Results of TETimaX Trial of Langerhans Cell Histiocytosis Treatment and Perspectives on the Role of Imatinib Mesylate in the Era of MAPK Signaling
Abstract
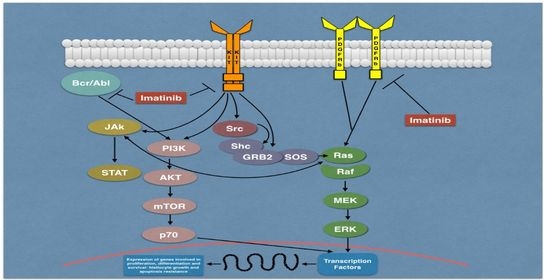
:1. Introduction
2. Materials and Methods
2.1. Immunohistochemistry Study
2.2. TETimaX Trial
3. Results
3.1. Immunohistochemistry Results
3.2. TETimaX Trial Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Allen, C.E.; Merad, M.; McClain, K.L. Langerhans-Cell Histiocytosis. N. Engl. J. Med. 2018, 379, 856–868. [Google Scholar] [CrossRef]
- Emile, J.F.; Abla, O.; Fraitag, S.; Horne, A.; Haroche, J.; Donadieu, J.; Requena-Caballero, L.; Jordan, M.B.; Abdel-Wahab, O.; Carl, E.; et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 2016, 27, 2672–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frater, J.L. The histiocytoses: As easy as ABC (or LCMRH). Blood 2016, 127, 2655–2656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, J.K.; Kang, S.; Kim, H.; Im, H.J.; Koh, K.N. Recent advances in the understanding of the molecular pathogenesis and targeted therapy options in Langerhans cell histiocytosis. Blood Res. 2021, 56, S65–S69. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Fantauzzo, K.A. The emerging complexity of PDGFRs: Activation, internalization and signal attenuation. Biochem. Soc. Trans. 2020, 48, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.C.; Blanke, C.D.; Druker, B.J.; Corless, C.L. Inhibition of KIT tyrosine kinase activity: A novel molecular approach to the treatment of KIT-positive malignancies. J. Clin. Oncol. 2002, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Uebelhoer, M.; Bewig, B.; Kreipe, H.; Nowak, D.; Magnussen, H.; Barth, J. Modulation of fibroblast activity in histiocytosis X by platelet-derived growth factor. Chest 1995, 107, 701–705. [Google Scholar] [CrossRef]
- Martinet, Y.; Bitterman, P.B.; Mornex, J.F.; Grotendorst, G.R.; Martin, G.R.; Crystal, R.G. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature 1986, 319, 158–160. [Google Scholar] [CrossRef]
- Caponetti, G.C.; Miranda, R.N.; Althof, P.A.; Dobesh, R.C.; Sanger, W.G.; Medeiros, L.J.; Greiner, T.C.; Weisenburger, D.D. Immunohistochemical and molecular cytogenetic evaluation of potential targets for tyrosine kinase inhibitors in Langerhans cell histiocytosis. Hum. Pathol. 2012, 43, 2223–2228. [Google Scholar] [CrossRef]
- Brown, R.E. Morphoproteomic Analysis of Osteolytic Langerhans Cell Histiocytosis with Therapeutic Implications. Ann. Clin. Lab. Sci. Spring 2005, 35, 131–136. [Google Scholar]
- Ray, P.; Krishnamoorthy, N.; Oriss, T.B.; Ray, A. Signaling of c-kit in dendritic cells influences adaptive immunity. Ann. N. Y. Acad. Sci. 2010, 1183, 104–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appel, S.; Boehmler, A.M.; Grünebach, F.; Müller, M.R.; Rupf, A.; Weck, M.M.; Hartmann, U.; Reichardt, V.L.; Kanz, L.; Brümmendorf, T.H.; et al. Imatinib mesylate affects the development and function of dendritic cells generated from CD34+ peripheral blood progenitor cells. Blood 2004, 103, 538–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zitvogel, L.; Rusakiewicz, S.; Routy, B.; Ayyoub, M.; Kroemer, G. Immunological off-target effects of imatinib. Nat. Rev. Clin. Oncol. 2016, 13, 431–446. [Google Scholar] [CrossRef]
- Montella, L.; Insabato, L.; Palmieri, G. Imatinib mesylate for cerebral Langerhans’-cell histiocytosis. N. Engl. J. Med. 2004, 351, 1034–1035. [Google Scholar] [CrossRef]
- Palmieri, G.; Marino, M.; Buonerba, C.; Federico, P.; Conti, S.; Milella, M.; Petillo, L.; Evoli, A.; Lalle, M.; Ceribelli, A.; et al. Imatinib mesylate in thymic epithelial malignancies. Cancer Chemother. Pharmacol. 2012, 69, 309–315. [Google Scholar] [CrossRef]
- Montella, L.; Insabato, L.; Strazzullo, V.; Di Vizio, D.; Baldi, A.; Palmieri, G. Platelet-Derived Growth Factor Receptor ß in Langerhans’-Cell Histiocytosis: Preliminary results of an immunohistochemical study. In Proceedings of the Histiocyte Society 20th Annual Meeting, Stockholm, Sweden, 12–14 September 2004. [Google Scholar]
- Janku, F.; Amin, H.M.; Yang, D.; Garrido-Laguna, I.; Trent, J.C.; Kurzrock, R. Response of histiocytoses to imatinib mesylate: Fire to ashes. J. Clin. Oncol. 2010, 28, e633–e636. [Google Scholar] [CrossRef]
- Utikal, J.; Ugurel, S.; Kurzen, H.; Erben, P.; Reiter, A.; Hochhaus, A.; Nebe, T.; Hildenbrand, R.; Haberkorn, U.; Goerdt, S.; et al. Imatinib as a treatment option for systemic non-Langerhans cell histiocytoses. Arch. Dermatol. 2007, 143, 736–740. [Google Scholar] [CrossRef]
- Gebhardt, C.; Averbeck, M.; Paasch, U.; Ugurel, S.; Kurzen, H.; Stumpp, P.; Simon, J.C.; Treudler, R. A case of cutaneous Rosai-Dorfman disease refractory to imatinib therapy. Arch. Dermatol. 2009, 145, 571–574. [Google Scholar] [CrossRef] [Green Version]
- Haroche, J.; Amoura, Z.; Charlotte, F.; Salvatierra, J.; Wechsler, B.; Graux, C.; Brousse, N.; Piette, J.C. Imatinib mesylate for platelet-derived growth factor receptor-beta-positive Erdheim-Chester histiocytosis. Blood 2008, 111, 5413–5415. [Google Scholar] [CrossRef] [PubMed]
- Badalian-Very, G.; Vergilio, J.A.; Degar, B.A.; MacConaill, L.E.; Brandner, B.; Calicchio, M.L.; Kuo, F.C.; Ligon, A.H.; Stevenson, K.E.; Kehoe, S.M.; et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood 2010, 116, 1919–1923. [Google Scholar] [CrossRef] [Green Version]
- Ny, L.; Hernberg, M.; Nyakas, M.; Koivunen, J.; Oddershede, L.; Yoon, M.; Wang, X.; Guyot, P.; Geisler, P.J. BRAF mutational status as a prognostic marker for survival in malignant melanoma: A systematic review and meta-analysis. Acta Oncol. 2020, 59, 833–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, W.L.; Qiu, M.Z.; He, C.Y.; Yang, L.Q.; Jin, Y.; Wang, Z.Q.; Li, Y.H.; Xu, R.H.; Wang, F.H. Clinicopathologic Features and Prognosis of BRAF Mutated Colorectal Cancer Patients. Front. Oncol. 2020, 10, 563407. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Jia, X.; Hu, X.; Pang, P.; Zhao, S.; Wang, Y.; Wang, J.; Zhang, Y.; Lyu, Z. The Coexistence of Genetic Mutations in Thyroid Carcinoma Predicts Histopathological Factors Associated With a Poor Prognosis: A Systematic Review and Network Meta-Analysis. Front. Oncol. 2020, 10, 540238. [Google Scholar] [CrossRef]
- Héritier, S.; Emile, J.F.; Barkaoui, M.A.; Thomas, C.; Fraitag, S.; Boudjemaa, S.; Renaud, F.; Moreau, A.; Peuchmaur, M.; Chassagne-Clément, C.; et al. BRAF Mutation Correlates With High-Risk Langerhans Cell Histiocytosis and Increased Resistance to First-Line Therapy. J. Clin. Oncol. 2016, 34, 3023–3030. [Google Scholar] [CrossRef] [Green Version]
- Abla, O.; Weitzman, S. Treatment of Langerhans cell histiocytosis: Role of BRAF/MAPK inhibition. Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 565–570. [Google Scholar] [CrossRef]
- Chakraborty, R.; Burke, T.M.; Hampton, O.A.; Zinn, D.J.; Lim, K.P.H.; Abhyankar, H.; Scull, B.; Kumar, V.; Kakkar, N.; Wheeler, D.A.; et al. Alternative genetic mechanisms of BRAF activation in Langerhans cell histiocytosis. Blood 2016, 128, 2533–2537. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, N.; Dogan, A.; Abdel-Wahab, O. Identification and targeting of kinase alterations in histiocytic neoplasms. Hematol. Oncol. Clin. N. Am. 2017, 31, 705–719. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ (accessed on 11 September 2021).
- Tóth, B.; Norbert Kiss, N.; Hársing, J.; Kárpáti, S.; Csomor, J.; Bödör, C.; Tímár, J.; Rásó, E. Frequent KIT mutations in skin lesions of patients with BRAF wild-type Langerhans cell histiocytosis. Virchows Arch. 2020, 477, 749–753. [Google Scholar] [CrossRef] [PubMed]


| Case | Sex/Age | LCH Site | PDGF-Rβ |
|---|---|---|---|
| 1 | M/10 | Ocular soft tissue | +1C |
| 2 | F/9 | Skin | +3C/N |
| 3 | M/76 | Skin | +2C |
| 4 | M/13 | Bone | +3C |
| 5 | M/19 | Bone | +3C |
| 6 | M/1 | Bone | +1C |
| 7 | M/45 | Oral mucosa | +3C |
| M/45 | Oral mucosa | +3C | |
| 8 | F/23 | Brain | +2C |
| 9 | M/5 | Eye | +2 |
| 10 | F/7 | Bone | +2 |
| Pt | Age | Sex | Presenting Sign/Symptom | Disease Involvement Sites | Best Responses and Timeframe | Treatments before Imatinib | Treatments after Imatinib | Follow-up after Imatinib | Current State Notes |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | M | Cough | Lung, multi-bone | CR Heart–lung transplantation avoided (10 yrs) | Vbl + Pdn | - | 12 years + 1 month | A |
| 2 | 46 | F | Cough, weight loss | Lung | PR (10 yrs) | Vbl + Pdn | - | 11 years + 6 months | A |
| 3 | 41 | F | Cough, headache, memory disturbances | Lung, brain, multi-bone | Brain and bone CR/lung PR (9 yrs) | Vbl + Pdn Cladribine Indomethacin | Cladribine Vbl + Pdn | 8 years + 6 months | A |
| 4 | 60 | M | Diplopia | Bone, retroperitoneal fibrosis | SD | Vbl + Pdn | IFN→vem | 9 years | D |
| 5 | 36 | M | Palpebral ptosis | Multi-bone | CR | Vbl + Pdn | Vbl + Pdn→VP16 | 12 years + 7 months | A b-raf wt |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montella, L.; Ottaviano, M.; Riccio, V.; Picozzi, F.; Facchini, G.; Insabato, L.; Giuliano, M.; Palmieri, G. Results of TETimaX Trial of Langerhans Cell Histiocytosis Treatment and Perspectives on the Role of Imatinib Mesylate in the Era of MAPK Signaling. Biomedicines 2021, 9, 1759. https://doi.org/10.3390/biomedicines9121759
Montella L, Ottaviano M, Riccio V, Picozzi F, Facchini G, Insabato L, Giuliano M, Palmieri G. Results of TETimaX Trial of Langerhans Cell Histiocytosis Treatment and Perspectives on the Role of Imatinib Mesylate in the Era of MAPK Signaling. Biomedicines. 2021; 9(12):1759. https://doi.org/10.3390/biomedicines9121759
Chicago/Turabian StyleMontella, Liliana, Margaret Ottaviano, Vittorio Riccio, Fernanda Picozzi, Gaetano Facchini, Luigi Insabato, Mario Giuliano, and Giovannella Palmieri. 2021. "Results of TETimaX Trial of Langerhans Cell Histiocytosis Treatment and Perspectives on the Role of Imatinib Mesylate in the Era of MAPK Signaling" Biomedicines 9, no. 12: 1759. https://doi.org/10.3390/biomedicines9121759
APA StyleMontella, L., Ottaviano, M., Riccio, V., Picozzi, F., Facchini, G., Insabato, L., Giuliano, M., & Palmieri, G. (2021). Results of TETimaX Trial of Langerhans Cell Histiocytosis Treatment and Perspectives on the Role of Imatinib Mesylate in the Era of MAPK Signaling. Biomedicines, 9(12), 1759. https://doi.org/10.3390/biomedicines9121759