.; Froment, P.; Guilbert, E.; et al. Expanding duplication of free fatty acid receptor-2 (GPR43) genes in the chicken genome. Genome Biol. Evol. 2015, 7, 1332–1348. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Ramsey, S.A.; Larson, M.K.; Berlow, N.E.; Ochola, D.; Shiprack, C.; Kashyap, A.; Seguin, B.; Keller, C.; Lohr, C.V. Elucidating the transcriptional program of feline injection-site sarcoma using a cross-species mRNA-sequencing approach. BMC Cancer 2019, 19, 311. [Google Scholar] [CrossRef] [PubMed]
- Read, J.E.; Cabrera-Sharp, V.; Offord, V.; Mirczuk, S.M.; Allen, S.P.; Fowkes, R.C.; de Mestre, A.M. Dynamic changes in gene expression and signalling during trophoblast development in the horse. Reproduction 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puhl, H.L., III; Won, Y.J.; Lu, V.B.; Ikeda, S.R. Human GPR42 is a transcribed multisite variant that exhibits copy number polymorphism and is functional when heterologously expressed. Sci. Rep. 2015, 5, 12880. [Google Scholar] [CrossRef] [Green Version]
- Regard, J.B.; Kataoka, H.; Cano, D.A.; Camerer, E.; Yin, L.; Zheng, Y.W.; Scanlan, T.S.; Hebrok, M.; Coughlin, S.R. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J. Clin. Investig. 2007, 117, 4034–4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layden, B.T.; Durai, V.; Newman, M.V.; Marinelarena, A.M.; Ahn, C.W.; Feng, G.; Lin, S.; Zhang, X.; Kaufman, D.B.; Jafari, N.; et al. Regulation of pancreatic islet gene expression in mouse islets by pregnancy. J. Endocrinol. 2010, 207, 265–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. PNAS USA 2004, 101, 1045–1050. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.; Mizukami, Y.; Miura, T.; Fujimoto, K.; Kobayashi, S.; Matsuzaki, M. Orphan G protein-coupled receptor, GPR41, induces apoptosis via a p53/Bax pathway during ischemic hypoxia and reoxygenation. J. Biol. Chem. 2001, 276, 26453–26460. [Google Scholar] [CrossRef] [Green Version]
- Azhar Aziz, M.; Al Mahri, S.; Al Ghamdi, A.; Mohammad, S. Role of free fatty acid receptor (FFAR3) in growth and proliferation of colorectal cancer cell line. Int. J. Cancer Res. 2019, 15, 17–22. [Google Scholar] [CrossRef]
- Stoddart, L.A.; Smith, N.J.; Jenkins, L.; Brown, A.J.; Milligan, G. Conserved polar residues in transmembrane domains V, VI, and VII of free fatty acid receptor 2 and free fatty acid receptor 3 are required for the binding and function of short chain fatty acids. J. Biol. Chem. 2008, 283, 32913–32924. [Google Scholar] [CrossRef] [Green Version]
- Nohr, M.K.; Egerod, K.L.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Moller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137. [Google Scholar] [CrossRef]
- Colina, C.; Puhl, H.L., III; Ikeda, S.R. Selective tracking of FFAR3-expressing neurons supports receptor coupling to N-type calcium channels in mouse sympathetic neurons. Sci. Rep. 2018, 8, 17379. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Kimura, I.; Wakabayashi, M.; Tsumoto, H.; Ozawa, K.; Hara, T.; Takei, Y.; Hirasawa, A.; Ishihama, Y.; Tsujimoto, G. Short-chain fatty acid receptor GPR41-mediated activation of sympathetic neurons involves synapsin 2b phosphorylation. FEBS Lett. 2012, 586, 1547–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Won, Y.J.; Lu, V.B.; Puhl, H.L., III; Ikeda, S.R. beta-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J. Neurosci. 2013, 33, 19314–19325. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Lee, J.H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef] [Green Version]
- Mukherji, A.; Kobiita, A.; Ye, T.; Chambon, P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 2013, 153, 812–827. [Google Scholar] [CrossRef] [Green Version]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; Delorey, T.M.; Howitt, M.R.; Katz, Y.; et al. A single-cell survey of the small intestinal epithelium. Nature 2017, 551, 333–339. [Google Scholar] [CrossRef]
- Nadjsombati, M.S.; McGinty, J.W.; Lyons-Cohen, M.R.; Jaffe, J.B.; DiPeso, L.; Schneider, C.; Miller, C.N.; Pollack, J.L.; Nagana Gowda, G.A.; Fontana, M.F.; et al. Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity 2018, 49, 33–41.e37. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.; O’Leary, C.E.; von Moltke, J.; Liang, H.E.; Ang, Q.Y.; Turnbaugh, P.J.; Radhakrishnan, S.; Pellizzon, M.; Ma, A.; Locksley, R.M. A metabolite-triggered tuft cell-ILC2 circuit drives small intestinal remodeling. Cell 2018, 174, 271–284.e214. [Google Scholar] [CrossRef] [Green Version]
- Han, J.H.; Kim, I.S.; Jung, S.H.; Lee, S.G.; Son, H.Y.; Myung, C.S. The effects of propionate and valerate on insulin responsiveness for glucose uptake in 3T3-L1 adipocytes and C2C12 myotubes via G protein-coupled receptor 41. PLoS ONE 2014, 9, e95268. [Google Scholar] [CrossRef] [Green Version]
- Mielenz, M.; Seybold, C.; Sauerwein, H. Effects of short-term infusion with propionate on the mRNA expression of a putative G-protein coupled receptor 41 (GPR41) in adipose tissue of goats. Livest. Sci. 2008, 116, 328–331. [Google Scholar] [CrossRef]
- Lemor, A.; Mielenz, M.; Altmann, M.; von Borell, E.; Sauerwein, H. mRNA abundance of adiponectin and its receptors, leptin and visfatin and of G-protein coupled receptor 41 in five different fat depots from sheep. J. Anim. Physiol.Anim. Nutr. 2010, 94, e96–e101. [Google Scholar] [CrossRef]
- Zou, J.; Chassaing, B.; Singh, V.; Pellizzon, M.; Ricci, M.; Fythe, M.D.; Kumar, M.V.; Gewirtz, A.T. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe 2018, 23, 41–53.e44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary fiber confers protection against flu by shaping Ly6c(-) patrolling monocyte hematopoiesis and CD8(+) T cell metabolism. Immunity 2018, 48, 992–1005.e1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.; Oshima, K.; Huang, Y.W.; Agle, K.A.; Drobyski, W.R.; Chen, X.; Zhang, J.; Yearsley, M.M.; Yu, J.; Wang, L.S. Loss of FFAR2 promotes colon cancer by epigenetic dysregulation of inflammation suppressors. Int. J. Cancer 2018, 143, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Villa, S.R.; Mishra, R.K.; Zapater, J.L.; Priyadarshini, M.; Gilchrist, A.; Mancebo, H.; Schiltz, G.E.; Layden, B.T. Homology modeling of FFA2 identifies novel agonists that potentiate insulin secretion. J. Investig. Med. 2017, 65, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Swaminath, G.; Jaeckel, P.; Guo, Q.; Cardozo, M.; Weiszmann, J.; Lindberg, R.; Wang, Y.; Schwandner, R.; Li, Y. Allosteric rescuing of loss-of-function FFAR2 mutations. FEBS Lett. 2010, 584, 4208–4214. [Google Scholar] [CrossRef] [Green Version]
- Frost, G.; Cai, Z.; Raven, M.; Otway, D.T.; Mushtaq, R.; Johnston, J.D. Effect of short chain fatty acids on the expression of free fatty acid receptor 2 (Ffar2), Ffar3 and early-stage adipogenesis. Nutr. Diabetes 2014, 4, e128. [Google Scholar] [CrossRef] [Green Version]
- Schofield, Z.V.; Croker, D.; Robertson, A.A.B.; Massey, N.L.; Donovan, C.; Tee, E.; Edwards, D.; Woodruff, T.M.; Halai, R.; Hansbro, P.M.; et al. Characterisation of small molecule ligands 4CMTB and 2CTAP as modulators of human FFA2 receptor signalling. Sci. Rep. 2018, 8, 17819. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Ang, Z.; Xiong, D.; Wu, M.; Ding, J.L. FFAR2-FFAR3 receptor heteromerization modulates short-chain fatty acid sensing. Faseb J. 2018, 32, 289–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Mikami, D.; Uwada, J.; Yazawa, T.; Kamiyama, K.; Kimura, H.; Taniguchi, T.; Iwano, M. A short-chain fatty acid, propionate, enhances the cytotoxic effect of cisplatin by modulating GPR41 signaling pathways in HepG2 cells. Oncotarget 2018, 9, 31342–31354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Mikami, D.; Kimura, H.; Kamiyama, K.; Morikawa, Y.; Yokoi, S.; Kasuno, K.; Takahashi, N.; Taniguchi, T.; Iwano, M. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem. Biophys. Res. Commun. 2017, 486, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 2018, 8, 74. [Google Scholar] [CrossRef]
- Thirunavukkarasan, M.; Wang, C.; Rao, A.; Hind, T.; Teo, Y.R.; Siddiquee, A.A.; Goghari, M.A.I.; Kumar, A.P.; Herr, D.R. Short-chain fatty acid receptors inhibit invasive phenotypes in breast cancer cells. PLoS ONE 2017, 12, e0186334. [Google Scholar] [CrossRef]
- Hu, S.; Kuwabara, R.; de Haan, B.J.; Smink, A.M.; de Vos, P. Acetate and Butyrate Improve beta-cell Metabolism and Mitochondrial Respiration under Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 1542. [Google Scholar] [CrossRef] [Green Version]
- Hudson, B.D.; Due-Hansen, M.E.; Christiansen, E.; Hansen, A.M.; Mackenzie, A.E.; Murdoch, H.; Pandey, S.K.; Ward, R.J.; Marquez, R.; Tikhonova, I.G.; et al. Defining the molecular basis for the first potent and selective orthosteric agonists of the FFA2 free fatty acid receptor. J. Biol. Chem. 2013, 288, 17296–17312. [Google Scholar] [CrossRef] [Green Version]
- Strausberg, R.L.; Feingold, E.A.; Grouse, L.H.; Derge, J.G.; Klausner, R.D.; Collins, F.S.; Wagner, L.; Shenmen, C.M.; Schuler, G.D.; Altschul, S.F.; et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. PNAS USA 2002, 99, 16899–16903. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Schiller, M.R. The carboxy-terminus, a key regulator of protein function. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 85–102. [Google Scholar] [CrossRef]
- Liaw, C.W.; Connolly, D.T. Sequence polymorphisms provide a common consensus sequence for GPR41 and GPR42. DNA Cell Biol. 2009, 28, 555–560. [Google Scholar] [CrossRef]
- Guha, R. On exploring structure-activity relationships. Methods Mol. Biol. 2013, 993, 81–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durham, E.; Dorr, B.; Woetzel, N.; Staritzbichler, R.; Meiler, J. Solvent accessible surface area approximations for rapid and accurate protein structure prediction. J. Mol. Model. 2009, 15, 1093–1108. [Google Scholar] [CrossRef] [Green Version]
- Stoddart, L.A.; Smith, N.J.; Milligan, G. International Union of Pharmacology. LXXI. Free fatty acid receptors FFA1, -2, and -3: Pharmacology and pathophysiological functions. Pharmacol. Rev. 2008, 60, 405–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhutia, Y.D.; Ganapathy, V. Short, but Smart: SCFAs Train T Cells in the Gut to Fight Autoimmunity in the Brain. Immunity 2015, 43, 629–631. [Google Scholar] [CrossRef] [Green Version]
- Sergeev, E.; Hansen, A.H.; Pandey, S.K.; MacKenzie, A.E.; Hudson, B.D.; Ulven, T.; Milligan, G. Non-equivalence of Key Positively Charged Residues of the Free Fatty Acid 2 Receptor in the Recognition and Function of Agonist Versus Antagonist Ligands. J. Biol. Chem. 2016, 291, 303–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzonero, M.; Dupont, S.; Babel, M.; Beaumont, S.; Bienvenu, N.; Blanque, R.; Cherel, L.; Christophe, T.; Crescenzi, B.; De Lemos, E.; et al. Discovery and optimization of an azetidine chemical series as a free fatty acid receptor 2 (FFA2) antagonist: From hit to clinic. J. Med. Chem. 2014, 57, 10044–10057. [Google Scholar] [CrossRef] [PubMed]
- Namour, F.; Galien, R.; Van Kaem, T.; Van der Aa, A.; Vanhoutte, F.; Beetens, J.; Van’t Klooster, G. Safety, pharmacokinetics and pharmacodynamics of GLPG0974, a potent and selective FFA2 antagonist, in healthy male subjects. Br. J. Clin. Pharmacol. 2016, 82, 139–148. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, X.; Kayser, F.; Liu, J.; Wang, Z.; Wanska, M.; Greenberg, J.; Weiszmann, J.; Ge, H.; Tian, H.; et al. The first synthetic agonists of FFA2: Discovery and SAR of phenylacetamides as allosteric modulators. Bioorgan. Med. Chem. Lett. 2010, 20, 493–498. [Google Scholar] [CrossRef]
- Hudson, B.D.; Ulven, T.; Milligan, G. The therapeutic potential of allosteric ligands for free fatty acid sensitive GPCRs. Curr. Top. Med. Chem. 2013, 13, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.; Schwandner, R.; Swaminath, G.; Weiszmann, J.; Cardozo, M.; Greenberg, J.; Jaeckel, P.; Ge, H.; Wang, Y.; Jiao, X.; et al. Identification and functional characterization of allosteric agonists for the G protein-coupled receptor FFA2. Mol. Pharmacol. 2008, 74, 1599–1609. [Google Scholar] [CrossRef] [Green Version]
- Swaminath, G.; Jaeckel, P.; Guo, Q.; Cardozo, M.; Weiszmann, J.; Lindberg, R.; Wang, Y.; Schwandner, R.; Li, Y. Mutational analysis of G-protein coupled receptor--FFA2. Biochem. Biophys. Res. Commun. 2011, 405, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Park, B.O.; Kim, S.H.; Kong, G.Y.; Kim, D.H.; Kwon, M.S.; Lee, S.U.; Kim, M.O.; Cho, S.; Lee, S.; Lee, H.J.; et al. Selective novel inverse agonists for human GPR43 augment GLP-1 secretion. Eur. J. Pharmacol. 2016, 771, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.J.; Milligan, G. Allostery at G protein-coupled receptor homo- and heteromers: Uncharted pharmacological landscapes. Pharmacol. Rev. 2010, 62, 701–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, N.J.; Ward, R.J.; Stoddart, L.A.; Hudson, B.D.; Kostenis, E.; Ulven, T.; Morris, J.C.; Trankle, C.; Tikhonova, I.G.; Adams, D.R.; et al. Extracellular loop 2 of the free fatty acid receptor 2 mediates allosterism of a phenylacetamide ago-allosteric modulator. Mol. Pharmacol. 2011, 80, 163–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, H.; Akiba, Y.; Narimatsu, K.; Maruta, K.; Kuri, A.; Iwamoto, K.I.; Kuwahara, A.; Kaunitz, J.D. FFA3 activation stimulates duodenal bicarbonate secretion and prevents NSAID-induced enteropathy via the GLP-2 Pathway in Rats. Dig. Dis. Sci. 2017, 62, 1944–1952. [Google Scholar] [CrossRef]
- Arora, T.; Rudenko, O.; Egerod, K.L.; Husted, A.S.; Kovatcheva-Datchary, P.; Akrami, R.; Kristensen, M.; Schwartz, T.W.; Backhed, F. Microbial fermentation of flaxseed fibers modulates the transcriptome of GPR41-expressing enteroendocrine cells and protects mice against diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E453–E463. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Toth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Galvao, I.; Tavares, L.P.; Correa, R.O.; Fachi, J.L.; Rocha, V.M.; Rungue, M.; Garcia, C.C.; Cassali, G.; Ferreira, C.M.; Martins, F.S.; et al. The metabolic sensor GPR43 receptor plays a role in the control of Klebsiella pneumoniae infection in the lung. Front. Immunol. 2018, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Furio, L.; Mecheri, R.; van der Does, A.M.; Lundeberg, E.; Saveanu, L.; Chen, Y.; van Endert, P.; Agerberth, B.; Diana, J. Pancreatic beta-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 2015, 43, 304–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Le Leu, R.K.; Christophersen, C.T.; Somashekar, R.; Conlon, M.A.; Meng, X.Q.; Winter, J.M.; Woodman, R.J.; McKinnon, R.; Young, G.P. Manipulation of the gut microbiota using resistant starch is associated with protection against colitis-associated colorectal cancer in rats. Carcinogenesis 2016, 37, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Nastasi, C.; Candela, M.; Bonefeld, C.M.; Geisler, C.; Hansen, M.; Krejsgaard, T.; Biagi, E.; Andersen, M.H.; Brigidi, P.; Odum, N.; et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 2015, 5, 16148. [Google Scholar] [CrossRef]
- Chun, E.; Lavoie, S.; Fonseca-Pereira, D.; Bae, S.; Michaud, M.; Hoveyda, H.R.; Fraser, G.L.; Gallini Comeau, C.A.; Glickman, J.N.; Fuller, M.H.; et al. Metabolite-sensing receptor Ffar2 regulates colonic group 3 innate lymphoid cells and gut immunity. Immunity 2019, 51, 871–884.e876. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Gurav, A.; Paschall, A.V.; Coe, G.L.; Chaudhary, K.; Cai, Y.; Kolhe, R.; Martin, P.; Browning, D.; Huang, L.; et al. An essential role of Ffar2 (Gpr43) in dietary fibre-mediated promotion of healthy composition of gut microbiota and suppression of intestinal carcinogenesis. Oncogenesis 2016, 5, e238. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Sun, M.; Chen, F.; Cao, A.T.; Liu, H.; Zhao, Y.; Huang, X.; Xiao, Y.; Yao, S.; Zhao, Q.; et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017, 10, 946–956. [Google Scholar] [CrossRef] [Green Version]
- Pirozzi, C.; Francisco, V.; Guida, F.D.; Gomez, R.; Lago, F.; Pino, J.; Meli, R.; Gualillo, O. Butyrate modulates inflammation in Chondrocytes via GPR43 receptor. Cell Physiol. Biochem. 2018, 51, 228–243. [Google Scholar] [CrossRef]
- Fujiwara, H.; Docampo, M.D.; Riwes, M.; Peltier, D.; Toubai, T.; Henig, I.; Wu, S.J.; Kim, S.; Taylor, A.; Brabbs, S.; et al. Microbial metabolite sensor GPR43 controls severity of experimental GVHD. Nat. Commun. 2018, 9, 3674. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Ferguson, G.J.; Kulkarni, S.; Damoulakis, G.; Anderson, K.; Bohlooly, Y.M.; Stephens, L.; Hawkins, P.T.; Curi, R. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS ONE 2011, 6, e21205. [Google Scholar] [CrossRef]
- Petersen, N.; Reimann, F.; Bartfeld, S.; Farin, H.F.; Ringnalda, F.C.; Vries, R.G.; van den Brink, S.; Clevers, H.; Gribble, F.M.; de Koning, E.J. Generation of L cells in mouse and human small intestine organoids. Diabetes 2014, 63, 410–420. [Google Scholar] [CrossRef] [Green Version]
- Bjorkman, L.; Martensson, J.; Winther, M.; Gabl, M.; Holdfeldt, A.; Uhrbom, M.; Bylund, J.; Hojgaard Hansen, A.; Pandey, S.K.; Ulven, T.; et al. The neutrophil response induced by an agonist for free fatty Acid receptor 2 (GPR43) is primed by tumor necrosis factor alpha and by receptor uncoupling from the cytoskeleton but attenuated by tissue recruitment. Mol. Cell Biol. 2016, 36, 2583–2595. [Google Scholar] [CrossRef] [Green Version]
- Seljeset, S.; Siehler, S. Receptor-specific regulation of ERK1/2 activation by members of the “free fatty acid receptor” family. J. Recept. Signal. Transduct. Res. 2012, 32, 196–201. [Google Scholar] [CrossRef]
- Tough, I.R.; Forbes, S.; Cox, H.M. Signaling of free fatty acid receptors 2 and 3 differs in colonic mucosa following selective agonism or coagonism by luminal propionate. Neurogastroenterol. Motil. 2018, 30, e13454. [Google Scholar] [CrossRef]
- Fuller, M.; Li, X.; Fisch, R.; Bughara, M.; Wicksteed, B.; Kovatcheva-Datchary, P.; Layden, B.T. FFA2 contribution to gestational glucose tolerance is not disrupted by antibiotics. PLoS ONE 2016, 11, e0167837. [Google Scholar] [CrossRef]
- Fuller, M.; Priyadarshini, M.; Gibbons, S.M.; Angueira, A.R.; Brodsky, M.; Hayes, M.G.; Kovatcheva-Datchary, P.; Backhed, F.; Gilbert, J.A.; Lowe, W.L., Jr.; et al. The short-chain fatty acid receptor, FFA2, contributes to gestational glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E840–E851. [Google Scholar] [CrossRef] [Green Version]
- Ørgaard, A.; Jepsen, S.L.; Holst, J.J. Short-chain fatty acids and regulation of pancreatic endocrine secretion in mice. Islets 2019, 11, 103–111. [Google Scholar] [CrossRef]
- Zadeh-Tahmasebi, M.; Duca, F.A.; Rasmussen, B.A.; Bauer, P.V.; Cote, C.D.; Filippi, B.M.; Lam, T.K. Activation of short and long chain fatty acid sensing machinery in the ileum lowers glucose production in vivo. J. Biol. Chem. 2016, 291, 8816–8824. [Google Scholar] [CrossRef] [Green Version]
- Forbes, S.; Stafford, S.; Coope, G.; Heffron, H.; Real, K.; Newman, R.; Davenport, R.; Barnes, M.; Grosse, J.; Cox, H. Selective FFA2 agonism appears to act via intestinal PYY to reduce transit and food intake but does not improve glucose tolerance in mouse models. Diabetes 2015, 64, 3763–3771. [Google Scholar] [CrossRef] [Green Version]
- Akiba, Y.; Inoue, T.; Kaji, I.; Higashiyama, M.; Narimatsu, K.; Iwamoto, K.; Watanabe, M.; Guth, P.H.; Engel, E.; Kuwahara, A.; et al. Short-chain fatty acid sensing in rat duodenum. J. Physiol. 2015, 593, 585–599. [Google Scholar] [CrossRef] [Green Version]
- Engelstoft, M.S.; Park, W.M.; Sakata, I.; Kristensen, L.V.; Husted, A.S.; Osborne-Lawrence, S.; Piper, P.K.; Walker, A.K.; Pedersen, M.H.; Nohr, M.K.; et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol. Metab. 2013, 2, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.; Aronow, B.J.; Rochman, Y.; Rochman, M.; Kc, K.; Dexheimer, P.J.; Putnam, P.; Mukkada, V.; Foote, H.; Rehn, K.; et al. Single-cell RNA sequencing identifies inflammatory tissue T cells in eosinophilic esophagitis. J. Clin. Investig. 2019, 129, 2014–2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; van Esch, B.C.A.M.; Henricks, P.A.J.; Garssen, J.; Folkerts, G. Time and concentration dependent effects of short chain fatty acids on Lipopolysaccharide- or tumor necrosis factor α-induced Endothelial activation. Front. Pharmacol. 2018, 9, 233. [Google Scholar] [CrossRef]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 2019, 50, 432–445.e437. [Google Scholar] [CrossRef] [Green Version]
- Chang, G.; Zhang, H.; Wang, Y.; Ma, N.; Chandra, R.A.; Ye, G.; Zhuang, S.; Zhu, W.; Shen, X. Microbial community shifts elicit inflammation in the caecal mucosa via the GPR41/43 signalling pathway during subacute ruminal acidosis. BMC Vet. Res. 2019, 15, 298. [Google Scholar] [CrossRef]
- Marino, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 2017, 18, 552–562. [Google Scholar] [CrossRef]
- Sanford, J.A.; O’Neill, A.M.; Zouboulis, C.C.; Gallo, R.L. Short-chain fatty acids from Cutibacterium acnes activate both a canonical and epigenetic inflammatory response in human Sebocytes. J. Immunol. 2019, 202, 1767–1776. [Google Scholar] [CrossRef] [Green Version]
- Egerod, K.L.; Engelstoft, M.S.; Grunddal, K.V.; Nohr, M.K.; Secher, A.; Sakata, I.; Pedersen, J.; Windelov, J.A.; Fuchtbauer, E.M.; Olsen, J.; et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 2012, 153, 5782–5795. [Google Scholar] [CrossRef] [Green Version]
- Kaji, I.; Karaki, S.; Tanaka, R.; Kuwahara, A. Density distribution of free fatty acid receptor 2 (FFA2)-expressing and GLP-1-producing enteroendocrine L cells in human and rat lower intestine, and increased cell numbers after ingestion of fructo-oligosaccharide. J. Mol. Histol. 2011, 42, 27–38. [Google Scholar] [CrossRef]
- Karaki, S.; Kuwahara, A. Propionate-induced epithelial K(+) and Cl(-)/HCO3(-) secretion and free fatty acid receptor 2 (FFA2, GPR43) expression in the guinea pig distal colon. Pflugers Archiv. 2011, 461, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.Y.; Zhang, X.; Miyamoto, J.; Kimura, I.; Taknaka, T.; Furusawa, K.; Jomori, T.; Fujimoto, K.; Uematsu, S.; Miki, T. Gut carbohydrate inhibits GIP secretion via a microbiota/SCFA/FFAR3 pathway. J. Endocrinol. 2018, 239, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peiris, M.; Aktar, R.; Raynel, S.; Hao, Z.; Mumphrey, M.B.; Berthoud, H.R.; Blackshaw, L.A. Effects of obesity and gastric bypass surgery on nutrient sensors, endocrine cells, and mucosal innervation of the mouse colon. Nutrients 2018, 10, 1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, H.; Jiao, A.R.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Huang, Z.Q.; Luo, Y.H.; Luo, J.Q.; Mao, X.B.; et al. Stimulation of intestinal growth with distal ileal infusion of short-chain fatty acid: A reevaluation in a pig model. RSC Adv. 2017, 7, 30792–30806. [Google Scholar] [CrossRef] [Green Version]
- Slawinska, A.; Dunislawska, A.; Plowiec, A.; Radomska, M.; Lachmanska, J.; Siwek, M.; Tavaniello, S.; Maiorano, G. Modulation of microbial communities and mucosal gene expression in chicken intestines after galactooligosaccharides delivery In Ovo. PLoS ONE 2019, 14, e0212318. [Google Scholar] [CrossRef]
- Lavoie, S.; Chun, E.; Bae, S.; Brennan, C.A.; Gallini Comeau, C.A.; Lang, J.K.; Michaud, M.; Hoveyda, H.R.; Fraser, G.L.; Fuller, M.H.; et al. Expression of FFAR2 by Dendritic Cells Prevents Their Expression of IL27 and is Required For Maintenance of Mucosal Barrier and Immune Response Against Colorectal Tumors in Mice. Gastroenterology 2020, 158, 1359–1372.e9. [Google Scholar] [CrossRef]
- Breton, J.; Ple, C.; Guerin-Deremaux, L.; Pot, B.; Lefranc-Millot, C.; Wils, D.; Foligne, B. Intrinsic immunomodulatory effects of low-digestible carbohydrates selectively extend their anti-inflammatory prebiotic potentials. Biomed. Res. Int. 2015, 2015, 162398. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Xiao, Y.; Huang, X.; Chen, F.; Sun, M.; Bilotta, A.J.; Xu, L.; Lu, Y.; Yao, S.; Zhao, Q.; et al. Microbiota metabolite short-chain fatty acids facilitate mucosal adjuvant activity of cholera toxin through GPR43. J. Immunol. 2019, 203, 282–292. [Google Scholar] [CrossRef]
- Pan, P.; Skaer, C.W.; Stirdivant, S.M.; Young, M.R.; Stoner, G.D.; Lechner, J.F.; Huang, Y.W.; Wang, L.S. Beneficial regulation of metabolic profiles by black raspberries in human colorectal cancer patients. Cancer Prev. Res. (Phila.) 2015, 8, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.; Skaer, C.W.; Wang, H.-T.; Stirdivant, S.M.; Young, M.R.; Oshima, K.; Stoner, G.D.; Lechner, J.F.; Huang, Y.-W.; Wang, L.-S. Black raspberries suppress colonic adenoma development in ApcMin/+ mice: Relation to metabolite profiles. Carcinogenesis 2015, 36, 1245–1253. [Google Scholar] [CrossRef] [Green Version]
- Hatanaka, H.; Tsukui, M.; Takada, S.; Kurashina, K.; Choi, Y.L.; Soda, M.; Yamashita, Y.; Haruta, H.; Hamada, T.; Ueno, T.; et al. Identification of transforming activity of free fatty acid receptor 2 by retroviral expression screening. Cancer Sci. 2010, 101, 54–59. [Google Scholar] [CrossRef]
- Han, M.; Song, P.; Huang, C.; Rezaei, A.; Farrar, S.; Brown, M.A.; Ma, X. Dietary grape seed proanthocyanidins (GSPs) improve weaned intestinal microbiota and mucosal barrier using a piglet model. Oncotarget 2016, 7, 80313–80326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Wang, Q.; Wu, Q.; Mao-Draayer, Y.; Kim, C.H. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci. Rep. 2019, 9, 8837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez Soto, E.J.; Gambino, L.O.; Mustafa, E.R. Free fatty acid receptor 3 is a key target of short chain fatty acid. What is the impact on the sympathetic nervous system? Channels (Austin) 2014, 8, 169–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaji, I.; Akiba, Y.; Konno, K.; Watanabe, M.; Kimura, S.; Iwanaga, T.; Kuri, A.; Iwamoto, K.; Kuwahara, A.; Kaunitz, J.D. Neural FFA3 activation inversely regulates anion secretion evoked by nicotinic ACh receptor activation in rat proximal colon. J. Physiol. 2016, 594, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- Kaji, I.; Akiba, Y.; Furuyama, T.; Adelson, D.W.; Iwamoto, K.; Watanabe, M.; Kuwahara, A.; Kaunitz, J.D. Free fatty acid receptor 3 activation suppresses neurogenic motility in rat proximal colon. Neurogastroenterol. Motil. 2018, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Kyrou, I.; Tan, B.K.; Dimitriadis, G.K.; Ramanjaneya, M.; Tripathi, G.; Patel, V.; James, S.; Kawan, M.; Chen, J.; et al. Short-chain fatty acid acetate stimulates adipogenesis and mitochondrial biogenesis via GPR43 in brown adipocytes. Endocrinology 2016, 157, 1881–1894. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, A.; Nakatani, A.; Hasegawa, S.; Irie, J.; Ozawa, K.; Tsujimoto, G.; Suganami, T.; Itoh, H.; Kimura, I. The short chain fatty acid receptor GPR43 regulates inflammatory signals in adipose tissue M2-type macrophages. PLoS ONE 2017, 12, e0179696. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, J.; Ohue-Kitano, R.; Mukouyama, H.; Nishida, A.; Watanabe, K.; Igarashi, M.; Irie, J.; Tsujimoto, G.; Satoh-Asahara, N.; Itoh, H.; et al. Ketone body receptor GPR43 regulates lipid metabolism under ketogenic conditions. PNAS USA 2019, 116, 23813–23821. [Google Scholar] [CrossRef] [Green Version]
- Muredda, L.; Kępczyńska, M.A.; Zaibi, M.S.; Alomar, S.Y.; Trayhurn, P. IL-1β and TNFα inhibit GPR120 (FFAR4) and stimulate GPR84 (EX33) and GPR41 (FFAR3) fatty acid receptor expression in human adipocytes: Implications for the anti-inflammatory action of n-3 fatty acids. Arch. Physiol. Biochem. 2018, 124, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Regard, J.B.; Sato, I.T.; Coughlin, S.R. Anatomical profiling of G protein-coupled receptor expression. Cell 2008, 135, 561–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellahcene, M.; O’Dowd, J.F.; Wargent, E.T.; Zaibi, M.S.; Hislop, D.C.; Ngala, R.A.; Smith, D.M.; Cawthorne, M.A.; Stocker, C.J.; Arch, J.R. Male mice that lack the G-protein-coupled receptor GPR41 have low energy expenditure and increased body fat content. Br. J. Nutr. 2013, 109, 1755–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sykaras, A.G.; Demenis, C.; Case, R.M.; McLaughlin, J.T.; Smith, C.P. Duodenal enteroendocrine I-cells contain mRNA transcripts encoding key endocannabinoid and fatty acid receptors. PLoS ONE 2012, 7, e42373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Zhou, A.; Cheung, P.C.K.; Zheng, B.; Zeng, S.; Lin, S. Expression of GPR43 in brown adipogenesis is enhanced by rosiglitazone and controlled by PPARgamma/RXR Heterodimerization. PPAR Res. 2018, 2018, 1051074. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Ge, Q.; Bindels, L.B.; Sohet, F.M.; Cani, P.D.; Brichard, S.M.; Delzenne, N.M. Evaluation of the relationship between GPR43 and adiposity in human. Nutr. Metab. 2013, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Daniele, G.; Eldor, R.; Merovci, A.; Clarke, G.D.; Xiong, J.; Tripathy, D.; Taranova, A.; Abdul-Ghani, M.; DeFronzo, R.A. Chronic reduction of plasma free fatty acid improves mitochondrial function and whole-body insulin sensitivity in obese and type 2 diabetic individuals. Diabetes 2014, 63, 2812–2820. [Google Scholar] [CrossRef] [Green Version]
- Kimura, I.; Miyamoto, J.; Ohue-Kitano, R.; Watanabe, K.; Yamada, T.; Onuki, M.; Aoki, R.; Isobe, Y.; Kashihara, D.; Inoue, D.; et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 2020, 367. [Google Scholar] [CrossRef]
- Ximenes, H.M.; Hirata, A.E.; Rocha, M.S.; Curi, R.; Carpinelli, A.R. Propionate inhibits glucose-induced insulin secretion in isolated rat pancreatic islets. Cell Biochem. Funct. 2007, 25, 173–178. [Google Scholar] [CrossRef]
- Bodis, K.; Kahl, S.; Simon, M.C.; Zhou, Z.; Sell, H.; Knebel, B.; Tura, A.; Strassburger, K.; Burkart, V.; Mussig, K.; et al. Reduced expression of stearoyl-CoA desaturase-1, but not free fatty acid receptor 2 or 4 in subcutaneous adipose tissue of patients with newly diagnosed type 2 diabetes mellitus. Nutr. Diabetes 2018, 8, 49. [Google Scholar] [CrossRef] [Green Version]







| S. No. | Cell Line/Type | Species | Tissues/Cells | Expression | Reference | |
|---|---|---|---|---|---|---|
| FFAR2 | FFAR3 | |||||
| 1 | 3T3-L1 | Mice | Adipose Tissue | Yes | Yes | Author *, [30,31,53,75,119] |
| 2 | 3T3-L442A | Mice | Adipose Tissue | Yes | Yes | [53] |
| 3 | αTC1 | Mice | Pancreatic α-cells | Lesser Extent | No | [73] |
| 4 | βTC1 | Mice | Pancreatic β-cells | Yes | Yes | [73] |
| 5 | βTC3 | Mice | Pancreatic β-cells | Yes | No | [26,117] |
| 6 | βTCtet | Mice | Pancreatic Islet β-cells | Yes | Yes | [73] |
| 7 | AML-12 | Mice | Liver | Yes | Yes | Author * |
| 8 | AR42J | Rat | Pancreatic Exocrine | Lesser Extent | No | [73] |
| 9 | BaF3 | Mice | B Lymphocytes | Yes | Very low | [18] |
| 10 | C2BBe1 | Human | Clone of Caco-2 | Yes | NA | [8,25,32] |
| 11 | C2C12 | Mice | Muscle | Yes | Yes | Author * |
| 12 | Caco-2 | Human | Colon | Low level | NA | Author *, [8,34] |
| 13 | Cardiomyocytes | Mice | Heart | Yes | Yes | [23] |
| 14 | CBS | Human | Colorectal | No | NA | [34] |
| 15 | CHO-K1 | Hamster | Ovary | Yes | Yes | [54,55,118,120] |
| 16 | CMEC/D3 | Human | Brain Endothelium | NA | Yes | [64] |
| 17 | CMT93 | Mice | Rectal Cell | Yes | Yes | Author * |
| 18 | COS-7 | Monkey | Kidney | Yes | Yes | [54] |
| 19 | FET | Human | Colon | No | NA | [30,34] |
| 20 | GLUTag | Mice | Intestinal Enteroendocrine Cells | Yes | Yes | Author *, [11,121] |
| 21 | hMADS | Human | Adipose tissue-derived stem cells | Yes | Yes | [15] |
| 22 | H9C2 | Rat | Heart/Myocardium | Yes | Yes | Author * |
| 23 | HBMEC | Human | Primary Brain Microvascular Endothelial Cells | NA | Yes | [64] |
| 24 | HCT116 | Human | Colon | No | Yes | [34,99] |
| 25 | HCT8 | Human | Colon | No | NA | [34] |
| 26 | HEK293T | Human | Embryonic Kidney | Yes | Yes | [29,54,103,122] |
| 27 | HeLa | Human | Cervix | Yes | NA | [29] |
| 28 | Hepa1-6 | Mice | Liver | Yes | Yes | Author * |
| 29 | HEPG2 | Human | Liver | Yes | Yes | Authors * |
| 30 | HLE | Human | Liver cells | Yes | Yes | [123] |
| 31 | HRCEs | Human | Kidney tissues | Yes | Yes | [124] |
| 32 | HT-29 | Human | Colon | Yes | NA | Author *, [8,34] |
| 33 | HuH-7 | Human | Hepatocellular | Yes | Yes | [123] |
| 34 | HuTu-80 | Human | Duodenum Epithelium | Yes | Yes | [125] |
| 35 | INS1 | Rat | Pancreatic Islet | Yes | NA | [25,32] |
| 36 | JHH-4 | Human | Hepatic cell | Yes | Yes | [123] |
| 37 | Jurkat T cells | Human | T-Lymphocyte Cells | NA | Yes | [41] |
| 38 | K562 | Human | Bone Marrow | Very low | NA | [18] |
| 39 | L-10 | Mice | Lymphoid Cells | Yes | No | [73] |
| 40 | Ltk | Mice | Adipose fibroblast cell | Yes | No | [34,73] |
| 41 | MCF7 | Human | Mammary Gland | Yes | Yes | [29,65] |
| 42 | MDA-MB-231 | Human | Breast Epithelial cells | Yes | Yes | [126] |
| 43 | MDA-MB-436 | Human | Breast Epithelial cells | Yes | Yes | [34,126] |
| 44 | MEFs | Mice | Embryonic Fibroblast | Yes | No | [30] |
| 45 | Min6 | Mice | Pancreatic Endocrinal Cells | Yes | Yes | [16,32,73,74,117,127] |
| 46 | NCI-H716 | Human | Intestinal Endocrinal L-cell | Yes | Yes | [105,125] |
| 47 | NCM-640 | Human | Colon Epithelial cells | Very Low | NA | [8] |
| 48 | Neuro2A | Mice | Brain | Barely | Barely | [23] |
| 49 | NIH-3T3 | Mice | Embryonic Cells | No | No | [73] |
| 50 | Ob-Luc | Mice | Adipocytes | NA | Yes | [97] |
| 51 | Raw264.7 | Mice | Macrophages | Yes | NA | Author *, [29] |
| 52 | SK-N-SH | Human | Brain | Yes | Yes | Author * |
| 53 | SW480 | Human | Colon | No | NA | [29,34,54,103,122] |
| 54 | SW620 | Human | Colon | No | NA | [34] |
| 55 | SW872 | Human | Liposarcoma | Yes | No | [128] |
| 56 | T-84 | Human | Colon Epithelial cells | Very Low | NA | [8] |
| 57 | THP-1 | Human | Monocyte | Yes | Yes | Author * |
| 58 | U937 | Human | Myeloid Lymphocytes | Yes | NA | [18,54,55,118,120] |
| S. No. | Tissue/Organ | Research Findings | Ref. |
|---|---|---|---|
| Human | |||
| 1 | Intestinal L-cells | -Secrete GLP-1 and PYY in response to glucose via FFAR2 signaling. | [3,11,76,162] |
| 2 | Primary Neutrophils | -Cmp1 and CATPB function as an agonist and antagonist for the neutrophil FFAR2 respectively. -Cmp1 and acetate activates the phospholipase C-inositol phosphate 3 (IP3) Ca2+ signaling while CATPB inhibits it. -Cmp1 act as a potent activator of the NADPH -oxidase in TNF-α-primed neutrophils with increased release of superoxide. -Moreover, Cmp1 triggered NADPH oxidase activity was inhibited by PTX. | [163] |
| 3 | Primary Monocytes | -Non-responders of Cmp1 shows no transient rise in intracellular Ca+2. -Human monocyte FFAR2 reduces inflammatory cytokine expression in response to acetate. -FFAR2 modulates p38-MAPK, Akt, and ERK signaling in response to acetate and FFAR2 agonist (CFMB). | [22,163,164] |
| 4 | Primary Lymphocytes | -Non-responders to Cmp1 with no transient rise in intracellular Ca+2. | [22,163] |
| 5 | Peripheral blood mononuclear cells (PBMCs) | -mRNA expression of FFAR2 upregulate in PBMCs in Type 1 Diabetes (T1D) patient via NFκB. -Overexpression induced cell apoptosis through ERK signaling. -Stimulated PBMCs for cytokine production in the presence of lipopolysaccharides (LPS) with and/or without acetate along with anti-FFAR2 antibody. | [29,42,163] |
| 6 | Primary Adipocytes | -FFAR2 expressed in the human multipotent adipose tissue-derived stem cells (hMADS). -SCFA acetate (luminal and systemic) are responsible for the antilipolytic response. -Treating with Gi-sensitive PTX inhibitors prevents anti-lipolytic response develop by acetate. -A mixture SCFA reduces plasma FFA in DIO mice along with beige adipogenesis marker. -So, colonic or systemic acetate modulation helps in improving the insulin resistance in human adipocytes via FFAR2 mediated attenuation of HSL phosphorylation. | [15,19,29,42] |
| 7 | Colon | -Luminal propionate stimulates FFAR2 pathway through PYY mediation confirmed by Y1 and Y2 antagonist (BIBO3304 and BIIE0246). -FFAR2 signaling expressed evenly in the entire intestine mostly at colon in the presence of FFAR2 agonist PA. | [15,19,165] |
| Mouse/Rodent | |||
| 1 | Pancreatic β-cells | -mRNA expression of FFAR2 upregulated through increase in pancreas β-cell expansion. -Increased β-cell contributes to more insulin secretion. -FFAR2 KO mice reduces gestational pancreatic β-cell expansion during pregnancy. -FFAR2 KO mice gestational glucose tolerance worsened even under antibiotic treatment and further deteriorated during second pregnancy. -Antibiotic modulation of gut microbiota does not disrupt the contribution of FFAR2 to gestational glucose tolerance. -FFAR2 acts as a novel target for β-cells adaptation to pregnancy-induced insulin resistance during to maintain normal glucose homeostasis. -FFAR2 a novel therapeutic target to stimulate β-cell growth and Proliferation. | [25,96,166,167] |
| 2 | Primary Pancreatic Islet | -SCFAs such as Acetate, propionate, and butyrate administration have no effect on insulin and glucagon secretion regardless of glucose level. -CFMB (FFAR2 agonist) has a significant effect in increasing the somatostatin and insulin secretion whereas no effect was observed in glucagon synthesis. -Mediate an inhibition of insulin secretion by coupling to Gi-type G Proteins -Under type 2 diabetic condition acetate concentration increases in pancreatic islet and systemic circulation -FFAR2 antagonist might increase insulin secretion in type 2 diabetes -Double knock-out of FFAR2 and FFAR3 altered the glucose tolerance in diabetic condition. | [28,168] |
| 3 | Ileum | -Bacterial metabolites, propionate, activate ileal mucosal FFAR2 to decrease hepatic glucose production. -Propionate stimulate GLP-1r dependent neuronal network to regulate glucose production activated through ileal FFAR2 signaling. -Regulate glucose homeostasis. | [28,169] |
| 4 | Macrophages | -Inducing apoptosis of infiltrated macrophages to pancrease through upregulation of FFAR2. -Improved glucose homeostasis in diabetic mice by treating with FFAR2 agonist, acetate and phenylacetamide 1. | [29,169] |
| 5 | Peripheral blood mononuclear cells (PBMCs) | -Dextran sodium Sulphate (DSS) -induced colon shortening, mucosal thickness, inflammatory cell infiltration, and crypt damage were ameliorated by acetate treatment in C57BL/6 mice. -Stimulated PBMCs in FFAR2 KO mice for cytokine production in the presence of lipopolysaccharides (LPS) with and/or without acetate. -DSS-induced colitis is exaberated in FFAR2 KO mice through increase in pro-inflammatory cytokines such as TNF-α and IL-17 with decrease of anti-inflammatory cytokine IL-10 in the colonic mucusa. | [29,42] |
| 6 | Neutrophil | -FFAR2 recognizes propionate and butyrate and expressed abundantly in polymorphonuclear (PMN) leukocytes. -FFAR2 mediated SCFA-induced chemotaxis through p38 MAPK signaling pathway. -Inhibiting FFAR2 mediated signaling a promising way for inhibiting the migration of PMN at the site of intestinal inflammation. -Under influenza infection, in FFAR2 KO along with wild type mice showed decrease neutrophil infiltration to airway. | [43,114] |
| 7 | Immune Cells | -FFAR2 KO mice develops unresolving or exaberated inflammation in colitis, arthritis and asthma mice model. -FFAR2 KO mice shows inflammatory action related to increase in the production of inflammatory mediators by increased in immune cell recruitment. -Germ-free mice, which are devoid of bacteria and express little or no SCFAs, showed similar dysregulation of certain inflammatory response. -SCFA-FFAR2 interaction has profound effect on normal resolution of certain inflammatory response with a molecular link between diet, gastrointestinal bacterial metabolism and immune response. | [35,40,43] |
| 8 | Monocytes | -Mice monocyte showed increased in IL-1α and IL-1β cytokine expression in response to acetate. -Even in FFAR2/3 KO mouse monocyte display elevate cytokine response on treatment with SCFAs. -SCFA does not act through FFAR2 to modulate mice monocyte inflammatory responses. | [22,35] |
| 9 | L-cells | -GLP-1 synthesis was enhanced in the presence of phosphodiesterase inhibitor isobutyl methyl xanthine (IBMX). -FFAR2 expression in small intestine and colonic L-cells as compare to non-L-cell population. -Induces GLP-1 and PYY secretion via glucose dependent mechanism. -SCFAs triggered Ca2+ elevation in L-cells with enhanced GLP-1 and PYY secretion through Gq-mediated pathway, implicating FFAR2 signaling involvement. -Synthetic phenylacetamide agonist of FFAR2, CFMB, mobilizes more intracellular Ca2+ in L-cells and elevates GLP-1 hormone secretion, in the presence of DPPIVi but not in its absence in mice. | [11,22,162,170] |
| 10 | Colonic Mucosa | -FFAR2 express in the colonic mucosa -Withdrawal of ceftriaxone antibiotic leads to reduction in SCFA concentration and increase in increased number of conditionally pathogenic Enterobacteria, E. coli, Clostridium, Staphylococcus spp., and hemolytic bacteria in colonic gut. -FFAR2 immune regulation mechanism get hamper with increase in cytokine concentration in colonic mucosa. -Increase histopathology condition of colitis with goblet cell dysfunction, colonic dilatation and wall thickening, ultimate leads to IBD. | [78] |
| 11 | Enterochromaffin cells | -FFAR2 agonist PA1 (Phenylacetamide 1) in a dose-dependent manner stimulate HCO3- secretion, even prior exposed to DPPIV inhibitor NVP728. -HCO3- secretion stimulate by activated FFAR2 through muscarinic and 5-HT4 receptor signaling rather than through VIP, CCK and GLP-2 pathway. -Moreover, SCFAs (mostly acetate) activate FFAR2 and FFAR3 followed by 5-HT and GLP-2 release. | [171] |
| 12 | Mast cell | -Rat intestinal lamina propria mast cells expressed FFAR2 along with 5-hydroxytryptophan (5-HT). -The activated mucosal FFAR2 act on the nearby nerve endings at 5-HT3 serotogenic receptors. -SCFAs stimulate PYY and 5-HT secretion from ileum and colonic endocrine cells by activating FFAR2 receptor. | [76,171] |
| 13 | Stomach | -The villi and microvilli of gastric brush cells reveal expression of FFAR2 (at gene and protein level) in the mice stomach. | [7,76,172] |
| 14 | Lungs | -Expressed in the mice lungs. -SCFAs modulate allergy airway inflammation in mice lungs via FFAR2 signaling. | [6,7,152,172] |
| 15 | Muscle | -Expressed FFAR2 in smooth muscle cells of small resistance vessels. -SCFAs produced from gut microbiota modulate the blood glucose level. | [6,20] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, S.P.; Karunakar, P.; Taraphder, S.; Yadav, H. Free Fatty Acid Receptors 2 and 3 as Microbial Metabolite Sensors to Shape Host Health: Pharmacophysiological View. Biomedicines 2020, 8, 154. https://doi.org/10.3390/biomedicines8060154
Mishra SP, Karunakar P, Taraphder S, Yadav H. Free Fatty Acid Receptors 2 and 3 as Microbial Metabolite Sensors to Shape Host Health: Pharmacophysiological View. Biomedicines. 2020; 8(6):154. https://doi.org/10.3390/biomedicines8060154
Chicago/Turabian StyleMishra, Sidharth P., Prashantha Karunakar, Subhash Taraphder, and Hariom Yadav. 2020. "Free Fatty Acid Receptors 2 and 3 as Microbial Metabolite Sensors to Shape Host Health: Pharmacophysiological View" Biomedicines 8, no. 6: 154. https://doi.org/10.3390/biomedicines8060154
APA StyleMishra, S. P., Karunakar, P., Taraphder, S., & Yadav, H. (2020). Free Fatty Acid Receptors 2 and 3 as Microbial Metabolite Sensors to Shape Host Health: Pharmacophysiological View. Biomedicines, 8(6), 154. https://doi.org/10.3390/biomedicines8060154