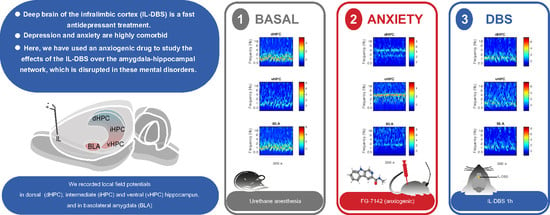
The Oscillatory Profile Induced by the Anxiogenic Drug FG-7142 in the Amygdala–Hippocampal Network Is Reversed by Infralimbic Deep Brain Stimulation: Relevance for Mood Disorders
Abstract
:1. Introduction
1.1. Depression and Anxiety as Comorbid Disorders
1.2. Prefrontal Deep Brain Stimulation in the Treatment of Depression
1.3. The Amygdala–Hippocampal–Prefrontal Network in Depression and Anxiety
1.4. FG-7142 as an Anxiogenic Drug
1.5. Brain Oscillations as a Measure of Network Dynamics
2. Materials and Methods
2.1. Animal Model and Surgical Procedures
2.2. Drug Administration
2.3. Recording and Stimulation Procedure
2.4. Histological Analysis
2.5. Data Analysis
2.5.1. Spectral Analysis
2.5.2. Wavelet Analysis
2.5.3. Cross-Frequency Coupling
2.6. Statistical Analysis
3. Results
3.1. Spectral Analysis and Peak Frequency
3.2. Relative Power of Slow Waves, Delta and Theta Band
3.3. Theta Segments
3.4. Effects of FG-7142 and IL-DBS on Local Gamma Power
3.5. Phase Synchronization
3.6. Cross-Frequency Coupling
3.6.1. Amplitude–Amplitude Correlation
3.6.2. Phase-Amplitude Coupling
4. Discussion
4.1. Study Limitations
4.2. Effects of FG-7142 and IL-DBS on the Spectral Composition
4.3. Communication in the Amygdala–Hippocampal Network
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cryan, J.F.; Holmes, A. The ascent of mouse: Advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005, 4, 775–790. [Google Scholar] [CrossRef]
- W.H.O. Depression and Other Common Mental Disorders: Global Health Estimates; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Nock, M.K.; Hwang, I.; Sampson, N.A.; Kessler, R.C. Mental disorders, comorbidity and suicidal behavior: Results from the National Comorbidity Survey Replication. Mol. Psychiatry 2010, 15, 868–876. [Google Scholar] [CrossRef]
- Nock, M.K.; Green, J.G.; Hwang, I.; McLaughlin, K.A.; Sampson, N.A.; Zaslavsky, A.M.; Kessler, R.C. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents. JAMA Psychiatry 2013, 70, 300. [Google Scholar] [CrossRef] [Green Version]
- Diagnostic and Statistical Manual of Mental Disorders (DSM–5), 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013.
- Zhou, Y.; Cao, Z.; Yang, M.; Xi, X.; Guo, Y.; Fang, M.; Cheng, L.; Du, Y. Comorbid generalized anxiety disorder and its association with quality of life in patients with major depressive disorder. Sci. Rep. 2017, 7, 40511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamers, F.; van Oppen, P.; Comijs, H.C.; Smit, J.H.; Spinhoven, P.; van Balkom, A.J.L.M.; Nolen, W.A.; Zitman, F.G.; Beekman, A.T.F.; Penninx, B.W.J.H. Comorbidity patterns of anxiety and depressive disorders in a large cohort study. J. Clin. Psychiatry 2011, 72, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Licinio, J. Research and treatment approaches to depression. Nat. Rev. Neurosci. 2001, 2, 243–351. [Google Scholar] [CrossRef] [PubMed]
- Artigas, F.; Bortolozzi, A.; Celada, P. Can we increase speed and efficacy of antidepressant treatments? Part I: General aspects and monoamine-based strategies. Eur. Neuropsychopharmacol. 2018, 28, 445–456. [Google Scholar] [CrossRef]
- Holtzheimer, P.E.; Mayberg, H.S. Stuck in a rut: Rethinking depression and its treatment. Trends Neurosci. 2011, 34, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef]
- Lozano, A.M.; Lipsman, N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 2013, 77, 406–424. [Google Scholar] [CrossRef] [Green Version]
- Mayberg, H.S.; Lozano, A.M.; Voon, V.; McNeely, H.E.; Seminowicz, D.; Hamani, C.; Schwalb, J.M.; Kennedy, S.H. Deep brain stimulation for treatment-resistant depression. Neuron 2005, 45, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Lozano, A.M.; Mayberg, H.H.S.; Giacobbe, P.; Hamani, C.; Craddock, R.C.; Kennedy, S.S.H. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol. Psychiatry 2008, 64, 461–467. [Google Scholar] [CrossRef]
- Crowell, A.L.; Riva-Posse, P.; Holtzheimer, P.E.; Garlow, S.J.; Kelley, M.E.; Gross, R.E.; Denison, L.; Quinn, S.; Mayberg, H.S. Long-term outcomes of subcallosal cingulate deep brain stimulation for treatment-resistant depression. Am. J. Psychiatry 2019, 176, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.H.; Giacobbe, P.; Rizvi, S.J.; Placenza, F.M.; Nishikawa, Y.; Mayberg, H.S.; Lozano, A.M. Deep brain stimulation for treatment-resistant depression: Follow-up after 3 to 6 years. Am. J. Psychiatry 2011, 168, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Herrington, T.M.; Cheng, J.J.; Eskandar, E.N. Mechanisms of deep brain stimulation. J. Neurophysiol. 2016, 115, 19–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashkan, K.; Rogers, P.; Bergman, H.; Ughratdar, I. Insights into the mechanisms of deep brain stimulation. Nat. Rev. Neurol. 2017, 13, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Malone, D.A.; Dougherty, D.D.; Rezai, A.R.; Carpenter, L.L.; Friehs, G.M.; Eskandar, E.N.; Rauch, S.L.; Rasmussen, S.A.; Machado, A.G.; Kubu, C.S.; et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol. Psychiatry 2009, 65, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Bewernick, B.H.; Hurlemann, R.; Matusch, A.; Kayser, S.; Grubert, C.; Hadrysiewicz, B.; Axmacher, N.; Lemke, M.; Cooper-Mahkorn, D.; Cohen, M.X.; et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol. Psychiatry 2010, 67, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, T.E.; Cohen, M.X.; Frick, C.; Kosel, M.; Brodesser, D.; Axmacher, N.; Joe, A.Y.; Kreft, M.; Lenartz, D.; Sturm, V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 2008, 33, 368–377. [Google Scholar] [CrossRef]
- Krüger, S.; Seminowicz, D.; Goldapple, K.; Kennedy, S.H.; Mayberg, H.S. State and trait influences on mood regulation in bipolar disorder: Blood flow differences with an acute mood challenge. Biol. Psychiatry 2003, 54, 1274–1283. [Google Scholar] [CrossRef]
- Simpson, J.R.; Drevets, W.C.; Snyder, A.Z.; Gusnard, D.A.; Raichle, M.E. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc. Natl. Acad. Sci. USA 2001, 98, 688–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zald, D.H.; Mattson, D.L.; Pardo, J.V. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc. Natl. Acad. Sci. USA 2002, 99, 2450–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puigdemont, D.; Pérez-Egea, R.; Portella, M.J.; Molet, J.; De Diego-Adeliño, J.; Gironell, A.; Radua, J.; Gómez-Anson, B.; Rodríguez, R.; Serra, M.; et al. Deep brain stimulation of the subcallosal cingulate gyrus: Further evidence in treatment-resistant major depression. Int. J. Neuropsychopharmacol. 2012, 15, 121–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Zhang, H.; Qin, Y.; Tian, T.; Xu, B.; Chen, J.; Zhou, X.; Zeng, L.; Fang, L.; Qi, X.; et al. A systematic review and meta-analysis of deep brain stimulation in treatment-resistant depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2018, 82, 224–232. [Google Scholar] [CrossRef]
- Khairuddin, S.; Ngo, F.Y.; Lim, W.L.; Aquili, L.; Khan, N.A.; Fung, M.-L.; Chan, Y.-S.; Temel, Y.; Lim, L.W. A decade of progress in deep brain stimulation of the subcallosal cingulate for the treatment of depression. J. Clin. Med. 2020, 9, 3260. [Google Scholar] [CrossRef]
- Holtzheimer, P.E.; Husain, M.M.; Lisanby, S.H.; Taylor, S.F.; Whitworth, L.A.; McClintock, S.; Slavin, K.V.; Berman, J.; McKhann, G.M.; Patil, P.G.; et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: A multisite, randomised, sham-controlled trial. Lancet Psychiatry 2017, 4, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, N.; Yu, L.; Zhao, M. Efficacy and feasibility of deep brain stimulation for patients with depression. Medicine 2021, 100, e26044. [Google Scholar] [CrossRef] [PubMed]
- Leonard, C.M. Finding prefrontal cortex in the rat. Brain Res. 2016, 1645, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Uylings, H.B.M.; Groenewegen, H.J.; Kolb, B. Do rats have a prefrontal cortex? Behav. Brain Res. 2003, 146, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Preuss, T.M. Do Rats Have Prefrontal Cortex? The Rose-Woolsey-Akert Program Reconsidered. J. Cogn. Neurosci. 1995, 7, 1–24. [Google Scholar] [CrossRef]
- Heidbreder, C.A.; Groenewegen, H.J. The medial prefrontal cortex in the rat: Evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci. Biobehav. Rev. 2003, 27, 555–579. [Google Scholar] [CrossRef]
- Schaeffer, D.J.; Hori, Y.; Gilbert, K.M.; Gati, J.S.; Menon, R.S.; Everling, S. Divergence of rodent and primate medial frontal cortex functional connectivity. Proc. Natl. Acad. Sci. USA 2020, 117, 21681–21689. [Google Scholar] [CrossRef]
- Vertes, R.P. Differential Projections of the Infralimbic and Prelimbic Cortex in the Rat. Synapse 2004, 51, 32–58. [Google Scholar] [CrossRef]
- Johansen-Berg, H.; Gutman, D.A.; Behrens, T.E.J.; Matthews, P.M.; Rushworth, M.F.S.; Katz, E.; Lozano, A.M.; Mayberg, H.S. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb. Cortex 2008, 18, 1374–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strange, B.A.; Witter, M.P.; Lein, E.S.; Moser, E.I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014, 15, 655–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, R.M.; Gratton, A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J. Neurosci. 2018, 19, 2834–2840. [Google Scholar] [CrossRef] [Green Version]
- Hamani, C.; Diwan, M.; Macedo, C.E.; Brandão, M.L.; Shumake, J.; Gonzalez-Lima, F.; Raymond, R.; Lozano, A.M.; Fletcher, P.J.; Nobrega, J.N. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol. Psychiatry 2010, 67, 117–124. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, L.; Linge, R.; Campa, L.; Valdizán, E.M.; Pazos, Á.; Díaz, Á.; Adell, A. Behavioral, neurochemical and molecular changes after acute deep brain stimulation of the infralimbic prefrontal cortex. Neuropharmacology 2016, 108, 91–102. [Google Scholar] [CrossRef]
- Torres-Sanchez, S.; Perez-Caballero, L.; Mico, J.A.; Celada, P.; Berrocoso, E. Effect of Deep Brain Stimulation of the ventromedial prefrontal cortex on the noradrenergic system in rats. Brain Stimul. 2018, 11, 222–230. [Google Scholar] [CrossRef]
- Hastings, R.S.; Parsey, R.V.; Oquendo, M.A.; Arango, V.; Mann, J.J. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology 2004, 29, 952–959. [Google Scholar] [CrossRef]
- Shin, L.M.; Rauch, S.L.; Pitman, R.K. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann. N. Y. Acad. Sci. 2006, 1071, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Videbech, P.; Ravnkilde, B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am. J. Psychiatry 2004, 161, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Macqueen, G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 2004, 29, 417–426. [Google Scholar]
- Cha, J.; Greenberg, T.; Song, I.; Blair Simpson, H.; Posner, J.; Mujica-Parodi, L.R. Abnormal hippocampal structure and function in clinical anxiety and comorbid depression. Hippocampus 2016, 26, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grillon, C. Associative learning deficits increase symptoms of anxiety in humans. Biol. Psychiatry 2002, 51, 851–858. [Google Scholar] [CrossRef]
- Kalisch, R.; Schubert, M.; Jacob, W.; Keßler, M.S.; Hemauer, R.; Wigger, A.; Landgraf, R.; Auer, D.P. Anxiety and hippocampus volume in the rat. Neuropsychopharmacology 2006, 31, 925–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapolsky, R.M. The possibility of neurotoxicity in the hippocampus in major depression: A primer on neuron death. Biol. Psychiatry 2000, 48, 755–765. [Google Scholar] [CrossRef]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of Depression; Elsevier: Amsterdam, The Netherlands, 2002; Volume 34, pp. 13–25. [Google Scholar]
- Amaral, D.G.; Witter, M.P. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience 1989, 31, 571–591. [Google Scholar] [CrossRef]
- Moser, M.-B.; Moser, E.I. Functional differentiation in the hippocampus. Hippocampus 1998, 8, 608–619. [Google Scholar] [CrossRef]
- Bannerman, D.; Rawlins, J.N.; McHugh, S.; Deacon, R.M.; Yee, B.; Bast, T.; Zhang, W.-N.; Pothuizen, H.H.; Feldon, J. Regional dissociations within the hippocampus—memory and anxiety. Neurosci. Biobehav. Rev. 2004, 28, 273–283. [Google Scholar] [CrossRef]
- Lothmann, K.; Deitersen, J.; Zilles, K.; Amunts, K.; Herold, C. New boundaries and dissociation of the mouse hippocampus along the dorsal-ventral axis based on glutamatergic, GABAergic and catecholaminergic receptor densities. Hippocampus 2020, 31, 56–78. [Google Scholar] [CrossRef]
- Phelps, E.A.; LeDoux, J.E. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 2005, 48, 175–187. [Google Scholar] [CrossRef] [Green Version]
- LeDoux, J. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 2003, 23, 727–738. [Google Scholar] [CrossRef]
- Rosenkranz, J.A.; Venheim, E.R.; Padival, M. Chronic stress causes amygdala hyperexcitability in rodents. Biol. Psychiatry 2010, 67, 1128–1136. [Google Scholar] [CrossRef] [Green Version]
- Roozendaal, B.; McEwen, B.S.; Chattarji, S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009, 10, 423–433. [Google Scholar] [CrossRef]
- Vyas, A.; Jadhav, S.; Chattarji, S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience 2006, 143, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.B.; Simmons, A.N.; Feinstein, J.S.; Paulus, M.P. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am. J. Psychiatry 2007, 164, 318–327. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Siemer, M.; Gotlib, I.H. Amygdala volume in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Mol. Psychiatry 2008, 13, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Sibille, E.; Wang, Y.; Joeyen-Waldorf, J.; Gaiteri, C.; Surget, A.; Oh, S.; Belzung, C.; Tseng, G.C.; Lewis, D.A. A molecular signature of depression in the amygdala. Am. J. Psychiatry 2009, 166, 100–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaveau, P.; Jabourian, M.; Lemogne, C.; Guionnet, S.; Bergouignan, L.; Fossati, P. Brain effects of antidepressants in major depression: A meta-analysis of emotional processing studies. J. Affect. Disord. 2011, 130, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Bellani, M.; Dusi, N.; Yeh, P.H.; Soares, J.C.; Brambilla, P. The effects of antidepressants on human brain as detected by imaging studies. Focus on major depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2011, 35, 1544–1552. [Google Scholar] [CrossRef]
- Castro, J.E.; Varea, E.; Márquez, C.; Cordero, M.I.; Poirier, G.; Sandi, C. Role of the amygdala in antidepressant effects on hippocampal cell proliferation and survival and on depression-like behavior in the rat. PLoS ONE 2010, 5, e8618. [Google Scholar] [CrossRef] [Green Version]
- Arnsten, A.F.T. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- Cervera-Ferri, A.; Teruel-Martí, V.; Barceló-Molina, M.; Martínez-Ricós, J.; Luque-García, A.; Martínez-Bellver, S.; Adell, A. Characterization of oscillatory changes in hippocampus and amygdala after deep brain stimulation of the infralimbic prefrontal cortex. Physiol. Rep. 2016, 4, e12854. [Google Scholar] [CrossRef] [Green Version]
- Harro, J. Animals, anxiety, and anxiety disorders: How to measure anxiety in rodents and why. Behav. Brain Res. 2018, 352, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, F.; Ho, J.; Woo, J.H.; Lim, C.L.; Poon, D.J.J.; Lamba, B.; Claridge-Chang, A. Concordance and incongruence in preclinical anxiety models: Systematic review and meta-analyses. Neurosci. Biobehav. Rev. 2016, 68, 504–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braestrup, C.; Nielsen, M.; Olsen, C.E. Urinary and brain β-carboline-3-carboxylates as potent inhibitors of brain benzodiazepine receptors. Proc. Natl. Acad. Sci. USA 1980, 77, 2288–2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, A.K.; Lowry, C.A. Pharmacology of the β-Carboline FG-7142, a Partial Inverse Agonist at the Benzodiazepine Allosteric Site of the GABAA Receptor: Neurochemical, Neurophysiological, and Behavioral Effects. CNS Drug Rev. 2007, 13, 475–501. [Google Scholar] [CrossRef]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 252–529. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Cole, J.C.; Aboualfa, K.; Stephenson, L.H. Ethopharmacological analysis of the effects of putative “anxiogenic” agents in the mouse elevated plus-maze. Pharmacol. Biochem. Behav. 1995, 52, 805–813. [Google Scholar] [CrossRef]
- Lawther, A.J.; Clissold, M.L.; Ma, S.; Kent, S.; Lowry, C.A.; Gundlach, A.L.; Hale, M.W. Anxiogenic drug administration and elevated plus-maze exposure in rats activate populations of relaxin-3 neurons in the nucleus incertus and serotonergic neurons in the dorsal raphe nucleus. Neuroscience 2015, 303, 270–284. [Google Scholar] [CrossRef]
- Singewald, N.; Salchner, P.; Sharp, T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol. Psychiatry 2003, 53, 275–283. [Google Scholar] [CrossRef]
- Dorow, R. FG 7142 and its anxiety-inducing effects in humans. Br. J. Clin. Pharmacol. 1987, 23, 781–782. [Google Scholar]
- Horowski, R. FG 7142: Is this validated tool to study anxiety now forgotten? J. Neural Transm. 2020, 127, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.K.; Johnson, P.L.; Hay-Schmidt, A.; Mikkelsen, J.D.; Shekhar, A.; Lowry, C.A. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience 2005, 133, 983–997. [Google Scholar] [CrossRef]
- Corda, M.G.; Blaker, W.D.; Mendelson, W.B.; Guidotti, A.; Costa, E. β-Carbolines enhance shock-induced suppression of drinking in rats. Proc. Natl. Acad. Sci. USA 1983, 80, 2072–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens, D.N.; Kehr, W.; Schneider, H.H.; Schmiechen, R. β-Carbolines with agonistic and inverse agonistic properties at benzodiazepine receptors of the rat. Neurosci. Lett. 1984, 47, 333–338. [Google Scholar] [CrossRef]
- Stephens, D.N.; Kehr, W. β-Carbolines can enhance or antagonize the effects of punishment in mice. Psychopharmacology 1985, 85, 143–147. [Google Scholar] [CrossRef]
- Thiébot, M.H.; Dangoumau, L.; Richard, G.; Puech, A.J. Safety signal withdrawal: A behavioural paradigm sensitive to both “anxiolytic” and “anxiogenic” drugs under identical experimental conditions. Psychopharmacology 1991, 103, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Richardson, R. A developmental dissociation in reinstatement of an extinguished fear response in rats. Neurobiol. Learn. Mem. 2007, 88, 48–57. [Google Scholar] [CrossRef]
- Short, K.R.; Maier, S.F. Stressor controllability, social interaction, and benzodiazepine systems. Pharmacol. Biochem. Behav. 1993, 45, 827–835. [Google Scholar] [CrossRef]
- Hackler, E.A.; Turner, G.H.; Gresch, P.J.; Sengupta, S.; Deutch, A.Y.; Avison, M.J.; Gore, J.C.; Sanders-Bush, E. 5-Hydroxytryptamine2C receptor contribution to m-chlorophenylpiperazine and N-methyl-β-carboline-3-carboxamide-induced anxiety-like behavior and limbic brain activation. J. Pharmacol. Exp. Ther. 2007, 320, 1023–1029. [Google Scholar] [CrossRef] [Green Version]
- Stephens, D.N.; Schneider, H.H.; Kehr, W.; Jensen, L.H.; Petersen, E.; Honore, T. Modulation of anxiety by β-carbolines and other benzodiazepine receptor ligands: Relationship of pharmacological to biochemical measures of efficacy. Brain Res. Bull. 1987, 19, 309–318. [Google Scholar] [CrossRef]
- Pellow, S.; File, S.E. The effects of putative anxiogenic compounds (FG 7142, CGS 8216 and Ro 15-1788) on the rat corticosterone response. Physiol. Behav. 1985, 35, 587–590. [Google Scholar] [CrossRef]
- Thiébot, M.H.; Soubrié, P.; Sanger, D. Anxiogenic properties of beta-CCE and FG 7142: A review of promises and pitfalls. Psychopharmacology 1988, 94, 452–463. [Google Scholar] [CrossRef]
- Takamatsu, H.; Noda, A.; Kurumaji, A.; Murakami, Y.; Tatsumi, M.; Ichise, R.; Nishimura, S. A PET study following treatment with a pharmacological stressor, FG7142, in conscious rhesus monkeys. Brain Res. 2003, 980, 275–280. [Google Scholar] [CrossRef]
- Ongini, E.; Barzaghi, C.; Marzanatti, M. Intrinsic and antagonistic effects of β-carboline FG 7142 on behavioral and EEG actions of benzodiazepines and pentobarbital in cats. Eur. J. Pharmacol. 1983, 95, 125–129. [Google Scholar] [CrossRef]
- Singewald, N.; Sharp, T. Neuroanatomical targets of anxiogenic drugs in the hindbrain as revealed by Fos immunocytochemistry. Neuroscience 2000, 98, 759–770. [Google Scholar] [CrossRef]
- Lyss, P.J.; Andersen, S.L.; Leblanc, C.J.; Teicher, M.H. Degree of neuronal activation following FG-7142 changes across regions during development. Dev. Brain Res. 1999, 116, 201–203. [Google Scholar] [CrossRef]
- Funk, D.; Li, Z.; Coen, K.; Lê, A.D. Effects of pharmacological stressors on c-fos and CRF mRNA in mouse brain: Relationship to alcohol seeking. Neurosci. Lett. 2008, 444, 254–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzsáki, G.; Watson, B.O. Brain rhythms and neural syntax: Implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin. Neurosci. 2012, 14, 345–367. [Google Scholar]
- Buzsáki, G.; Draguhn, A. Neuronal olscillations in cortical networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, W. Temporal Coherence: A Versatile Code for the Definition of Relations. In The Senses: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2008; Volume 2, pp. 1–9. ISBN 9780123708809. [Google Scholar]
- Watson, B.O.; Buzsáki, G. Neural syntax in mental disorders. Biol. Psychiatry 2015, 77, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Yener, G.G.; Başar, E. Brain oscillations as biomarkers in neuropsychiatric disorders. Suppl. Clin. Neurophysiol. 2013, 15, 343–363. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Claustre, Y.; Rouquier, L.; Desvignes, C.; Leonetti, M.; Montégut, J.; Aubin, N.; Allouard, N.; Bougault, I.; Oury-Donat, F.; Steinberg, R. Effects of the vasopressin (V1B) receptor antagonist, SSR149415, and the corticotropin-releasing factor 1 receptor antagonist, SSR125543, on FG 7142-induced increase in acetylcholine and norepinephrine release in the rat. Neuroscience 2006, 141, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Zhou, Y.; Sun, Y.; Liu, H.; Zhang, D.; Liu, P.; Shu, H. Acetylcholine muscarinic receptors in ventral hippocampus modulate stress-induced anxiety-like behaviors in mice. Front. Mol. Neurosci. 2020, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Torrence, C.; Compo, G.P. A practical guide to wavelet analysis. Bull. Am. Meteorol. Soc. 1997, 79, 61–78. [Google Scholar] [CrossRef] [Green Version]
- Vinck, M.; Oostenveld, R.; van Wingerden, M.; Battaglia, F.; Pennartz, C.M.A. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 2011, 55, 1548–1565. [Google Scholar] [CrossRef]
- Tort, A.B.L.; Komorowski, R.; Eichenbaum, H.; Kopell, N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J. Neurophysiol. 2010, 104, 1195–1210. [Google Scholar] [CrossRef] [PubMed]
- Bland, B.H.; Oddie, S.D. Theta band oscillation and synchrony in the hippocampal formation and associated structures: The case for its role in sensorimotor integration. Behav. Brain Res. 2001, 127, 119–136. [Google Scholar] [CrossRef]
- McNaughton, N.; Swart, C.; Neo, P.; Bates, V.; Glue, P. Anti-anxiety drugs reduce conflict-specific “theta”—A possible human anxiety-specific biomarker. J. Affect. Disord. 2013, 148, 104–111. [Google Scholar] [CrossRef]
- Holtzheimer, P.E. Subcallosal Cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch. Gen. Psychiatry 2012, 69, 150. [Google Scholar] [CrossRef]
- Clement, E.A.; Richard, A.; Thwaites, M.; Ailon, J.; Peters, S.; Dickson, C.T. Cyclic and sleep-like spontaneous alternations of brain state under urethane anaesthesia. PLoS ONE 2008, 3, e2004. [Google Scholar] [CrossRef]
- Steriade, M.; Nunez, A.; Amzica, F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: Depolarizing and hyperpolarizing components. J. Neurosci. 1993, 13, 3252–3265. [Google Scholar] [CrossRef] [Green Version]
- Nuñez, A.; Cervera-Ferri, A.; Olucha-Bordonau, F.; Ruiz-Torner, A.; Teruel, V. Nucleus incertus contribution to hippocampal theta rhythm generation. Eur. J. Neurosci. 2006, 23, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Cervera-Ferri, A.; Guerrero-Martínez, J.; Bataller-Mompeán, M.; Taberner-Cortes, A.; Martínez-Ricós, J.; Ruiz-Torner, A.; Teruel-Martí, V. Theta synchronization between the hippocampus and the nucleus incertus in urethane-anesthetized rats. Exp. Brain Res. 2011, 211, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Bellver, S.; Cervera-Ferri, A.; Martínez-Ricós, J.; Ruiz-Torner, A.; Luque-García, A.; Blasco-Serra, A.; Guerrero-Martínez, J.; Bataller-Mompeán, M.; Teruel-Martí, V. Regular theta-firing neurons in the nucleus incertus during sustained hippocampal activation. Eur. J. Neurosci. 2015, 41, 1049–1067. [Google Scholar] [CrossRef] [PubMed]
- Kohtala, S.; Theilmann, W.; Rosenholm, M.; Penna, L.; Karabulut, G.; Uusitalo, S.; Järventausta, K.; Yli-Hankala, A.; Yalcin, I.; Matsui, N.; et al. Cortical excitability and activation of trkb signaling during rebound slow oscillations are critical for rapid antidepressant responses. Mol. Neurobiol. 2019, 56, 4163–4174. [Google Scholar] [CrossRef] [Green Version]
- Drugan, R.C.; Maier, S.F.; Skolnick, P.; Paul, S.M.; Crawley, J.N. An anxiogenic benzodiazepine receptor ligand induces learned helplessness. Eur. J. Pharmacol. 1985, 113, 453–457. [Google Scholar] [CrossRef]
- Lukkes, J.L.; Engelman, G.H.; Zelin, N.S.; Hale, M.W.; Lowry, C.A. Post-weaning social isolation of female rats, anxiety-related behavior, and serotonergic systems. Brain Res. 2012, 1443, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.L.; Samuels, B.C.; Fitz, S.D.; Federici, L.M.; Hammes, N.; Early, M.C.; Truitt, W.; Lowry, C.A.; Shekhar, A. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol. Behav. 2012, 107, 733–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGregor, I.S.; Lee, A.M.; Westbrook, R.F. Stress-induced changes in respiratory quotient, energy expenditure and locomotor activity in rats: Effects of midazolam. Psychopharmacology 1994, 116, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Gokan, H.; Oh-Nishi, A.; Suhara, T.; Watanabe, S.; Minamimoto, T. Vocalizations associated with anxiety and fear in the common marmoset (Callithrix jacchus). Behav. Brain Res. 2014, 275, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Marín, R.H.; Martijena, I.D.; Arce, A. Effect of diazepam and a β-carboline on open-field and T-maze behaviors in 2-day-old chicks. Pharmacol. Biochem. Behav. 1997, 58, 915–921. [Google Scholar] [CrossRef]
- Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: A pharmacological study. Behav. Brain Res. 2011, 222, 15–25. [Google Scholar] [CrossRef]
- Vollmayr, B.; Gass, P. Learned helplessness: Unique features and translational value of a cognitive depression model. Cell Tissue Res. 2013, 354, 171–178. [Google Scholar] [CrossRef]
- Etiévant, A.; Oosterhof, C.; Bétry, C.; Abrial, E.; Novo-Perez, M.; Rovera, R.; Scarna, H.; Devader, C.; Mazella, J.; Wegener, G.; et al. Astroglial control of the antidepressant-like effects of prefrontal cortex deep brain stimulation. EBioMedicine 2015, 2, 898–908. [Google Scholar] [CrossRef] [Green Version]
- Massimini, M. The sleep slow oscillation as a traveling wave. J. Neurosci. 2004, 24, 6862–6870. [Google Scholar] [CrossRef]
- Murphy, M.; Riedner, B.A.; Huber, R.; Massimini, M.; Ferrarelli, F.; Tononi, G. Source modeling sleep slow waves. Proc. Natl. Acad. Sci. USA 2009, 106, 1608–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esser, S.K.; Hill, S.L.; Tononi, G. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep 2007, 30, 1617–1630. [Google Scholar] [CrossRef] [Green Version]
- Riedner, B.A.; Vyazovskiy, V.V.; Huber, R.; Massimini, M.; Esser, S.; Murphy, M.; Tononi, G. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep 2007, 30, 1643–1657. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Riedner, B.A.; Cirelli, C.; Tononi, G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep 2007, 30, 1631–1642. [Google Scholar] [CrossRef]
- Maquet, P.; Degueldre, C.; Delfiore, G.; Aerts, J.; Péters, J.M.; Luxen, A.; Franck, G. Functional neuroanatomy of human slow wave sleep. J. Neurosci. 1997, 17, 2801–2812. [Google Scholar] [CrossRef] [Green Version]
- Voget, M.; Rummel, J.; Avchalumov, Y.; Sohr, R.; Haumesser, J.K.; Rea, E.; Mathé, A.A.; Hadar, R.; van Riesen, C.; Winter, C. Altered local field potential activity and serotonergic neurotransmission are further characteristics of the Flinders sensitive line rat model of depression. Behav. Brain Res. 2015, 291, 299–305. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, T. Synaptic plasticity-related neural oscillations on hippocampus-prefrontal cortex pathway in depression. Neuroscience 2015, 292, 170–180. [Google Scholar] [CrossRef]
- Armitage, R. Microarchitectural findings in sleep EEG in depression: Diagnostic implications. Biol. Psychiatry 1995, 37, 72–84. [Google Scholar] [CrossRef]
- Benca, R.M.; Obermeyer, W.H.; Thisted, R.A.; Gillin, J.C. Sleep and Psychiatric disorders: A meta-analysis. Arch. Gen. Psychiatry 1992, 49, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Dijk, D.J. Slow-wave sleep deficiency and enhancement: Implications for insomnia and its management. World J. Biol. Psychiatry 2010, 11, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Duncan, W.C.; Zarate, C.A. Ketamine, sleep, and depression: Current status and new questions. Curr. Psychiatry Rep. 2013, 15, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlers, C.L.; Havstad, J.W.; Kupfer, D.J. Estimation of the time course of slow-wave sleep over the night in depressed patients: Effects of clomipramine and clinical response. Biol. Psychiatry 1996, 39, 171–181. [Google Scholar] [CrossRef]
- Argyropoulos, S.V.; Hicks, J.A.; Nash, J.R.; Bell, C.J.; Rich, A.S.; Nutt, D.J.; Wilson, S. Redistribution of slow wave activity of sleep during pharmacological treatment of depression with paroxetine but not with nefazodone. J. Sleep Res. 2009, 18, 342–348. [Google Scholar] [CrossRef]
- Landsness, E.C.; Goldstein, M.R.; Peterson, M.J.; Tononi, G.; Benca, R.M. Antidepressant effects of selective slow wave sleep deprivation in major depression: A high-density EEG investigation. J. Psychiatr. Res. 2011, 45, 1019–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, W.C.; Sarasso, S.; Ferrarelli, F.; Selter, J.; Riedner, B.A.; Hejazi, N.S.; Yuan, P.; Brutsche, N.; Manji, H.K.; Tononi, G.; et al. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int. J. Neuropsychopharmacol. 2013, 16, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G. Theta oscillations in the hippocampus. Neuron 2002, 33, 325–340. [Google Scholar] [CrossRef] [Green Version]
- Hasselmo, M.E. What is the function of hippocampal theta rhythm? Linking bahavioral data to phasic properties of field potential and unit recording data. Hippocampus 2005, 15, 936–949. [Google Scholar] [CrossRef] [Green Version]
- Wells, C.E.; Amos, D.P.; Jeewajee, A.; Douchamps, V.; Rodgers, J.; O’Keefe, J.; Burgess, N.; Lever, C. Novelty and anxiolytic drugs dissociate two components of hippocampal theta in behaving rats. J. Neurosci. 2013, 33, 8650–8667. [Google Scholar] [CrossRef] [Green Version]
- O’Keefe, J.; Nadel, L. The Hippocampus as a Cognitive Map; Oxford University Press: Oxford, UK, 1978; ISBN 9780198572060. [Google Scholar]
- Buzsáki, G.; Moser, E.I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013, 16, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Sainsbury, R.S.; Heynen, A.; Montoya, C.P. Behavioral correlates of hippocampal type 2 theta in the rat. Physiol. Behav. 1987, 39, 513–519. [Google Scholar] [CrossRef]
- Hegde, P.; Singh, K.; Chaplot, S.; Shankaranarayana Rao, B.S.; Chattarji, S.; Kutty, B.M.; Laxmi, T.R. Stress-induced changes in sleep and associated neuronal activity in rat hippocampus and amygdala. Neuroscience 2008, 153, 20–30. [Google Scholar] [CrossRef]
- Hegde, P.; Jayakrishnan, H.R.; Chattarji, S.; Kutty, B.M.; Laxmi, T.R. Chronic stress-induced changes in REM sleep on theta oscillations in the rat hippocampus and amygdala. Brain Res. 2011, 1382, 155–164. [Google Scholar] [CrossRef]
- Jacinto, L.R.; Reis, J.S.; Dias, N.S.; Cerqueira, J.J.; Correia, J.H.; Sousa, N. Stress affects theta activity in limbic networks and impairs novelty-induced exploration and familiarization. Front. Behav. Neurosci. 2013, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Jacinto, L.R.; Cerqueira, J.J.; Sousa, N. Patterns of theta activity in limbic anxiety circuit preceding exploratory behavior in approach-avoidance conflict. Front. Behav. Neurosci. 2016, 10, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, A.; Topiwala, M.A.; Gordon, J.A. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 2010, 65, 257–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, R.T.; Seidenbecher, T.; Kluge, C.; Bergado, J.; Stork, O.; Pape, H.C. Dissociated theta phase synchronization in amygdalo-hippocampal circuits during various stages of fear memory. Eur. J. Neurosci. 2007, 25, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Seidenbecher, T.; Lesting, J. Amygdala-hippocampal theta synchrony in learning, memory and disease. In Insights into the Amygdala: Structure, Functions and Implications for Disorders; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; ISBN 9781622570119. [Google Scholar]
- Lesting, J.; Narayanan, R.T.; Kluge, C.; Sangha, S.; Seidenbecher, T.; Pape, H.C. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS ONE 2011, 6, e21714. [Google Scholar] [CrossRef] [Green Version]
- Popa, D.; Duvarci, S.; Popescu, A.T.; Lena, C.; Pare, D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc. Natl. Acad. Sci. USA 2010, 107, 6516–6519. [Google Scholar] [CrossRef] [Green Version]
- Yartsev, M.M. Distinct or gradually changing spatial and nonspatial representations along the dorsoventral axis of the hippocampus. J. Neurosci. 2010, 30, 7758–7760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, A.; Miller, J.; Qasim, S.E.; Watrous, A.J.; Zhang, H.; Stein, J.M.; Inman, C.S.; Gross, R.E.; Willie, J.T.; Lega, B.; et al. Functionally distinct high and low theta oscillations in the human hippocampus. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Mikulovic, S.; Restrepo, C.E.; Siwani, S.; Bauer, P.; Pupe, S.; Tort, A.B.L.; Kullander, K.; Leão, R.N. Ventral hippocampal OLM cells control type 2 theta oscillations and response to predator odor. Nat. Commun. 2018, 9, 3638. [Google Scholar] [CrossRef]
- Gray, J.A.; McNaughton, N. Comparison between the behavioural effects of septal and hippocampal lesions: A review. Neurosci. Biobehav. Rev. 1983, 7, 119–188. [Google Scholar] [CrossRef]
- McNaughton, N.; Kocsis, B.; Hajós, M. Elicited hippocampal theta rhythm: A screen for anxiolytic and procognitive drugs through changes in hippocampal function? Behav. Pharmacol. 2007, 18, 329–346. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, N. Development of a theoretically-derived human anxiety syndrome biomarker. Transl. Neurosci. 2014, 5, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Yeung, M.; Dickson, C.T.; Treit, D. Intrahippocampal infusion of the I h blocker ZD7288 slows evoked theta rhythm and produces anxiolytic-like effects in the elevated plus maze. Hippocampus 2013, 23, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Crone, N.E.; Niebur, E.; Franaszczuk, P.J.; Hsiao, S.S. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J. Neurosci. 2008, 28, 11526–11536. [Google Scholar] [CrossRef] [Green Version]
- Buzsáki, G.; Wang, X.-J. Mechanisms of Gamma Oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar] [CrossRef] [Green Version]
- Sirota, A.; Montgomery, S.; Fujisawa, S.; Isomura, Y.; Zugaro, M.; Buzsáki, G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 2008, 60, 683–697. [Google Scholar] [CrossRef] [Green Version]
- Bi, K.; Chattun, M.R.; Liu, X.; Wang, Q.; Tian, S.; Zhang, S.; Lu, Q.; Yao, Z. Abnormal early dynamic individual patterns of functional networks in low gamma band for depression recognition. J. Affect. Disord. 2018, 238, 366–374. [Google Scholar] [CrossRef]
- Lee, P.-S.; Chen, Y.-S.; Hsieh, J.-C.; Su, T.-P.; Chen, L.-F. Distinct neuronal oscillatory responses between patients with bipolar and unipolar disorders: A magnetoencephalographic study. J. Affect. Disord. 2010, 123, 270–275. [Google Scholar] [CrossRef]
- Liu, T.Y.; Chen, Y.S.; Su, T.P.; Hsieh, J.C.; Chen, L.F. Abnormal early gamma responses to emotional faces differentiate unipolar from bipolar disorder patients. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, P.J.; Watson, B.O. Gamma oscillations as a biomarker for major depression: An emerging topic. Transl. Psychiatry 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.R.; Zarate, C.A. Electrophysiological biomarkers of antidepressant response to ketamine in treatment-resistant depression: Gamma power and long-term potentiation. Pharmacol. Biochem. Behav. 2020, 189, 172856. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.E.; Summerfelt, A.; Buchanan, R.W.; O’Donnell, P.; Thaker, G.K.; Weiler, M.A.; Lahti, A.C. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology 2010, 35, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Maksimow, A.; Särkelä, M.; Långsjö, J.W.; Salmi, E.; Kaisti, K.K.; Yli-Hankala, A.; Hinkka-Yli-Salomäki, S.; Scheinin, H.; Jääskeläinen, S.K. Increase in high frequency EEG activity explains the poor performance of EEG spectral entropy monitor during S-ketamine anesthesia. Clin. Neurophysiol. 2006, 117, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaraswamy, S.D.; Shaw, A.D.; Jackson, L.E.; Hall, J.; Moran, R.; Saxena, N. Evidence that subanesthetic doses of ketamine cause sustained disruptions of NMDA and AMPA-mediated frontoparietal connectivity in humans. J. Neurosci. 2015, 35, 11694–11706. [Google Scholar] [CrossRef] [Green Version]
- Shaw, A.D.; Muthukumaraswamy, S.D.; Saxena, N.; Sumner, R.L.; Adams, N.E.; Moran, R.J.; Singh, K.D. Generative modelling of the thalamo-cortical circuit mechanisms underlying the neurophysiological effects of ketamine. Neuroimage 2020, 221, 117189. [Google Scholar] [CrossRef]
- Zacharias, N.; Musso, F.; Müller, F.; Lammers, F.; Saleh, A.; London, M.; de Boer, P.; Winterer, G. Ketamine effects on default mode network activity and vigilance: A randomized, placebo-controlled crossover simultaneous fMRI/EEG study. Hum. Brain Mapp. 2020, 41, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Matveychuk, D.; Thomas, R.K.; Swainson, J.; Khullar, A.; MacKay, M.-A.; Baker, G.B.; Dursun, S.M. Ketamine as an antidepressant: Overview of its mechanisms of action and potential predictive biomarkers. Ther. Adv. Psychopharmacol. 2020, 10, 204512532091665. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, D.F.; Luckenbaugh, D.A.; Niciu, M.J.; Richards, E.M.; Slonena, E.E.; Vande Voort, J.L.; Brutsche, N.E.; Zarate, C.A. Effect of baseline anxious depression on initial and sustained antidepressant response to Ketamine. J. Clin. Psychiatry 2014, 75, e932–e938. [Google Scholar] [CrossRef]
- Taylor, J.H.; Landeros-Weisenberger, A.; Coughlin, C.; Mulqueen, J.; Johnson, J.A.; Gabriel, D.; Reed, M.O.; Jakubovski, E.; Bloch, M.H. Ketamine for social anxiety disorder: A randomized, placebo-controlled crossover trial. Neuropsychopharmacology 2018, 43, 325–333. [Google Scholar] [CrossRef]
- Cornwell, B.R.; Salvadore, G.; Furey, M.; Marquardt, C.A.; Brutsche, N.E.; Grillon, C.; Zarate, C.A. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol. Psychiatry 2012, 72, 555–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nugent, A.C.; Ballard, E.D.; Gould, T.D.; Park, L.T.; Moaddel, R.; Brutsche, N.E.; Zarate, C.A. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol. Psychiatry 2019, 24, 1040–1052. [Google Scholar] [CrossRef]
- Engin, E.; Treit, D.; Dickson, C.T. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience 2009, 161, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.K.; Senkowski, D.; Schneider, T.R. Multisensory integration through neural coherence. In The Neural Bases of Multisensory Processes; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781439812198. [Google Scholar]
- Sauseng, P.; Klimesch, W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci. Biobehav. Rev. 2008, 32, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Tadayonnejad, R.; MacNamara, A.; Ajilore, O.; DiGangi, J.; Phan, K.L.; Leow, A.; Klumpp, H. Resting-state theta band connectivity and graph analysis in generalized social anxiety disorder. NeuroImage Clin. 2017, 13, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Schutter, D.J.L.G.; Van Honk, J. Salivary cortisol levels and the coupling of midfrontal delta-beta oscillations. Int. J. Psychophysiol. 2005, 55, 127–129. [Google Scholar] [CrossRef] [Green Version]
- Knyazev, G.G. Cross-frequency coupling of brain oscillations: An impact of state anxiety. Int. J. Psychophysiol. 2011, 80, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Van Peer, J.M.; Roelofs, K.; Spinhoven, P. Cortisol administration enhances the coupling of midfrontal delta and beta oscillations. Int. J. Psychophysiol. 2008, 677, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.W.S. Spectral analysis of hippocampal EEG in the freely moving rat: Effects of centrally active drugs and relations to evoked potentials. Electroencephalogr. Clin. Neurophysiol. 1985, 60, 65–77. [Google Scholar] [CrossRef]
- Canolty, R.T.; Knight, R.T. The functional role of cross-frequency coupling. Trends Cogn. Sci. 2010, 14, 506–515. [Google Scholar] [CrossRef] [Green Version]
- Salimpour, Y.; Anderson, W.S. Cross-frequency coupling based neuromodulation for treating neurological disorders. Front. Neurosci. 2019, 13, 125. [Google Scholar] [CrossRef] [Green Version]
- Lisman, J. The theta/gamma discrete phase code occuring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus 2005, 15, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.; Buzsáki, G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr. Bull. 2008, 34, 974–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axmacher, N.; Henseler, M.M.; Jensen, O.; Weinreich, I.; Elger, C.E.; Fell, J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 3228–3233. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Period | dHPC | iHPC | vHPC | BLA |
|---|---|---|---|---|
| Basal | 1.063 ± 0.039 | 1.084 ± 0.042 | 0.979 ± 0.051 | 1.105 ± 0.045 |
| Saline | 1.042 ± 0.046 | 1.041 ± 0.063 | 1.048 ± 0.069 | 1.084 ± 0.042 |
| FG-7142 | 3.355 ± 0.128 *** | 3.574 ± 0.100 *** | 3.629 ± 0.244 *** | 3.202 ± 0.083 *** |
| DBS1 | 3.047 ± 0.087 *** | 3.356 ± 0.056 *** | 3.330 ± 0.087 *** | 3.526 ± 0.061 *** |
| DBS2 | 2.380 ± 0.098 *** | 2.292 ± 0.081 *** | 1.705 ± 0.085 *** | 1.653 ± 0.109 *** |
| DBS3 | 1.251 ± 0.051 *** | 1.421 ± 0.101 *** | 1.426 ± 0.061 *** | 1.359 ± 0.101 ** |
| DBS4 | 1.147 ± 0.058 | 1.230 ± 0.051 | 1.253 ± 0.098 ** | 1.398 ± 0.046 *** |
| DBS5 | 1.083 ± 0.060 | 1.147 ± 0.049 | 1.048 ± 0.070 | 1.147 ± 0.058 |
| POST-DBS | 1.063 ± 0.049 | 1.020 ± 0.060 | 1.048 ± 0.069 | 1.063 ± 0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vila-Merkle, H.; González-Martínez, A.; Campos-Jiménez, R.; Martínez-Ricós, J.; Teruel-Martí, V.; Blasco-Serra, A.; Lloret, A.; Celada, P.; Cervera-Ferri, A. The Oscillatory Profile Induced by the Anxiogenic Drug FG-7142 in the Amygdala–Hippocampal Network Is Reversed by Infralimbic Deep Brain Stimulation: Relevance for Mood Disorders. Biomedicines 2021, 9, 783. https://doi.org/10.3390/biomedicines9070783
Vila-Merkle H, González-Martínez A, Campos-Jiménez R, Martínez-Ricós J, Teruel-Martí V, Blasco-Serra A, Lloret A, Celada P, Cervera-Ferri A. The Oscillatory Profile Induced by the Anxiogenic Drug FG-7142 in the Amygdala–Hippocampal Network Is Reversed by Infralimbic Deep Brain Stimulation: Relevance for Mood Disorders. Biomedicines. 2021; 9(7):783. https://doi.org/10.3390/biomedicines9070783
Chicago/Turabian StyleVila-Merkle, Hanna, Alicia González-Martínez, Rut Campos-Jiménez, Joana Martínez-Ricós, Vicent Teruel-Martí, Arantxa Blasco-Serra, Ana Lloret, Pau Celada, and Ana Cervera-Ferri. 2021. "The Oscillatory Profile Induced by the Anxiogenic Drug FG-7142 in the Amygdala–Hippocampal Network Is Reversed by Infralimbic Deep Brain Stimulation: Relevance for Mood Disorders" Biomedicines 9, no. 7: 783. https://doi.org/10.3390/biomedicines9070783
APA StyleVila-Merkle, H., González-Martínez, A., Campos-Jiménez, R., Martínez-Ricós, J., Teruel-Martí, V., Blasco-Serra, A., Lloret, A., Celada, P., & Cervera-Ferri, A. (2021). The Oscillatory Profile Induced by the Anxiogenic Drug FG-7142 in the Amygdala–Hippocampal Network Is Reversed by Infralimbic Deep Brain Stimulation: Relevance for Mood Disorders. Biomedicines, 9(7), 783. https://doi.org/10.3390/biomedicines9070783