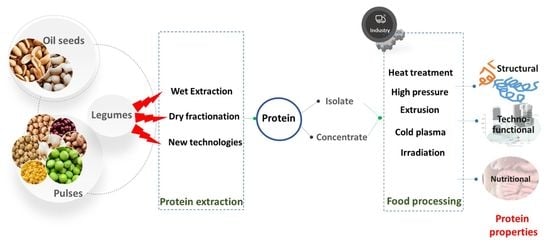
Legume Protein Extracts: The Relevance of Physical Processing in the Context of Structural, Techno-Functional and Nutritional Aspects of Food Development
Abstract
:1. Introduction
2. Legume Proteins
2.1. Classification and Composition
2.2. Protein Quality of Legumes
2.3. Extraction Techniques
3. Processing Affects Structural and Functional Properties of Proteins
3.1. Effect of Thermal Processing
3.1.1. Effect of Heat Treatment
3.1.2. The Effects of Extrusion Treatment
3.2. Effect of Non-Thermal Technologies
3.2.1. Effect of High-Pressure Treatment
3.2.2. Cold Plasma
3.2.3. Irradiation
4. Effect of Extraction and Processing on the Nutritional Characteristics
4.1. Effect of Extraction
4.2. Effect of Processing
4.2.1. Effect of Thermal Processing
Heat Treatment
Extrusion Treatment
4.2.2. Effect of Non-Thermal Processing
The Effect of High Hydrostatic Pressure Processing
Cold Plasma
Irradiation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Available online: https://www.fao.org/news/story/en/item/35571/icode/ (accessed on 14 September 2022).
- Anzani, C.; Boukid, F.; Drummond, L.; Mullen, A.M.; Álvarez, C. Optimising the use of proteins from rich meat co-products and non-meat alternatives: Nutritional, technological and allergenicity challenges. Food Res. Int. 2020, 137, 109575. [Google Scholar] [CrossRef] [PubMed]
- Ingerslev, A.K.; Rasmussen, L.; Zhou, P.; Nørgaard, J.V.; Theil, P.K.; Jensen, S.K.; Lærke, H.N. Effects of dairy and plant protein on growth and growth biomarkers in a piglet model. Food Funct. 2021, 12, 11625–11640. [Google Scholar] [CrossRef] [PubMed]
- Kurpad, A.V. Protein: Quality and Sources. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; pp. 123–130. [Google Scholar]
- Zhang, C.; Guan, X.; Yu, S.; Zhou, J.; Chen, J. Production of meat alternatives using live cells, cultures and plant proteins. Curr. Opin. Food Sci. 2022, 43, 43–52. [Google Scholar] [CrossRef]
- Onwezen, M.C.; Bouwman, E.P.; Reinders, M.J.; Dagevos, H. A systematic review on consumer acceptance of alternative proteins: Pulses, algae, insects, plant-based meat alternatives, and cultured meat. Appetite 2021, 159, 105058. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, J.; Soret, S. Sustainability of plant-based diets: Back to the future. Am. J. Clin. Nutr. 2014, 100, 476S–482S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duranti, M. Grain legume proteins and nutraceutical properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef]
- Sim, S.Y.J.; SRV, A.; Chiang, J.H.; Henry, C.J. Plant Proteins for Future Foods: A Roadmap. Foods 2021, 10, 1967. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef]
- Gutiérrez-Uribe, J.A.; Guajardo-Flores, D.; López-Barrios, L. Legumes in the Diet. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P.M., Toldra, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 539–543. [Google Scholar]
- Hasanuzzaman, M. Legume Crops: Prospects; BoD—Books on Demand: London, UK, 2020; p. 182. [Google Scholar]
- Simpson, M.G. 8—Diversity and Classification of Flowering Plants: Eudicots. In Plant Systematics, 2nd ed.; Simpson, M.G., Ed.; Elsevier Inc.: New York, NY, USA, 2010; pp. 275–448. [Google Scholar]
- Singhee, D. Review on Natural Dyes for Textiles from Wastes. In Chemistry and Technology of Natural and Synthetic Dyes and Pigments; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Semba, R.D.; Ramsing, R.; Rahman, N.; Kraemer, K.; Bloem, M.W. Legumes as a sustainable source of protein in human diets. Glob. Food Secur. 2021, 28, 100520. [Google Scholar] [CrossRef]
- Kelemu, S.; Cardona, C.; Segura, G. Antimicrobial and insecticidal protein isolated from seeds of Clitoria ternatea, a tropical forage legume. Plant Physiol. Biochem. 2004, 42, 867–873. [Google Scholar] [CrossRef]
- Somers, D.A.; Samac, D.A.; Olhoft, P.M. Recent Advances in Legume Transformation. Plant Physiol. 2003, 131, 892–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Research Council. Tropical Legumes: Resources for the Future; The Minerva Group, Ed.; The Minerva Group: New York, NY, USA; Hong Kong, China, 2002; p. 340. [Google Scholar]
- Gresshoff, P.M.; Hayashi, S.; Biswas, B.; Mirzaei, S.; Indrasumunar, A.; Reid, D.; Samuel, S.; Tollenaere, A.; van Hameren, B.; Hastwell, A.; et al. The value of biodiversity in legume symbiotic nitrogen fixation and nodulation for biofuel and food production. J. Plant Physiol. 2015, 172, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Food processing for the improvement of plant proteins digestibility. Crit. Rev. Food Sci. Nutr. 2020, 60, 3367–3386. [Google Scholar] [CrossRef] [PubMed]
- Sarwar Gilani, G.; Wu Xiao, C.; Cockell, K.A. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef] [Green Version]
- Falade, K.O.; Akeem, S.A. Physicochemical properties, protein digestibility and thermal stability of processed African mesquite bean (Prosopis africana) flours and protein isolates. Food Meas. 2020, 14, 1481–1496. [Google Scholar] [CrossRef]
- Drulyte, D.; Orlien, V. The Effect of Processing on Digestion of Legume Proteins. Foods 2019, 8, 224. [Google Scholar] [CrossRef] [Green Version]
- Rubio, L.A.; Clemente, A. In vivo (rat) and in vitro (Caco-2 cells) absorption of amino acids from legume protein isolates as compared to lactalbumin or casein. Arch. Anim. Nutr. 2009, 63, 413–426. [Google Scholar] [CrossRef]
- Arntfield, S.D.; Maskus, H.D. 9—Peas and other legume proteins. In Handbook of Food Proteins? 1st ed.; Phillips, G.O., Williams, P.A., Eds.; Elsevier Ltd.: London, UK, 2011; pp. 233–266. [Google Scholar]
- Abbas, Y.; Ahmad, A. Impact of processing on nutritional and antinutritional factors of legumes: A review. Desalination 2018, 2, 199–215. [Google Scholar]
- Patterson, C.A.; Curran, J.; Der, T. Effect of Processing on Antinutrient Compounds in Pulses. Cereal Chem. 2017, 94, 2–10. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Gowen, A.; McKenna, B. Pulse Foods: Processing, Quality and Nutraceutical Applications, 2nd ed.; Academic Press: Cambridge, MA, USA, 2020; p. 536. [Google Scholar]
- Zhu, H.; Tang, H.; Cheng, Y.; Li, Z.; Tong, L. Electrostatic separation technology for obtaining plant protein concentrates: A review. Trends Food Sci. Technol. 2021, 113, 66–76. [Google Scholar] [CrossRef]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Karthikeyan, S.; Saari, N. Effects of drying techniques on the physicochemical, functional, thermal, structural and rheological properties of mung bean (Vigna radiata) protein isolate powder. Food Res. Int. 2020, 138, 109783. [Google Scholar] [CrossRef] [PubMed]
- Cruz Solorio, A.; Garín Aguilar, M.; Valencia del Toro, G. Nutritional and functional properties of protein concentrate and protein isolates of foods. In Science within Food: Up-to-Date Advances on Research and Educational Ideas, 1st ed.; Méndez-Vilas, A., Ed.; FORMATEX: Badajos, Spain, 2020; p. 176. [Google Scholar]
- Schefer, S.; Oest, M.; Rohn, S. Interactions between Phenolic Acids, Proteins, and Carbohydrates—Influence on Dough and Bread Properties. Foods 2021, 10, 2798. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, K.; Thompson, R.; Burstin, J. Reserve accumulation in legume seeds. Comptes Rendus Biol. 2008, 331, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes. Rev. 2014, 15, 392–407. [Google Scholar] [CrossRef]
- Mani-López, E.; Palou, E.; López-Malo, A. Legume proteins, peptides, water extracts, and crude protein extracts as antifungals for food applications. Trends Food Sci. Technol. 2021, 112, 16–24. [Google Scholar] [CrossRef]
- Allen, L.H. Legumes. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Elsevier Ltd.: London, UK, 2013; pp. 74–79. [Google Scholar]
- Fujiwara, T.; Nambara, E.; Yamagishi, K.; Goto, D.B.; Naito, S. Storage Proteins. Arab. Book 2002, 1, e0020. [Google Scholar] [CrossRef] [Green Version]
- Belitz, H.; Grosch, W.; Schieberle, P. Food Chemistry, 3rd ed.; Translation from the Fifth German Edition; Springer: Berlin/Heidelberg, Germany, 2004; p. 1070. [Google Scholar]
- Alting, A.C.; van de Velde, F. 9—Proteins as clean label ingredients in foods and beverages. In Natural Food Additives, Ingredients and Flavourings, 1st ed.; Baines, D., Seal, R., Eds.; Elsevier Ltd.: London, UK, 2012; pp. 197–211. [Google Scholar]
- Goyal, A.K. (Ed.) Grain Legumes; BoD Books on Demand: Zagreb, Croatia, 2016; p. 188. [Google Scholar]
- Moreno-Valdespino, C.A.; Luna-Vital, D.; Camacho-Ruiz, R.M.; Mojica, L. Bioactive proteins and phytochemicals from legumes: Mechanisms of action preventing obesity and type-2 diabetes. Food Res. Int. 2020, 130, 108905. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Vitale, A.; Bollini, R. Legume storage Proteins. In Seed Development and Germination, 2nd ed.; Kigel, J., Ed.; Routledge: New York, NY, USA, 2017; pp. 73–95. [Google Scholar]
- Stone, A.K.; Nosworthy, M.G.; Chiremba, C.; House, J.D.; Nickerson, M.T. A comparative study of the functionality and protein quality of a variety of legume and cereal flours. Cereal Chem. 2019, 96, 1159–1169. [Google Scholar] [CrossRef]
- Sashikala, V.B.; Sreerama, Y.N.; Pratape, V.M.; Narasimha, H.V. Effect of thermal processing on protein solubility of green gram (Phaseolus aureus) legume cultivars. J. Food Sci. Technol. 2013, 52, 1552–1560. [Google Scholar] [CrossRef] [Green Version]
- Alonso, R.; Grant, G.; Dewey, P.; Marzo, F. Nutritional Assessment in Vitro and in Vivo of Raw and Extruded Peas (Pisum s ativum L.). J. Agric. Food Chem. 2000, 48, 2286–2290. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, C.W. Encyclopedia of Food Grains, 2nd ed.; Elsevier Science: San Diego, CA, USA, 2015; p. 1976. [Google Scholar]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- Diedericks, C.F.; Venema, P.; Mubaiwa, J.; Jideani, V.A.; van der Linden, E. Effect of processing on the microstructure and composition of Bambara groundnut (Vigna subterranea (L.) Verdc.) seeds, flour and protein isolates. Food Hydrocoll. 2020, 108, 106031. [Google Scholar] [CrossRef]
- Kigel, J. Seed Development and Germination, 1st ed.; Routledge: New York, NY, USA, 2017; p. 872. [Google Scholar]
- Keskin, S.O.; Ali, T.M.; Ahmed, J.; Shaikh, M.; Siddiq, M.; Uebersax, M.A. Physico-chemical and functional properties of legume protein, starch, and dietary fiber—A review. Legume Sci. 2022, 4, 117. [Google Scholar] [CrossRef]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: https://www.statpearls.com/ArticleLibrary/viewarticle/36202 (accessed on 14 September 2022).
- Baptista, A.; Pinho, O.; Pinto, E.; Casal, S.; Mota, C.; Ferreira, I.M. Characterization of protein and fat composition of seeds from common beans (Phaseolus vulgaris L.), cowpea (Vigna unguiculata L. Walp) and bambara groundnuts (Vigna subterranea L. Verdc) from Mozambique. Food Meas. 2016, 11, 442–450. [Google Scholar] [CrossRef]
- Margier, M.; Georgé, S.; Hafnaoui, N.; Remond, D.; Nowicki, M.; Du Chaffaut, L.; Amiot, M.; Reboul, E. Nutritional Composition and Bioactive Content of Legumes: Characterization of Pulses Frequently Consumed in France and Effect of the Cooking Method. Nutrients 2018, 10, 1668. [Google Scholar] [CrossRef] [Green Version]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; p. I. [Google Scholar]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Sayyar Khan, M. Nutritional quality of important food legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- Yao, D.N.; Kouassi, K.N.; Erba, D.; Scazzina, F.; Pellegrini, N.; Casiraghi, M.C. Nutritive Evaluation of the Bambara Groundnut Ci12 Landrace [Vigna subterranea (L.) Verdc. (Fabaceae)] Produced in Côte d’Ivoire. Int. J. Mol. Sci. 2015, 16, 21428–21441. [Google Scholar] [CrossRef] [Green Version]
- Khattab, R.Y.; Arntfield, S.D.; Nyachoti, C.M. Nutritional quality of legume seeds as affected by some physical treatments, Part 1: Protein quality evaluation. Food Sci. Technol. 2009, 42, 1107–1112. [Google Scholar] [CrossRef]
- Jahreis, G.; Brese, M.; Leiterer, M.; Schäfer, U.; Böhm, V. Jena Legume flours: Nutritionally important sources of protein and dietary fiber. Sci. Res. 2015, 63, 36–42. [Google Scholar]
- Liu, B.; Peng, J.; Zhang, S.; Zou, B.; Zhong, G. Chemical composition, in vitro starch digestibility and amino acid analysis of a underexplored kidney bean (Phaseolus vulgaris L.) grown in Chongqing, China. Int. J. Food Sci. Technol. 2013, 48, 527–532. [Google Scholar] [CrossRef]
- Fouad, A.A.; Rehab, F.M.A. Effect of germination time on proximate analysis, bioactive compounds and antioxidant activity of lentil (Lens culinaris Medik.) Sprouts. Acta Sci. Polonorum. Technol. Aliment. 2015, 14, 233–246. [Google Scholar] [CrossRef]
- Aremu, M.O.; Stephen Audu, S.; Lyambee Gav, B. Comparative Review of Crude Protein and Amino Acids of Leguminous Seeds Grown in Nigeria. Int. J. Sci. 2017, 3, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Arowora, K.A.; Popoola, C.A.; Yakubu, O.E.; Ugwuoke, K.C.; Aliyu, F. Proximate Composition and Amino Acids Contents in Selected Legumes and Hura crepitans seed in Wukari Taraba State. Covenant J. Phys. Life Sci. 2020, 8, 2354–3574. [Google Scholar] [CrossRef]
- Palander, S.; Laurinen, P.; Perttilä, S.; Valaja, J.; Partanen, K. Protein and amino acid digestibility and metabolizable energy value of pea (Pisum sativum), faba bean (Vicia faba) and lupin (Lupinus angustifolius) seeds for turkeys of different age. Anim. Feed Sci. Technol. 2006, 127, 89–100. [Google Scholar] [CrossRef]
- Lásztity, R.; Khalil, M.M.; Haraszi, R.; Baticz, O.; Tömösközi, S. Isolation, functional properties and potential use of protein preparations from lupin. Food Nahr. 2001, 45, 396–398. [Google Scholar] [CrossRef]
- Martineau-Côté, D.; Achouri, A.; Karboune, S.; L’Hocine, L. Faba Bean: An Untapped Source of Quality Plant Proteins and Bioactives. Nutrients 2022, 14, 1541. [Google Scholar] [CrossRef]
- Ghumman, A.; Kaur, A.; Singh, N. Functionality and digestibility of albumins and globulins from lentil and horse gram and their effect on starch rheology. Food Hydrocoll. 2016, 61, 843–850. [Google Scholar] [CrossRef]
- Santos-Hernández, M.; Alfieri, F.; Gallo, V.; Miralles, B.; Masi, P.; Romano, A.; Ferranti, P.; Recio, I. Compared digestibility of plant protein isolates by using the INFOGEST digestion protocol. Food Res. Int. 2020, 137, 109708. [Google Scholar] [CrossRef]
- Araüjo, A.H.; Cardoso, P.C.B.; Pereira, R.A.; Lima, L.M.; Oliveira, A.S.; Miranda, M.R.A.; Xavier-Filho, J.; Sales, M.P. In vitro digestibility of globulins from cowpea (Vigna unguiculata) and xerophitic algaroba (Prosopis juliflora) seeds by mammalian digestive proteinases: A comparative study. Food Chem. 2002, 78, 143–147. [Google Scholar] [CrossRef]
- Teka, T.A.; Retta, N.; Bultosa, G.; Admassu, H.; Astatkie, T. Protein fractions, in vitro protein digestibility and amino acid composition of select cowpea varieties grown in Ethiopia. Food Biosci. 2020, 36, 100634. [Google Scholar] [CrossRef]
- Singhal, A.; Karaca, A.C.; Tyler, R.; Nickerson, M. Pulse Proteins: From Processing to Structure-Function Relationships; IntechOpen: Rijeka, Croatia, 2016; p. 188. [Google Scholar]
- Prandi, B.; Zurlini, C.; Maria, C.I.; Cutroneo, S.; Di Massimo, M.; Bondi, M.; Brutti, A.; Sforza, S.; Tedeschi, T. Targeting the Nutritional Value of Proteins from Legumes By-Products Through Mild Extraction Technologies. Front. Nutr. 2021, 8, 695793. [Google Scholar] [CrossRef] [PubMed]
- Berghout, J.A.M.; Pelgrom, P.J.M.; Schutyser, M.A.I.; Boom, R.M.; van der Goot, A.J. Sustainability assessment of oilseed fractionation processes: A case study on lupin seeds. J. Food Eng. 2015, 150, 117–124. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Berghout, J.A.M.; van der Goot, A.J.; Boom, R.M.; Schutyser, M.A.I. Preparation of functional lupine protein fractions by dry separation. Food Sci. Technol. 2014, 59, 680–688. [Google Scholar] [CrossRef]
- Schutyser, M.A.I.; Pelgrom, P.J.M.; van der Goot, A.J.; Boom, R.M. Dry fractionation for sustainable production of functional legume protein concentrates. Trends Food Sci. Technol. 2015, 45, 327–335. [Google Scholar] [CrossRef]
- Czuchajowska, Z. Opportunities for pulses in food and non-food industrial processes by fractionation. In Linking Research and Marketing Opportunities for Pulses in the 21st Century: Proceedings of the Third International Food Legumes Research Conference, 3rd ed.; Springer Science & Business Media, Ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; p. 707. [Google Scholar]
- Do, D.T.; Singh, J. Legume Microstructure. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 15–21. [Google Scholar]
- Schutyser, M.A.I.; van der Goot, A.J. The potential of dry fractionation processes for sustainable plant protein production. Trends Food Sci. Technol. 2011, 22, 154–164. [Google Scholar] [CrossRef]
- Tabtabaei, S.; Konakbayeva, D.; Rajabzadeh, A.R.; Legge, R.L. Functional properties of navy bean (Phaseolus vulgaris) protein concentrates obtained by pneumatic tribo-electrostatic separation. Food Chem. 2019, 283, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Bayram, M.; Alameen, A. Protein Extraction Techniques from Cereals and Legumes. In Proceedings of the International Gap Agriculture and Livestock Congress, Sanliurfa, Turkey, 25–27 April 2018; pp. 948–953. [Google Scholar]
- Pelgrom, P.J.; Vissers, A.M.; Boom, R.M.; Schutyser, M.A. Dry fractionation for production of functional pea protein concentrates. Food Res. Int. 2013, 53, 232–239. [Google Scholar] [CrossRef]
- Assatory, A.; Vitelli, M.; Rajabzadeh, A.R.; Legge, R.L. Dry fractionation methods for plant protein, starch and fiber enrichment: A review. Trends Food Sci. Technol. 2019, 86, 340–351. [Google Scholar] [CrossRef]
- Jiang, L.; Hua, D.; Wang, Z.; Xu, S. Aqueous enzymatic extraction of peanut oil and protein hydrolysates. Food Bioprod. Process. 2010, 88, 233–238. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Krijgsman, A.J.; Fryer, P.J.; Zuidam, N.J. Intensification of protein extraction from soybean processing materials using hydrodynamic cavitation. Innov. Food Sci. Emerg. Technol. 2017, 41, 47–55. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Krijgsman, A.; Fryer, P.J.; Zuidam, N.J. Intensified soy protein extraction by ultrasound. Chem. Eng. Process. 2017, 113, 94–101. [Google Scholar] [CrossRef]
- Lu, W.; Chen, X.; Wang, J.; Yang, X.; Qi, J. Enzyme-assisted subcritical water extraction and characterization of soy protein from heat-denatured meal. J. Food Eng. 2016, 169, 250–258. [Google Scholar] [CrossRef]
- Tanger, C.; Engel, J.; Kulozik, U. Influence of extraction conditions on the conformational alteration of pea protein extracted from pea flour. Food Hydrocoll. 2020, 107, 105949. [Google Scholar] [CrossRef]
- Klupšaitė, D.; Juodeikienė, G. Legume: Composition, protein extraction and functional properties. A review. Chem. Technol. 2015, 66, 5–12. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Boom, R.M.; Schutyser, M.A.I. Method Development to Increase Protein Enrichment During Dry Fractionation of Starch-Rich Legumes. Food Bioprocess Technol. 2015, 8, 1495–1502. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, D.S.; Linnemann, A.R.; van Boekel, T.A.J.S. Towards Sustainable Production of Protein-Rich Foods: Appraisal of Eight Crops for Western Europe. PART II: Analysis of the Technological Aspects of the Production Chain. Crit. Rev. Food Sci. Nutr. 2003, 43, 481–506. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, M.M.N.; Butt, F.M.; Anjum, H. Nawaz composition analysis of some selected legumes for protein isolates recovery. J. Anim. Plant Sci. 2012, 22, 1156–1162. [Google Scholar]
- Pelgrom, P.J.M.; Wang, J.; Boom, R.M.; Schutyser, M.A.I. Pre- and post-treatment enhance the protein enrichment from milling and air classification of legumes. J. Food Eng. 2015, 155, 53–61. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Khazaei, H.; Subedi, M.; Nickerson, M.; Martínez-Villaluenga, C.; Frias, J.; Vandenberg, A. Seed Protein of Lentils: Current Status, Progress, and Food Applications. Foods 2019, 8, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, K.C.; Bento, J.A.C.; Caliari, M.; Bassinello, P.Z.; Berrios, J.D.J. Dry bean proteins: Extraction methods, functionality, and application in products for human consumption. Cereal Chem. 2022, 99, 67–77. [Google Scholar] [CrossRef]
- Sandberg, A. Developing Functional Ingredients: A Case Study of Pea Protein. In Functional Foods—Concept to Product, 2nd ed.; Woodhead Publishing: Sawston, UK, 2011; p. 1. [Google Scholar]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; de Wit, M.; Boom, R.M.; Schutyser, M.A.I. Lupine protein enrichment by milling and electrostatic separation. Innov. Food Sci. Emerg. Technol. 2016, 33, 596–602. [Google Scholar] [CrossRef]
- Xing, Q.; de Wit, M.; Kyriakopoulou, K.; Boom, R.M.; Schutyser, M.A.I. Protein enrichment of defatted soybean flour by fine milling and electrostatic separation. Innov. Food Sci. Emerg. Technol. 2018, 50, 42–49. [Google Scholar] [CrossRef]
- Allotey, D.K.; Kwofie, E.M.; Adewale, P.; Lam, E.; Ngadi, M. A meta-analysis of pulse-protein extraction technologies: Impact on recovery and purity. J. Food Eng. 2022, 327, 111048. [Google Scholar] [CrossRef]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef]
- Xing, Q.; Utami, D.P.; Demattey, M.B.; Kyriakopoulou, K.; de Wit, M.; Boom, R.M.; Schutyser, M.A.I. A two-step air classification and electrostatic separation process for protein enrichment of starch-containing legumes. Innov. Food Sci. Emerg. Technol. 2020, 66, 102480. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Boom, R.M.; Schutyser, M.A.I. Functional analysis of mildly refined fractions from yellow pea. Food Hydrocoll. 2015, 44, 12–22. [Google Scholar] [CrossRef]
- Kamal, H.; Le, C.F.; Salter, A.M.; Ali, A. Extraction of protein from food waste: An overview of current status and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2455–2475. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Xu, L.; Ma, H. An efficient ultrasound-assisted extraction method of pea protein and its effect on protein functional properties and biological activities. LWT Food Sci. Technol. 2020, 127, 109348. [Google Scholar] [CrossRef]
- Fukase, H.; Ohdaira, E.; Masuzawa, N.; Ide, M. Effect of Ultrasound in Soybean Protein Extraction. Jpn. J. Appl. Phys. 1994, 33, 3088–3090. [Google Scholar] [CrossRef]
- Aguilar-Acosta, L.A.; Serna-Saldivar, S.O.; Rodríguez-Rodríguez, J.; Escalante-Aburto, A.; Chuck-Hernández, C. Effect of Ultrasound Application on Protein Yield and Fate of Alkaloids during Lupin Alkaline Extraction Process. Biomolecules 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Peyrano, F.; Speroni, F.; Avanza, M.V. Physicochemical and functional properties of cowpea protein isolates treated with temperature or high hydrostatic pressure. Innov. Food Sci. Emerg. Technol. 2016, 33, 38–46. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, J.; Ge, G.; Zhao, M.; Sun, W. Impact of heating treatments on physical stability and lipid-protein co-oxidation in oil-in-water emulsion prepared with soy protein isolates. Food Hydrocoll. 2020, 100, 105167. [Google Scholar] [CrossRef]
- Tang, C.; Sun, X.; Yin, S. Physicochemical, functional and structural properties of vicilin-rich protein isolates from three Phaseolus legumes: Effect of heat treatment. Food Hydrocoll. 2009, 23, 1771–1778. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Jiang, L.; Qi, B.; Zhou, L. Relationship between Secondary Structure and Surface Hydrophobicity of Soybean Protein Isolate Subjected to Heat Treatment. J. Chem. 2014, 2014, 475389. [Google Scholar] [CrossRef] [Green Version]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Rashedi Ismail-Fitry, M.; Zarei, M.; Karthikeyan, S.; Caballero-Briones, F.; Saari, N. Structural and rheological changes of texturized mung bean protein induced by feed moisture during extrusion. Food Chem. 2021, 344, 128643. [Google Scholar] [CrossRef]
- Mozafarpour, R.; Koocheki, A.; Milani, E.; Varidi, M. Extruded soy protein as a novel emulsifier: Structure, interfacial activity and emulsifying property. Food Hydrocoll. 2019, 93, 361–373. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, X.; Cheng, Y.; Cui, B.; Abd El-Aty, A.M. Impact of high moisture contents on the structure and functional properties of pea protein isolate during extrusion. Food Hydrocoll. 2022, 127, 107508. [Google Scholar] [CrossRef]
- Beck, S.M.; Knoerzer, K.; Arcot, J. Effect of low moisture extrusion on a pea protein isolate’s expansion, solubility, molecular weight distribution and secondary structure as determined by Fourier Transform Infrared Spectroscopy (FTIR). J. Food Eng. 2017, 214, 166–174. [Google Scholar] [CrossRef]
- Yin, S.; Tang, C.; Wen, Q.; Yang, X.; Li, L. Functional properties and in vitro trypsin digestibility of red kidney bean (Phaseolus vulgaris L.) protein isolate: Effect of high-pressure treatment. Food Chem. 2008, 110, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Jung, S.; Aluko, R.E. Physicochemical and functional properties of high pressure-treated isolated pea protein. Innov. Food Sci. Emerg. Technol. 2018, 45, 179–185. [Google Scholar] [CrossRef]
- Ahmed, J.; Al-Ruwaih, N.; Mulla, M.; Rahman, M.H. Effect of high pressure treatment on functional, rheological and structural properties of kidney bean protein isolate. Food Sci. Technol. 2018, 91, 191–197. [Google Scholar] [CrossRef]
- Mehr, H.M.; Koocheki, A. Effect of atmospheric cold plasma on structure, interfacial and emulsifying properties of Grass pea (Lathyrus sativus L.) protein isolate. Food Hydrocoll. 2020, 106, 105899. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Zhang, J.; Nasiru, M.M.; Wang, Y.; Fu, L. Atmospheric cold plasma treatment of soybean protein isolate: Insights into the structural, physicochemical, and allergenic characteristics. J. Food Sci. 2021, 86, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Dong, S.; Han, F.; Li, Y.; Chen, G.; Li, L.; Chen, Y. Effects of Dielectric Barrier Discharge (DBD) Cold Plasma Treatment on Physicochemical and Functional Properties of Peanut Protein. Food Bioprocess Technol. 2017, 11, 344–354. [Google Scholar] [CrossRef]
- Mallikarjunan, N.; Marathe, S.; Rajalakshmi, D.; Mahesh, S.; Jamdar, S.N.; Sharma, A. Effect of ionizing radiation on structural and functional attributes of red kidney bean (Phaseolus vulgaris L.) lectin. Food Sci. Technol. 2014, 59, 300–307. [Google Scholar] [CrossRef]
- Vaz, A.F.M.; Souza, M.P.; Medeiros, P.L.; Melo, A.M.M.A.; Silva-Lucca, R.A.; Santana, L.A.; Oliva, M.L.V.; Perez, K.R.; Cuccovia, I.M.; Correia, M.T.S. Low-dose gamma irradiation of food protein increases its allergenicity in a chronic oral challenge. Food Chem. Toxicol. 2013, 51, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Ghribi, A.M.; Gafsi, I.M.; Blecker, C.; Danthine, S.; Attia, H.; Besbes, S. Effect of drying methods on physico-chemical and functional properties of chickpea protein concentrates. J. Food Eng. 2015, 165, 179–188. [Google Scholar] [CrossRef]
- Bußler, S.; Steins, V.; Ehlbeck, J.; Schlüter, O. Impact of thermal treatment versus cold atmospheric plasma processing on the techno-functional protein properties from Pisum sativum ‘Salamanca’. J. Food Eng. 2015, 167, 166–174. [Google Scholar] [CrossRef]
- Maurya, A.; Mhatre, S.; Kalha, R.; Dabade, A. Effect of gamma radiation treatment on physico-chemical and functional properties of soya proteins isolate. Ann. Food Sci. Technol. 2020, 21, 274–285. Available online: https://afst.valahia.ro/wp-content/uploads/2022/09/I.4_Maurya.pdf (accessed on 25 September 2022).
- Ngui, S.P.; Nyobe, C.E.; Bassogog, C.B.B.; Tang, E.N.; Minka, S.R.; Mune, M.A.M. Influence of pH and temperature on the physicochemical and functional properties of Bambara bean protein isolate. Heliyon 2021, 7, e07824. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mora, P.; Peñas, E.; Frias, J.; Gomez, R.; Martinez-Villaluenga, C. High-pressure improves enzymatic proteolysis and the release of peptides with angiotensin I converting enzyme inhibitory and antioxidant activities from lentil proteins. Food Chem. 2015, 171, 224–232. [Google Scholar] [CrossRef]
- Saricaoglu, F.T. Application of high-pressure homogenization (HPH) to modify functional, structural and rheological properties of lentil (Lens culinaris) proteins. Int. J. Biol. Macromol. 2020, 144, 760–769. [Google Scholar] [CrossRef]
- Guo, F.; Xiong, Y.L.; Qin, F.; Jian, H.; Huang, X.; Chen, J. Surface Properties of Heat-Induced Soluble Soy Protein Aggregates of Different Molecular Masses. J. Food Sci. 2015, 80, C279–C287. [Google Scholar] [CrossRef]
- Puppo, C.; Chapleau, N.; Speroni, F.; de Lamballerie-Anton, M.; Michel, F.; Añón, C.; Anton, M. Physicochemical Modifications of High-Pressure-Treated Soybean Protein Isolates. J. Agric. Food Chem. 2004, 52, 1564–1571. [Google Scholar] [CrossRef]
- Chao, D.; Aluko, R.E. Modification of the structural, emulsifying, and foaming properties of an isolated pea protein by thermal pretreatment. CYTA J. Food 2018, 16, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, X.; Liu, F.; Ding, Y.; Wang, R.; Luo, X.; Li, Y.; Chen, Z. Study of the functional properties and anti-oxidant activity of pea protein irradiated by electron beam. Innov. Food Sci. Emerg. Technol. 2017, 41, 124–129. [Google Scholar] [CrossRef]
- Peyrano, F.; de Lamballerie, M.; Avanza, M.V.; Speroni, F. Rheological characterization of the thermal gelation of cowpea protein isolates: Effect of pretreatments with high hydrostatic pressure or calcium addition. Food Sci. Technol. 2019, 115, 108472. [Google Scholar] [CrossRef]
- Sim, S.Y.J.; Karwe, M.V.; Moraru, C.I. High pressure structuring of pea protein concentrates. J. Food Process Eng. 2019, 42, e13261. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, W.; Feizollahi, E.; Roopesh, M.S.; Chen, L. Improvement of pea protein gelation at reduced temperature by atmospheric cold plasma and the gelling mechanism study. Innov. Food Sci. Emerg. Technol. 2021, 67, 102567. [Google Scholar] [CrossRef]
- Salazar-Villanea, S.; Hendriks, W.H.; Bruininx, E.M.A.M.; Gruppen, H.; van der Poel, A.F.B. Protein structural changes during processing of vegetable feed ingredients used in swine diets: Implications for nutritional value. Nutr. Res. Rev. 2016, 29, 126–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, M.; Adhikari, B.; Aldred, P.; Panozzo, J.; Kasapis, S. Physicochemical and functional properties of lentil protein isolates prepared by different drying methods. Food Chem. 2011, 129, 1513–1522. [Google Scholar] [CrossRef]
- Al-Ali, H.A.; Shah, U.; Hackett, M.J.; Gulzar, M.; Karakyriakos, E.; Johnson, S.K. Technological strategies to improve gelation properties of legume proteins with the focus on lupin. Innov. Food Sci. Emerg. Technol. 2021, 68, 102634. [Google Scholar] [CrossRef]
- Mession, J.; Chihi, M.L.; Sok, N.; Saurel, R. Effect of globular pea proteins fractionation on their heat-induced aggregation and acid cold-set gelation. Food Hydrocoll. 2015, 46, 233–243. [Google Scholar] [CrossRef]
- Dalbhagat, C.G.; Mahato, D.K.; Mishra, H.N. Effect of extrusion processing on physicochemical, functional and nutritional characteristics of rice and rice-based products: A review. Trends Food Sci. Technol. 2019, 85, 226–240. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B. Chemical cross-linking and molecular aggregation of soybean protein during extrusion cooking at low and high moisture content. Food Sci. Technol. 2011, 44, 957–962. [Google Scholar] [CrossRef]
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional aspects of food extrusion: A review. Int. J. Food Sci. Technol. 2007, 42, 916–929. [Google Scholar] [CrossRef]
- Ojokoh, A.O.; Yimin, W. Effect of Fermentation on Chemical Composition and Nutritional Quality of Extruded and Fermented Soya Products. Int. J. Food Eng. 2011, 7, 1857. [Google Scholar] [CrossRef]
- Alam, M.S.; Kaur, J.; Khaira, H.; Gupta, K. Extrusion and Extruded Products: Changes in Quality Attributes as Affected by Extrusion Process Parameters: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 445–473. [Google Scholar] [CrossRef]
- Samard, S.; Gu, B.; Ryu, G.-H. Effects of extrusion types, screw speed and addition of wheat gluten on physicochemical characteristics and cooking stability of meat analogues. J. Sci. Food Agric. 2019, 99, 4922–4931. [Google Scholar] [CrossRef] [PubMed]
- Philipp, C.; Oey, I.; Silcock, P.; Beck, S.M.; Buckow, R. Impact of protein content on physical and microstructural properties of extruded rice starch-pea protein snacks. J. Food Eng. 2017, 212, 165–173. [Google Scholar] [CrossRef]
- Phongthai, S.; D’Amico, S.; Schoenlechner, R.; Homthawornchoo, W.; Rawdkuen, S. Effects of protein enrichment on the properties of rice flour based gluten-free pasta. Food Sci. Technol. 2017, 80, 378–385. [Google Scholar] [CrossRef]
- Yu, L.; Ramaswamy, H.S.; Boye, J. Protein rich extruded products prepared from soy protein isolate-corn flour blends. Food Sci. Technol. 2013, 50, 279–289. [Google Scholar] [CrossRef]
- Bueno, A.S.; Pereira, C.M.; Menegassi, B.; Arêas, J.A.G.; Castro, I.A. Effect of extrusion on the emulsifying properties of soybean proteins and pectin mixtures modelled by response surface methodology. J. Food Eng. 2009, 90, 504–510. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Meng, S.; Wang, Q. Rheological properties of pea protein isolate-amylose/amylopectin mixtures and the application in the high-moisture extruded meat substitutes. Food Hydrocoll. 2021, 117, 106732. [Google Scholar] [CrossRef]
- Carmo, C.S.D.; Knutsen, S.H.; Malizia, G.; Dessev, T.; Geny, A.; Zobel, H.; Myhrer, K.S.; Varela, P.; Sahlstrøm, S. Meat analogues from a faba bean concentrate can be generated by high moisture extrusion. Future Foods A Dedic. J. Sustain. Food Sci. 2021, 3, 100014. [Google Scholar] [CrossRef]
- Silva, A.C.C.; Arêas, E.P.G.; Silva, M.A.; Arêas, J.A.G. Effects of Extrusion on the Emulsifying Properties of Rumen and Soy Protein. Food Biophys. 2010, 5, 94–102. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Eisner, P.; Schweiggert-Weisz, U. Effect of high moisture extrusion cooking on protein-protein interactions of pea (Pisum sativum L.) protein isolates. Int. J. Food Sci. Technol. 2015, 50, 1390–1396. [Google Scholar] [CrossRef]
- Samard, S.; Ryu, G. Physicochemical and functional characteristics of plant protein-based meat analogs. J. Food Process. Preserv. 2019, 43, e14123. [Google Scholar] [CrossRef]
- Ma, K.K.; Greis, M.; Lu, J.; Nolden, A.A.; Mcclements, D.J.; Kinchla, A.J. Functional Performance of Plant Proteins. Foods 2022, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.R.; Sorgentini, D.A.; Añón, M.C. Relation between Solubility and Surface Hydrophobicity as an Indicator of Modifications during Preparation Processes of Commercial and Laboratory-Prepared Soy Protein Isolates. J. Agric. Food Chem. 2000, 48, 3159–3165. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B. Effects of high hydrostatic pressure on the quality and functionality of protein isolates, concentrates, and hydrolysates derived from pulse legumes: A review. Trends Food Sci. Technol. 2021, 107, 466–479. [Google Scholar] [CrossRef]
- Queirós, R.P.; Saraiva, J.A.; da Silva, J.A.L. Tailoring structure and technological properties of plant proteins using high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 2018, 58, 1538–1556. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, F.; Lanciotti, R. Applications of high and ultra high pressure homogenization for food safety. Front. Microbiol. 2016, 7, 1132. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, J.; Mulla, M.; Al-Ruwaih, N.; Arfat, Y.A. Effect of high-pressure treatment prior to enzymatic hydrolysis on rheological, thermal, and antioxidant properties of lentil protein isolate. Legum. Sci. 2019, 1, e10. [Google Scholar] [CrossRef] [Green Version]
- Molina, E.; Papadopoulou, A.; Ledward, D.A. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocoll. 2001, 15, 263. [Google Scholar] [CrossRef]
- Ishak, N.F.; Hashim, N.A.; Othman, M.H.D.; Monash, P.; Zuki, F.M. Recent progress in the hydrophilic modification of alumina membranes for protein separation and purification. Ceram. Int. 2017, 43, 915–925. [Google Scholar] [CrossRef]
- Puppo, M.C.; Speroni, F.; Chapleau, N.; de Lamballerie, M.; Añón, M.C.; Anton, M. Effect of high-pressure treatment on emulsifying properties of soybean proteins. Food Hydrocoll. 2005, 19, 289–296. [Google Scholar] [CrossRef]
- Ucar, Y.; Ceylan, Z.; Durmus, M.; Tomar, O.; Cetinkaya, T. Application of cold plasma technology in the food industry and its combination with other emerging technologies. Trends Food Sci. Technol. 2021, 114, 355–371. [Google Scholar] [CrossRef]
- Saremnezhad, S.; Soltani, M.; Faraji, A.; Hayaloglu, A.A. Chemical changes of food constituents during cold plasma processing: A review. Food Res. Int. 2021, 147, 110552. [Google Scholar] [CrossRef] [PubMed]
- Niemira, B.A. Cold Plasma Decontamination of Foods. Annu. Rev. Food Sci. Technol. 2012, 3, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Niemira, B.A. Chapter 18—Decontamination of Foods by Cold Plasma. In Emerging Technologies for Food Processing, 2nd ed.; Sun, D., Ed.; Elsevier Ltd.: London, UK, 2015; pp. 327–333. [Google Scholar]
- Ji, Y.; Wang, Z.; Pei, F.; Shi, J.; Li, J.; Gunosewoyo, H.; Yang, F.; Tang, J.; Xie, X.; Yu, L. Introducing nitrogen atoms to amidoalkylindoles: Potent and selective cannabinoid type 2 receptor agonists with improved aqueous solubility. Med. Chem. Commun. 2019, 10, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.; Mohácsi-Farkas, C. History and future of food irradiation. Trends Food Sci. Technol. 2011, 22, 121–126. [Google Scholar] [CrossRef]
- Farkas, J. Irradiation for better foods. Trends Food Sci. Technol. 2006, 17, 148–152. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.; Song, K.B. Effect of γ-irradiation on the physicochemical properties of soy protein isolate films. Radiat Phys. Chem. 2005, 72, 35–40. [Google Scholar] [CrossRef]
- Singh Pulses: An overview. J. Food Sci. Technol. 2017, 54, 853–857. [CrossRef] [Green Version]
- Joye, I. Protein Digestibility of Cereal Products. Foods 2019, 8, 199. [Google Scholar] [CrossRef] [Green Version]
- Meinlschmidt, P.; Ueberham, E.; Lehmann, J.; Reineke, K.; Schlüter, O.; Schweiggert-Weisz, U.; Eisner, P. The effects of pulsed ultraviolet light, cold atmospheric pressure plasma, and gamma-irradiation on the immunoreactivity of soy protein isolate. Innov. Food Sci. Emerg. Technol. 2016, 38, 374–383. [Google Scholar] [CrossRef]
- Basak, S.; Annapure, U.S. Recent trends in the application of cold plasma for the modification of plant proteins—A review. Future Foods A Dedic. J. Sustain. Food Sci. 2022, 5, 100119. [Google Scholar] [CrossRef]
- Hall, A.E.; Moraru, C.I. Effect of High Pressure Processing and heat treatment on in vitro digestibility and trypsin inhibitor activity in lentil and faba bean protein concentrates. Food Sci. Technol. 2021, 152, 112342. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Makkar, H.P.; Becker, K. The effect of ionising radiation on antinutritional factors and the nutritional value of plant materials with reference to human and animal food. Food Chem. 2002, 78, 187. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Guo, A. Animal and Plant Protein Oxidation: Chemical and Functional Property Significance. Foods 2020, 10, 40. [Google Scholar] [CrossRef]
- Palanisamy, M.; Franke, K.; Berger, R.G.; Heinz, V.; Töpfl, S. High moisture extrusion of lupin protein: Influence of extrusion parameters on extruder responses and product properties. J. Sci. Food Agric. 2018, 99, 2175–2185. [Google Scholar] [CrossRef]
- Omosebi, M.O.; Osundahunsi, O.F.; Fagbemi, T.N. Effect of extrusion on protein quality, antinutritional factors, and digestibility of complementary diet from quality protein maize and soybean protein concentrate. J. Food Biochem. 2018, 42, e12508. [Google Scholar] [CrossRef]
- Joehnke, M.S.; Jeske, S.; Ispiryan, L.; Zannini, E.; Arendt, E.K.; Bez, J.; Sørensen, J.C.; Petersen, I.L. Nutritional and anti-nutritional properties of lentil (Lens culinaris) protein isolates prepared by pilot-scale processing. Food Chem. X 2021, 9, 100112. [Google Scholar] [CrossRef]
- Barbana, C.; Boye, J.I. In vitro protein digestibility and physico-chemical properties of flours and protein concentrates from two varieties of lentil (Lens culinaris). Food Funct. 2013, 4, 310–321. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; Boye, J.I. Comparative Study of the Effects of Processing on the Nutritional, Physicochemical and Functional Properties of Lentil. J. Food Process. Preserv. 2017, 41, e12824. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 2011, 43, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Laguna, L.; Picouet, P.; Guàrdia, M.D.; Renard, C.M.G.C.; Sarkar, A. In vitro gastrointestinal digestion of pea protein isolate as a function of pH, food matrices, autoclaving, high-pressure and re-heat treatments. Food Sci. Technol. 2017, 84, 511–519. [Google Scholar] [CrossRef]
- Tang, C.; Chen, L.; Ma, C. Thermal aggregation, amino acid composition and in vitro digestibility of vicilin-rich protein isolates from three Phaseolus legumes: A comparative study. Food Chem. 2009, 113, 957–963. [Google Scholar] [CrossRef]
- Gulati, P.; Brahma, S.; Rose, D.J. Chapter 13—Impacts of extrusion processing on nutritional components in cereals and legumes: Carbohydrates, proteins, lipids, vitamins, and minerals. In Extrusion Cooking, 2nd ed.; Ganjyal, G.M., Ed.; Elsevier Inc.: Edinburgh, UK, 2020; pp. 415–443. [Google Scholar]
- MacDonald, R.S.; Pryzbyszewski, J.; Hsieh, F.H. Soy Protein Isolate Extruded with High Moisture Retains High Nutritional Quality. J. Agric. Food Chem. 2009, 57, 3550–3555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Ha, M.J.; Shahbaz, H.M.; Kim, J.U.; Jang, H.; Park, J. High hydrostatic pressure treatment for manufacturing of red bean powder: A comparison with the thermal treatment. J. Food Eng. 2018, 238, 141–147. [Google Scholar] [CrossRef]
- Takai, E.; Kitamura, T.; Kuwabara, J.; Ikawa, S.; Yoshizawa, S.; Shiraki, K.; Kawasaki, H.; Arakawa, R.; Kitano, K. Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution. J. Phys. D Appl. Phys. 2014, 47, 285403. [Google Scholar] [CrossRef]
- Esteghlal, S.; Gahruie, H.H.; Niakousari, M.; Barba, F.J.; Bekhit, A.E.; Mallikarjunan, K.; Roohinejad, S. Bridging the Knowledge Gap for the Impact of Non-Thermal Processing on Proteins and Amino Acids. Foods 2019, 8, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariotti, F.; Gardner, C.D. Dietary Protein and Amino Acids in Vegetarian Diets—A Review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef] [Green Version]
- Kawada, K.; Kuramoto, N.; Mimori, S. Possibility that the Onset of Autism Spectrum Disorder is Induced by Failure of the Glutamine-Glutamate Cycle. Curr. Mol. Pharmacol. 2021, 14, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Strzyz, P. Forcing MT glutamylation. Nat. Rev. Mol. Cell Biol. 2021, 22, 509. [Google Scholar] [CrossRef]


| Amino Acids/Legumes | Chickpea | Soybean | Lentil | Bean | Faba Bean | Lupin | Cowpea | Pea | FAO |
|---|---|---|---|---|---|---|---|---|---|
| Alanine | 4.5–5.2 | 3.615–4.7 | 4.2–4.7 | 3.07–4.89 | 3.97–4.15 | 3.17–3.8 | 4.2–4.67 | 3.54–5.2 | NR * |
| Arginine | 8–9.2 | 6.18–8.3 | 7.6–7.8 | 5.29–6.08 | 8.96–9.46 | 8.51–14.1 | 6.66–7.69 | 4–8.6 | NR |
| Aspartic acid | 10.2–12.1 | 7.13–11.8 | 11.8–13.7 | 9.02–12.94 | 9.28–10.77 | 8.2–15.1 | 10.8–11.44 | 8.06–12.37 | NR |
| Cysteine | 0.4–1.7 | 1.5–2.065 | 0.7–0.9 | 0–0.94 | 0.85–1.33 | 1.12–1.72 | 0.28–0.32 | 0.35–1.8 | NR |
| Glutamic acid | 16.5–17.8 | 9.115–18.2 | 21.4–21.5 | 11.42–17.06 | 15.67–16.51 | 19.19–22.15 | 17.2–18.54 | 8.53–19.7 | NR |
| Glycine | 3.4–4.3 | 3.71–4.4 | 3.6 | 3.19–4.55 | 3.95–4.73 | 3.83–4.77 | 3.8–4.48 | 3.87–5.27 | NR |
| Histidine | 2.7–3.2 | 2.4–3 | 2.2–2.5 | 2.59–3.42 | 2.41–2.61 | 2.31–2.95 | 3.06–3.19 | 1.92–2.94 | 1.6 |
| Isoleucine | 4.1–5.2 | 4.2–5.9 | 3.8–4.1 | 3.42–5.21 | 3.67–3.94 | 2.89–4.62 | 3.75–3.84 | 3.09–4.5 | 3 |
| Leucine | 7.7–9.5 | 7.095–7.9 | 7.8 | 6.72–8.46 | 6.57–7.47 | 5.83–7.3 | 7.65–7.7 | 6.7–7.84 | 6.1 |
| Lysine | 6.7–7.8 | 6–6.58 | 7–7.3 | 4.91–6.48 | 5.97–7.08 | 4.35–4.92 | 5.74–7.5 | 3.41–8.1 | 4.8 |
| Methionine | 0.8–1.6 | 1.1–2.72 | 0.8 | 0.72–1.76 | 0.52–1.06 | 0.35–0.7 | 1.46–2.11 | 0.72–1.6 | NR |
| Phenylanine | 5–6.2 | 3.88–5.8 | 4.5–5 | 4.48–5.91 | 3.98–4.19 | 3.42–4 | 5.75–5.51 | 4.02–5.2 | NR |
| Proline | 3.5–4.4 | 3.63–5.3 | 3.5–4.9 | 2.95–6.49 | 3.86–4.27 | 4.4–5.72 | 4–5.91 | 2.11–5 | NR |
| Serine | 3.3–5.6 | 5.5–6.375 | 3.5–5.2 | 4.59–6.9 | 4.28–4.76 | 4.31–5.98 | 4.5–5.6 | 2.93–5.71 | NR |
| Threonine | 2.7–3.9 | 2.68–4.1 | 3–3.5 | 3.17–4.72 | 2.96–3.4 | 2.9–5.02 | 3.8–4.1 | 1.55–4.46 | 2.5 |
| Tryptophan | 0.6–1.4 | 1–7.64 | 0.7–1.2 | 1.11–1.18 | 0.85–0.87 | 0.49–1 | 0.7–1.11 | 0.61–3 | 0.66 |
| Tyrosine | 2.6–3.1 | 4–4.14 | 3.2–3.3 | 2.75–5.25 | 2.59–2.78 | 3.11–5.1 | 2.92–4.04 | 3.17–3.7 | NR |
| Valine | 3.9–5.2 | 4.4–5.245 | 4.5–5 | 4.58–5.38 | 3.41–4.31 | 2.46–4.2 | 4.68–5.1 | 3.97–5.11 | 4 |
| Physical Process | Effect | Protein Extract | References |
|---|---|---|---|
| Heat treatment | Unfolding and denaturation | Cowpea protein isolates | [108] |
| Soybean protein isolate | [109] | ||
| Denaturation and/or subsequent aggregation Alteration in secondary and tertiary conformational | Kidney, red and mung beans protein isolates | [110] | |
| No dissociation in the protein subunit | Mung bean protein isolate | [30] | |
| Increased α-helix decrease in β-sheets | Soybean Protein Isolate | [111] | |
| Extrusion treatment | Denaturation Increase degree of aggregation and crosslinking | Mung bean protein isolate | [112] |
| Increase in the proportion of β-turn structure Decrease of α-helix and β-sheet | Soy protein concentrate | [113] | |
| Pea protein isolate | [114] | ||
| Pea protein isolate | [115] | ||
| Increase in α-helix Decrease in β-sheet content | Mung bean protein isolate | [112]. | |
| High pressure | Unfolding/denaturation/ aggregation | Red kidney bean protein isolate | [116] |
| Cowpea protein isolates | [108] | ||
| Yellow field pea protein isolate | [117] | ||
| Changes in the secondary structure (β-sheets) | Red kidney bean protein isolate | [118] | |
| Cold plasma | Compact tertiary structure Higher ordered secondary structure Dissociation of globulins | Grass pea protein isolate | [119] |
| Oxidation/alteration of the secondary and tertiary structures | Soy protein isolate | [120] | |
| Alteration of the secondary structures | Peanut protein Isolate | [121] | |
| Irradiation | Modification of the secondary and tertiary structure | Red kidney bean phytohemagglutinin (lectin) | [122] |
| Insoluble amorphous aggregates and partially unfolded | Jack bean Concanavalin A | [123] |
| Properties | Treatment | Effect | References |
|---|---|---|---|
| Water-holding capacity | Heat treatment | Increase | [124] |
| High hydrostatic pressure | Increase | [108,118] | |
| Cold plasma | Increase | [121,125] | |
| Irradiation (X-ray irradiation) | Decrease | [126] | |
| Oil-holding capacity | Heat treatment | Increase | [127] |
| Cold plasma | Increase | [121,125] | |
| Protein solubility | Heat treatment | Increase/Decrease Factors: pH, Temperature | [30,127] |
| Extrusion | Decrease | [113,114] | |
| High hydrostatic pressure | Increase/Decrease Factor: Protein isolate’s nature, Pressure | [116,128,129] | |
| Cold plasma | Increase | [119,121,125] | |
| Irradiation (Gamma/X-ray irradiation) | Decrease | [126] | |
| Surface hydrophobicity | Heat treatment | Increase | [130] |
| Extrusion | Increase/Decrease Factor: Feed moisture, Temperature | [112,113] | |
| High hydrostatic pressure | Increase | [131] | |
| Emulsifying activity and stability | Heat treatment | Increase/Decrease Factor: pH | [30,110,124,132] |
| Extrusion | Stability: Increase | [113,114] | |
| High hydrostatic pressure | Increase/Decrease Factor: Pressure | [116,118,129] | |
| Cold plasma | Increase | [120,121] | |
| Irradiation (Gamma/Electron beam irradiation) | Increase | [126,133] | |
| Extrusion | Decrease | [114] | |
| High hydrostatic pressure | Increase | [129] | |
| Cold plasma | Increase | [120,121] | |
| Irradiation (X-ray irradiation) | Decrease | [126] | |
| Gelling capacity | Heat treatment | Increase | [30] |
| High hydrostatic pressure | Increase | [108,134,135] | |
| Cold plasma | Increase | [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neji, C.; Semwal, J.; Kamani, M.H.; Máthé, E.; Sipos, P. Legume Protein Extracts: The Relevance of Physical Processing in the Context of Structural, Techno-Functional and Nutritional Aspects of Food Development. Processes 2022, 10, 2586. https://doi.org/10.3390/pr10122586
Neji C, Semwal J, Kamani MH, Máthé E, Sipos P. Legume Protein Extracts: The Relevance of Physical Processing in the Context of Structural, Techno-Functional and Nutritional Aspects of Food Development. Processes. 2022; 10(12):2586. https://doi.org/10.3390/pr10122586
Chicago/Turabian StyleNeji, Chaima, Jyoti Semwal, Mohammad Hassan Kamani, Endre Máthé, and Péter Sipos. 2022. "Legume Protein Extracts: The Relevance of Physical Processing in the Context of Structural, Techno-Functional and Nutritional Aspects of Food Development" Processes 10, no. 12: 2586. https://doi.org/10.3390/pr10122586
APA StyleNeji, C., Semwal, J., Kamani, M. H., Máthé, E., & Sipos, P. (2022). Legume Protein Extracts: The Relevance of Physical Processing in the Context of Structural, Techno-Functional and Nutritional Aspects of Food Development. Processes, 10(12), 2586. https://doi.org/10.3390/pr10122586