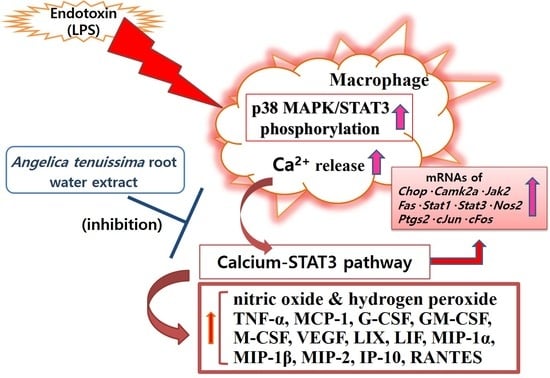
Conioselinum tenuissimum Root Extract Modulates Macrophage Activation via the Calcium–STAT3 Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AT
2.3. Diethylene Glycol Colorimetric Assay
2.4. MTT Assay
2.5. Griess Reagent Assay
2.6. Fluo-4 Calcium Assay
2.7. Dihydrorhodamine 123 Assay
2.8. Multiplex Cytokine Assay
2.9. Real-Time PCR
2.10. Flow Cytometry Assay
2.11. Statistical Analyses
3. Results
3.1. Total Flavonoid Content of AT
3.2. Cell Viability
3.3. NO Production
3.4. Calcium Release
3.5. Hydrogen Peroxide Production
3.6. Cytokine Production
3.7. The Level of Inflammatory Gene Expression
3.8. The Level of Phospho-STAT3 and Phospho-P38 MAPK
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jansen, K.U.; Anderson, A.S. The Role of Vaccines in Fighting Antimicrobial Resistance (AMR). Hum. Vaccines Immunother. 2018, 14, 2142–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez de la Lastra, J.M.; Anand, U.; Gonzalez-Acosta, S.; Lopez, M.R.; Dey, A.; Bontempi, E.; Morales delaNuez, A. Antimicrobial Resistance in the COVID-19 Landscape: Is there an Opportunity for Anti-Infective Antibodies and Antimicrobial Peptides? Front. Immunol. 2022, 13, 921483. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S. Protein Phylogenies and Signature Sequences: A Reappraisal of Evolutionary Relationships among Archaebacteria, Eubacteria, and Eukaryotes. Microbiol. Mol. Biol. Rev. 1998, 62, 1435–1491. [Google Scholar] [CrossRef] [Green Version]
- Koonin, E.V.; Makarova, K.S.; Wolf, Y.I. Evolutionary Genomics of Defense Systems in Archaea and Bacteria. Annu. Rev. Microbiol. 2017, 71, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Giordano, N.P.; Cian, M.B.; Dalebroux, Z.D. Outer Membrane Lipid Secretion and the Innate Immune Response to Gram-Negative Bacteria. Infect. Immun. 2020, 88, e00920-19. [Google Scholar] [CrossRef] [PubMed]
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8, 1. [Google Scholar] [CrossRef]
- Prins, J.M.; Lauw, F.N.; Derkx, B.H.; Speelman, P.; Kuijper, E.J.; Dankert, J.; van Deventer, S.J. Endotoxin Release and Cytokine Production in Acute and Chronic Meningococcaemia. Clin. Exp. Immunol. 1998, 114, 215–219. [Google Scholar] [CrossRef]
- Mathias, B.; Mira, J.C.; Rehfuss, J.P.; Rincon, J.C.; Ungaro, R.; Nacionales, D.C.; Lopez, M.C.; Baker, H.V.; Moldawer, L.L.; Larson, S.D. LPS Stimulation of Cord Blood Reveals a Newborn-Specific Neutrophil Transcriptomic Response and Cytokine Production. Shock 2017, 47, 606–614. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.Y.; Sadri, N.; Schneider, R.J. Endotoxic Shock in AUF1 Knockout Mice Mediated by Failure to Degrade Proinflammatory Cytokine mRNAs. Genes Dev. 2006, 20, 3174–3184. [Google Scholar] [CrossRef] [Green Version]
- Hagar, J.A.; Powell, D.A.; Aachoui, Y.; Ernst, R.K.; Miao, E.A. Cytoplasmic LPS Activates Caspase-11: Implications in TLR4-Independent Endotoxic Shock. Science 2013, 341, 1250–1253. [Google Scholar] [CrossRef]
- Barth, E.; Radermacher, P.; Thiemermann, C.; Weber, S.; Georgieff, M.; Albuszies, G. Role of Inducible Nitric Oxide Synthase in the Reduced Responsiveness of the Myocardium to Catecholamines in a Hyperdynamic, Murine Model of Septic Shock. Crit. Care Med. 2006, 34, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cano, I.; D’Amore, P.A. Update on the Role of the Endothelial Glycocalyx in Angiogenesis and Vascular Inflammation. Front. Cell. Dev. Biol. 2021, 9, 734276. [Google Scholar] [CrossRef]
- Muniandy, K.; Gothai, S.; Badran, K.M.H.; Suresh Kumar, S.; Esa, N.M.; Arulselvan, P. Suppression of Proinflammatory Cytokines and Mediators in LPS-Induced RAW 264.7 Macrophages by Stem Extract of Alternanthera Sessilis Via the Inhibition of the NF-kappaB Pathway. J. Immunol. Res. 2018, 2018, 3430684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Luo, G.; Yuan, J.; Wang, Y.; Yang, X.; Wang, X.; Li, G.; Liu, Z.; Zhong, N. Vitamin C Mitigates Oxidative Stress and Tumor Necrosis Factor-Alpha in Severe Community-Acquired Pneumonia and LPS-Induced Macrophages. Mediat. Inflamm. 2014, 2014, 426740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Choi, M.Y.; Do, E.j.; Kim, M.R. Comparative evaluation on biological activities of Ziingiber officinale Roscoe extracted from different solvents. Korea J. Herbol. 2021, 36, 19–29. [Google Scholar]
- Yoon, S.B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-Inflammatory Effects of Scutellaria Baicalensis Water Extract on LPS-Activated RAW 264.7 Macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef]
- Forman, H.J.; Torres, M. Redox Signaling in Macrophages. Mol. Asp. Med. 2001, 22, 189–216. [Google Scholar] [CrossRef]
- Lendeckel, U.; Venz, S.; Wolke, C. Macrophages: Shapes and Functions. ChemTexts 2022, 8, 12. [Google Scholar] [CrossRef]
- Forman, H.J.; Torres, M. Reactive Oxygen Species and Cell Signaling: Respiratory Burst in Macrophage Signaling. Am. J. Respir. Crit. Care Med. 2002, 166, S4–S8. [Google Scholar] [CrossRef]
- Chong, W.C.; Shastri, M.D.; Eri, R. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology. Int. J. Mol. Sci. 2017, 18, 771. [Google Scholar] [CrossRef]
- Sygitowicz, G.; Sitkiewicz, D. Molecular Mechanisms of Organ Damage in Sepsis: An Overview. Braz. J. Infect. Dis. 2020, 24, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Victor, V.M.; Rocha, M.; Esplugues, J.V.; De la Fuente, M. Role of Free Radicals in Sepsis: Antioxidant Therapy. Curr. Pharm. Des. 2005, 11, 3141–3158. [Google Scholar] [CrossRef] [PubMed]
- Recio, C.; Guerra, B.; Guerra-Rodriguez, M.; Aranda-Tavio, H.; Martin-Rodriguez, P.; de Mirecki-Garrido, M.; Brito-Casillas, Y.; Garcia-Castellano, J.M.; Estevez-Braun, A.; Fernandez-Perez, L. Signal Transducer and Activator of Transcription (STAT)-5: An Opportunity for Drug Development in Oncohematology. Oncogene 2019, 38, 4657–4668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qi, Y.F.; Yu, Y.R. STAT3: A Key Regulator in Liver Fibrosis. Ann. Hepatol. 2021, 21, 100224. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, H.; Chan, L.S. The Involvement of the JAK-STAT Signaling Pathway in Chronic Inflammatory Skin Disease Atopic Dermatitis. JAKSTAT 2013, 2, e24137. [Google Scholar] [CrossRef] [Green Version]
- Montero, P.; Milara, J.; Roger, I.; Cortijo, J. Role of JAK/STAT in Interstitial Lung Diseases; Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2021, 22, 6211. [Google Scholar] [CrossRef]
- Choi, H.G.; Je, I.G.; Kim, G.J.; Nam, J.W.; Shim, S.H.; Kim, S.H.; Choi, H. Chemical Constituents of the Root of Angelica tenuissima and their Anti-Allergic Inflammatory Activity. Nat. Prod. Commun. 2017, 12, 779–780. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Kim, D.; Yang, I.; Choi, B.; Lee, J.W.; Namkoong, S.; Koo, H.J.; Lee, S.R.; Park, M.R.; Lim, H.; et al. Decursin and Z-Ligustilide in Angelica tenuissima Root Extract Fermented by Aspergillus Oryzae Display Anti-Pigment Activity in Melanoma Cells. J. Microbiol. Biotechnol. 2018, 28, 1061–1067. [Google Scholar] [CrossRef]
- Park, Y.A.; Lee, S.R.; Lee, J.W.; Koo, H.J.; Jang, S.A.; Yun, S.W.; Kim, H.J.; Woo, J.S.; Park, M.R.; Kang, S.C.; et al. Suppressive Effect of Fermented Angelica tenuissima Root Extract Against Photoaging: Possible Involvement of Hemeoxygenase-1. J. Microbiol. Biotechnol. 2018, 28, 1391–1400. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Lee, J.Y.; Kim, Y.J.; Kim, H.J.; Park, W. Rubi Fructus Water Extract Alleviates LPS-Stimulated Macrophage Activation Via an ER Stress-Induced Calcium/CHOP Signaling Pathway. Nutrients 2020, 12, 3577. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.Y.; Kim, H.J.; Kim, D.H.; Lee, T.H.; Kang, M.S.; Park, W. Anti-Inflammatory Effects of Angelica Sinensis (Oliv.) Diels Water Extract on RAW 264.7 Induced with Lipopolysaccharide. Nutrients 2018, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, H.; Kim, H.; Lee, H.; Sung, Y.H.; Kim, S.E.; Chang, H.K.; Shin, M.C.; Shin, M.S.; Kim, C.J. Inhibitory Effect of Angelicae Tenuissimae Radix on Expressions of Cyclooxygenase-2 and Inducible Nitric Oxide Synthase in Mouse BV2 Microglial Cells. Neurol. Res. 2010, 32 (Suppl. S1), 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bae, S.; Kwon, K.Y.; Hwang, Y.I.; Kang, J.S.; Lee, W.J. A Combinational Effect of Acetaminophen and Oriental Herbs on the Regulation of Inflammatory Mediators in Microglia Cell Line, BV2. Anat. Cell Biol. 2015, 48, 244–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarino, A.V.; Kanno, Y.; Ferdinand, J.R.; O’Shea, J.J. Mechanisms of Jak/STAT Signaling in Immunity and Disease. J. Immunol. 2015, 194, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Tang, T.; Sheng, L.; Wang, Z.; Tao, H.; Zhang, Q.; Zhang, Y.; Qi, Z. Aloin Suppresses Lipopolysaccharideinduced Inflammation by Inhibiting JAK1STAT1/3 Activation and ROS Production in RAW264.7 Cells. Int. J. Mol. Med. 2018, 42, 1925–1934. [Google Scholar]
- Tkach, M.; Rosemblit, C.; Rivas, M.A.; Proietti, C.J.; Diaz Flaque, M.C.; Mercogliano, M.F.; Beguelin, W.; Maronna, E.; Guzman, P.; Gercovich, F.G.; et al. P42/p44 MAPK-Mediated Stat3Ser727 Phosphorylation is Required for Progestin-Induced Full Activation of Stat3 and Breast Cancer Growth. Endocr. Relat. Cancer 2013, 20, 197–212. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Stark, G.R. Roles of Unphosphorylated STATs in Signaling. Cell Res. 2008, 18, 443–451. [Google Scholar] [CrossRef]
- Simon, A.R.; Rai, U.; Fanburg, B.L.; Cochran, B.H. Activation of the JAK-STAT Pathway by Reactive Oxygen Species. Am. J. Physiol. 1998, 275, C1640–C1652. [Google Scholar] [CrossRef]
- Meares, G.P.; Liu, Y.; Rajbhandari, R.; Qin, H.; Nozell, S.E.; Mobley, J.A.; Corbett, J.A.; Benveniste, E.N. PERK-Dependent Activation of JAK1 and STAT3 Contributes to Endoplasmic Reticulum Stress-Induced Inflammation. Mol. Cell. Biol. 2014, 34, 3911–3925. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Liang, W.; Ye, Y.; Yi, B. PERK-Dependent Activation of the JAK2/STAT3 Pathway Contributes to High Glucose-Induced Extracellular Matrix Deposition in Renal Tubular Epithelial Cells. Int. J. Endocrinol. 2021, 2021, 8475868. [Google Scholar] [CrossRef]
- Ahyi, A.N.; Quinton, L.J.; Jones, M.R.; Ferrari, J.D.; Pepper-Cunningham, Z.A.; Mella, J.R.; Remick, D.G.; Mizgerd, J.P. Roles of STAT3 in Protein Secretion Pathways during the Acute-Phase Response. Infect. Immun. 2013, 81, 1644–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabas, I.; Seimon, T.; Timmins, J.; Li, G.; Lim, W. Macrophage Apoptosis in Advanced Atherosclerosis. Ann. N. Y. Acad. Sci. 2009, 1173 (Suppl. S1), E40–E45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Z.; Ron, D. Stress-Induced Phosphorylation and Activation of the Transcription Factor CHOP (GADD153) by p38 MAP Kinase. Science 1996, 272, 1347–1349. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Xu, R.; Feng, L.; Guo, W.; Cao, N.; Qian, C.; Teng, P.; Wang, L.; Wu, X.; Sun, Y.; et al. A Novel Chromone Derivative with Anti-Inflammatory Property Via Inhibition of ROS-Dependent Activation of TRAF6-ASK1-p38 Pathway. PLoS ONE 2012, 7, e37168. [Google Scholar] [CrossRef] [Green Version]
- Timmins, J.M.; Ozcan, L.; Seimon, T.A.; Li, G.; Malagelada, C.; Backs, J.; Backs, T.; Bassel-Duby, R.; Olson, E.N.; Anderson, M.E.; et al. Calcium/calmodulin-Dependent Protein Kinase II Links ER Stress with Fas and Mitochondrial Apoptosis Pathways. J. Clin. Invest. 2009, 119, 2925–2941. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Zhang, M.; Li, Y.; Mei, S.; Qin, J.; Yan, J. CJun/Ap1 is Upregulated in an Ang IIinduced Abdominal Aortic Aneurysm Formation Model and Mediates Chop Expression in Mouse Aortic Smooth Muscle Cells. Mol. Med. Rep. 2019, 19, 3459–3468. [Google Scholar]
- Klymenko, O.; Huehn, M.; Wilhelm, J.; Wasnick, R.; Shalashova, I.; Ruppert, C.; Henneke, I.; Hezel, S.; Guenther, K.; Mahavadi, P.; et al. Regulation and Role of the ER Stress Transcription Factor CHOP in Alveolar Epithelial Type-II Cells. J. Mol. Med. 2019, 97, 973–990. [Google Scholar] [CrossRef] [Green Version]
- Endo, M.; Mori, M.; Akira, S.; Gotoh, T. C/EBP Homologous Protein (CHOP) is Crucial for the Induction of Caspase-11 and the Pathogenesis of Lipopolysaccharide-Induced Inflammation. J. Immunol. 2006, 176, 6245–6253. [Google Scholar] [CrossRef] [Green Version]
- Stout, B.A.; Melendez, K.; Seagrave, J.; Holtzman, M.J.; Wilson, B.; Xiang, J.; Tesfaigzi, Y. STAT1 Activation Causes Translocation of Bax to the Endoplasmic Reticulum during the Resolution of Airway Mucous Cell Hyperplasia by IFN-Gamma. J. Immunol. 2007, 178, 8107–8116. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.; Cho, J.Y.; Lim, K.S.; Kim, K.P.; Kim, J.; Kim, B.H.; Hong, J.H.; Jang, I.J.; Shin, S.G.; Yu, K.S. Effects of Angelicae Tenuissima Radix, Angelicae Dahuricae Radix and Scutellariae Radix Extracts on Cytochrome P450 Activities in Healthy Volunteers. Basic Clin. Pharmacol. Toxicol. 2009, 105, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Obregon, D.; Ehrhart, J.; Deng, J.; Tian, J.; Hou, H.; Giunta, B.; Sawmiller, D.; Tan, J. Baicalein Reduces Beta-Amyloid and Promotes Nonamyloidogenic Amyloid Precursor Protein Processing in an Alzheimer’s Disease Transgenic Mouse Model. J. Neurosci. Res. 2013, 91, 1239–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.H.; Kwon, J.E.; Cho, Y.; Kim, I.; Kang, S.C. Anti-Inflammatory Effect of Angelica Gigas Via Heme Oxygenase (HO)-1 Expression. Nutrients 2015, 7, 4862–4874. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Du, J.R.; Wang, Y.; Kuang, X.; Wang, C.Y. Z-Ligustilide Attenuates Lipopolysaccharide-Induced Proinflammatory Response Via Inhibiting NF-kappaB Pathway in Primary Rat Microglia. Acta Pharmacol. Sin. 2010, 31, 791–797. [Google Scholar] [CrossRef] [PubMed]







| Gene Name | GenBank Accession Number |
|---|---|
| Chop | NM_007837 |
| Camk2a | NM_012920 |
| Stat1 | NM_009283.4 |
| Stat3 | NM_011486.5 |
| Jak2 | NM_001048177.3 |
| Fas | NM_007987 |
| c-Jun | NM_010591 |
| c-Fos | NM_010234 |
| Nos2 | NM_010927.3 |
| Ptgs2 | NM_011198 |
| β-Actin | NM_007393.3 |
| Inflammatory Factor | Normal (Media Only) | Control (LPS Alone) | Concentration (µg/mL) of AT with Lipopolysaccharide (LPS) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 100 | 200 | |||||||||||||||
| IL-6 (pg/mL) | 397.67 | ± | 68.05 | 21,494.67 | ± | 973.61 | 19,787.50 | ± | 797.22 | 17,699.33 | ± | 3085.14 | 17,879.83 | ± | 1655.03* | 16,650.00 | ± | 272.53** |
| TNF-α (pg/mL) | 342.50 | ± | 104.26 | 6740.63 | ± | 324.74 | 6420.00 | ± | 268.06 | 5346.00 | ± | 230.36** | 5745.50 | ± | 621.53* | 4817.83 | ± | 606.39** |
| G-CSF (pg/mL) | 521.00 | ± | 83.72 | 25,543.00 | ± | 238.10 | 24,842.50 | ± | 357.68* | 24,842.83 | ± | 323.51* | 24,810.50 | ± | 347.73* | 24,253.50 | ± | 248.47** |
| GM-CSF (pg/mL) | 51.67 | ± | 5.03 | 9191.88 | ± | 961.95 | 6978.33 | ± | 597.92* | 5821.33 | ± | 1745.5* | 7315.83 | ± | 712.23* | 5202.25 | ± | 1347** |
| M-CSF (pg/mL) | 38.67 | ± | 4.04 | 64.25 | ± | 0.87 | 59.88 | ± | 2.39* | 52.75 | ± | 2.22*** | 52.50 | ± | 1.78*** | 50.88 | ± | 10.02* |
| VEGF (pg/mL) | 282.67 | ± | 39.03 | 2258.50 | ± | 181.85 | 1641.00 | ± | 146.21** | 1567.00 | ± | 575.31 | 1324.67 | ± | 127.45** | 1329.33 | ± | 176.38** |
| IP-10 (pg/mL) | 925.67 | ± | 235.72 | 9146.33 | ± | 257.94 | 8075.17 | ± | 1031.92 | 7064.33 | ± | 816.24* | 6314.50 | ± | 1103.26** | 5472.33 | ± | 270.81** |
| LIF (pg/mL) | 48.00 | ± | 3.04 | 8874.63 | ± | 321.15 | 8004.38 | ± | 979.19 | 6841.63 | ± | 1219.48* | 6759.38 | ± | 790.78** | 5881.25 | ± | 354.84*** |
| LIX (pg/mL) | 227.00 | ± | 14.93 | 9632.00 | ± | 556.22 | 8041.67 | ± | 656.98* | 7785.50 | ± | 1082.63* | 7891.83 | ± | 365.63* | 7194.67 | ± | 642.9** |
| MCP-1 (pg/mL) | 265.33 | ± | 36.83 | 6632.75 | ± | 542.50 | 5690.33 | ± | 1434.00 | 4457.67 | ± | 1597.81* | 3903.75 | ± | 1278.68** | 3420.13 | ± | 313.43*** |
| MIP-1α (pg/mL) | 20,198.33 | ± | 733.50 | 26,577.30 | ± | 201.23 | 25,957.67 | ± | 465.5* | 25,611.50 | ± | 189.76** | 25,562.83 | ± | 443.83* | 24,959.83 | ± | 121.64*** |
| MIP-1β (pg/mL) | 17,552.50 | ± | 520.21 | 24,111.80 | ± | 145.97 | 23,903.67 | ± | 122.45 | 23,671.67 | ± | 75.18* | 23,840.00 | ± | 139.53* | 23,215.33 | ± | 553.15* |
| MIP-2 (pg/mL) | 120.67 | ± | 24.17 | 24,363.00 | ± | 89.64 | 23,902.00 | ± | 212.08* | 23,343.50 | ± | 265.26** | 23,749.50 | ± | 360.7* | 23,358.83 | ± | 576.98* |
| RANTES (pg/mL) | 76.00 | ± | 10.39 | 11,624.71 | ± | 674.17 | 11,397.50 | ± | 1156.28 | 10,413.83 | ± | 763.51* | 9724.33 | ± | 30.09** | 9703.50 | ± | 2096.06* |
| Chop mRNA (ratio) | 1.24 | ± | 0.80 | 17.83 | ± | 6.00 | 4.98 | ± | 1.18** | 4.69 | ± | 1.19** | 4.63 | ± | 1.36** | 4.57 | ± | 0.34** |
| Camk2a mRNA (ratio) | 1.51 | ± | 1.28 | 17.03 | ± | 6.96 | 6.32 | ± | 0.38* | 3.32 | ± | 0.12** | 6.06 | ± | 5.32* | 3.83 | ± | 0.2* |
| Stat-1 mRNA (ratio) | 1.08 | ± | 0.43 | 5.90 | ± | 2.12 | 1.28 | ± | 0.31*** | 0.86 | ± | 0.1*** | 0.81 | ± | 0.25*** | 1.49 | ± | 0.21*** |
| Stat-3 mRNA (ratio) | 1.38 | ± | 0.98 | 3.18 | ± | 1.45 | 1.63 | ± | 0.85* | 1.37 | ± | 0.52* | 1.34 | ± | 0.43* | 1.30 | ± | 0.36* |
| Jak2 mRNA (ratio) | 1.13 | ± | 0.73 | 3.99 | ± | 1.03 | 1.21 | ± | 0.08** | 1.20 | ± | 0.33*** | 1.19 | ± | 0.29** | 0.89 | ± | 0.17** |
| Fas mRNA (ratio) | 1.09 | ± | 0.59 | 366.74 | ± | 68.43 | 103.31 | ± | 2.7*** | 93.07 | ± | 9.9** | 86.25 | ± | 5.84*** | 79.15 | ± | 9.41*** |
| c-Jun mRNA (ratio) | 1.14 | ± | 0.53 | 15.22 | ± | 1.60 | 9.42 | ± | 1.75* | 6.65 | ± | 1.33* | 8.97 | ± | 3.09* | 6.18 | ± | 0.77* |
| c-Fos mRNA (ratio) | 1.16 | ± | 0.61 | 18.40 | ± | 4.62 | 8.33 | ± | 1.17* | 5.59 | ± | 0.67* | 8.02 | ± | 0.36* | 5.32 | ± | 1.07* |
| Nos2 mRNA (ratio) | 1.38 | ± | 1.78 | 3354.89 | ± | 2239.32 | 920.95 | ± | 687.25* | 884.32 | ± | 806.5* | 1046.78 | ± | 244.75* | 262.17 | ± | 123.65* |
| Ptgs2 mRNA (ratio) | 2.05 | ± | 2.56 | 23,427.87 | ± | 14,650.27 | 1886.16 | ± | 49.8* | 1260.22 | ± | 33.95* | 1361.26 | ± | 202.44* | 2966.61 | ± | 268.69* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-H.; Kim, D.-H.; Park, W. Conioselinum tenuissimum Root Extract Modulates Macrophage Activation via the Calcium–STAT3 Pathway. Processes 2022, 10, 2238. https://doi.org/10.3390/pr10112238
Kim T-H, Kim D-H, Park W. Conioselinum tenuissimum Root Extract Modulates Macrophage Activation via the Calcium–STAT3 Pathway. Processes. 2022; 10(11):2238. https://doi.org/10.3390/pr10112238
Chicago/Turabian StyleKim, Tae-Hun, Do-Hoon Kim, and Wansu Park. 2022. "Conioselinum tenuissimum Root Extract Modulates Macrophage Activation via the Calcium–STAT3 Pathway" Processes 10, no. 11: 2238. https://doi.org/10.3390/pr10112238
APA StyleKim, T. -H., Kim, D. -H., & Park, W. (2022). Conioselinum tenuissimum Root Extract Modulates Macrophage Activation via the Calcium–STAT3 Pathway. Processes, 10(11), 2238. https://doi.org/10.3390/pr10112238