Functional Compounds from Banana Peel Used to Decrease Oxidative Stress Effects
Abstract
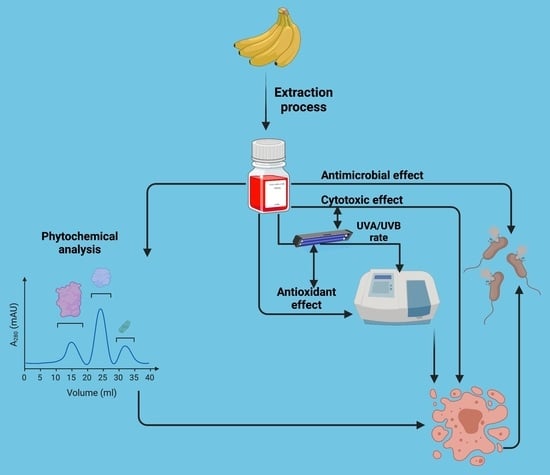
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Antioxidant Activities Determination—In Vitro
2.3. Quantitative Antibacterial Assay by Minimum Inhibitory Concentration (MIC) Determination
2.4. Solar Protection Factor (SPF) Determination—In Vitro
2.5. Quantification of Polyphenolic Compounds by Capillary Zone Electrophoresis
2.5.1. Reagents
2.5.2. Equipment and Method
2.6. In Vitro Antiproliferative Assay
2.7. Statistical Analysis
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manzoor, A.; Ahmad, S. Banana Peel: Characteristics and Consideration of Its Extract for Use in Meat Products Preservation: A Review. ACS Food Sci. Technol. 2021, 9, 1492–1506. [Google Scholar] [CrossRef]
- Jin, G.H.; Liu, Y.; Jin, S.Z.; Liu, X.D.; Liu, S.Z. UVB induced oxidative stress in human keratinocytes and protective effect of antioxidant agents. Radiat. Environ. Biophys. 2007, 46, 61–68. [Google Scholar] [CrossRef]
- Sánchez-Marzo, N.; Pérez-Sánchez, A.; Castillo, J.; Herranz-López, M.; Barrajón-Catalán, E.; Micol, V. Oxidative Stress and DNA damage in human keratinocytes by citrus and olive formulations. Free Radic. Biol. Med. 2017, 108, S82. [Google Scholar] [CrossRef]
- El Barnossi, A.; Moussaid, F.; Iraqi Housseini, A. Tangerine, banana and pomegranate peels valorisation for sustainable environment: A review. Biotechnol. Rep. 2020, 29, e00574. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneira, J.P.; Wu, T.; Ybanez, Q.; Dorado, A.A.; Migo, V.P.; Nayve, F.R.P., Jr.; Castillo-Israel, K.A.T. Microwave-Assisted Extraction of Pectin from “Saba” Banana Peel Waste: Optimization, Characterization, and Rheology Study. Int. J. Food Sci. 2020, 2020, 8879425. [Google Scholar] [CrossRef]
- Jamal, P.; Saheed, O.K.; Alam, Z. Bio-Valorization Potential of Banana Peels (Musa sapientum): An Overview. Asian J. Biotechnol. 2012, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chueh, C.C.; Lin, L.J.; Lin, W.C.; Huang, S.H.; Jan, M.S.; Chang, S.C.; Chung, W.S.; Lee, T. Antioxidant capacity of banana peel and its modulation of Nrf2-ARE associated gene expression in broiler chickens. Ital. J. Anim. Sci. 2019, 18, 1394–1403. [Google Scholar] [CrossRef] [Green Version]
- Vamanu, E.; Gatea, F.; Pelinescu, D.R. Bioavailability and Bioactivities of Polyphenols Eco Extracts from Coffee Grounds after In Vitro Digestion. Foods 2020, 9, 1281. [Google Scholar] [CrossRef]
- Abreu-Naranjo, R.; Paredes-Moreta, J.G.; Granda-Albuja, G.; Iturralde, G.; González-Paramás, A.M.; Alvarez-Suarez, J.M. Bioactive compounds, phenolic profile, antioxidant capacity and effectiveness against lipid peroxidation of cell membranes of Mauritia flexuosa L. fruit extracts from three biomes in the Ecuadorian Amazon. Heliyon 2020, 6, e05211. [Google Scholar] [CrossRef]
- Gomes, C.; Silva, A.C.; Marques, A.C.; Sousa Lobo, J.; Amaral, M.H. Biotechnology Applied to Cosmetics and Aesthetic Medicines. Cosmetics 2020, 7, 33. [Google Scholar] [CrossRef]
- Dabulici, C.M.; Sârbu, I.; Vamanu, E. The Bioactive Potential of Functional Products and Bioavailability of Phenolic Compounds. Foods 2020, 9, 953. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.N.; Kang, S.C. In vitro antioxidant activity of the water and ethanol extracts of Forsythia koreana flowers. Nat. Prod. Res. 2012, 26, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Idamokoro, E.M.; Masika, P.J.; Muchenje, V. A Report on the In Vitro Antioxidant Properties of Vachellia karroo Leaf Extract: A Plant Widely Grazed by Goats in the Central Eastern Cape of South Africa. Sustainability 2017, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Mihai, M.M.; Holban, A.M.; Giurcăneanu, C.; Popa, L.G.; Buzea, M.; Filipov, M.; Lazǎr, V.; Chifiriuc, M.C.; Popa, M.I. Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Rom. J. Morphol. Embryol. 2014, 55, 1401–1408. [Google Scholar]
- Sarbu, I.; Vassu, T.; Chifiriuc, M.C.; Bucur, M.; Stoica, I.; Stefana, P.; Rusu, E. Assessment the Activity of Some Enzymes and Antibiotic Substances Sensitivity on Pathogenic Bacteria Species. Rev. Chim. 2017, 68, 3015–3021. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Fernandes, A.R.; Sanchez-Lopez, E.; Sousa, I.M.d.O.; Figueiredo, M.C.; Ruiz, A.L.T.G.; Foglio, M.A.; Mazzola, P.G.; Souto, E.B. Evaluation of In Vitro Solar Protection Factor (SPF), Antioxidant Activity, and Cell Viability of Mixed Vegetable Extracts from Dirmophandra mollis Benth, Ginkgo biloba L., Ruta graveolens L., and Vitis vinífera L. Plants 2019, 8, 453. [Google Scholar] [CrossRef] [Green Version]
- Prochor, P.; Mierzejewska, Z.A. Influence of the Surface Roughness of PEEK GRF30 and Ti6Al4V SLM on the Viability of Primary Human Osteoblasts Determined by the MTT Test. Materials 2019, 12, 4189. [Google Scholar] [CrossRef] [Green Version]
- Cid-Ortega, S.; Monroy-Rivera, J.A. Extraction of Kaempferol and Its Glycosides Using Supercritical Fluids from Plant Sources: A Review. Food Technol. Biotechnol. 2018, 56, 480–493. [Google Scholar] [CrossRef]
- Yun, J.E.; Lee, H.; Ko, H.J.; Woo, E.R.; Lee, D.G. Fungicidal effect of isoquercitrin via inducing membrane disturbance. Biochim. Biophys. Acta (BBA)—Rev. Biomembr. 2015, 1848, 695–701. [Google Scholar] [CrossRef] [Green Version]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Cheng, S.; Liao, Y.; Huang, B.; Du, B.; Zeng, W.; Jiang, Y.; Duan, X.; Yang, Z. Comparative analysis of pigments in red and yellow banana fruit. Food Chem. 2018, 239, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.S.; Matheus, J.R.V.; de Souza, T.S.P.; Moreira, R.F.A.; Fai, A.E.C. Green-based active packaging: Opportunities beyond COVID-19, food applications, and perspectives in circular economy—A brief review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4881–4905. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Dinu, L.-D.; Luntraru, C.M.; Suciu, A. In Vitro Coliform Resistance to Bioactive Compounds in Urinary Infection, Assessed in a Lab Catheterization Model. Appl. Sci. 2021, 11, 4315. [Google Scholar] [CrossRef]
- Anal, A.K.; Jaisanti, S.; Noomhorm, A. Enhanced yield of phenolic extracts from banana peels (Musa acuminata Colla AAA) and cinnamon barks (Cinnamomum varum) and their antioxidative potentials in fish oil. J. Food Sci. Technol. 2014, 51, 2632–2639. [Google Scholar] [CrossRef] [Green Version]
- Al-Mqbali, L.R.A.; Hossain, M.A. Cytotoxic and antimicrobial potential of different varieties of ripe banana used traditionally to treat ulcers. Toxicol. Rep. 2019, 6, 1086–1090. [Google Scholar] [CrossRef]
- Panda, S.K.; Castro, A.; Jouneghani, R.S.; Leyssen, P.; Neyts, J.; Swennen, R.; Luyten, W. Antiviral and Cytotoxic Activity of Different Plant Parts of Banana (Musa spp.). Viruses 2020, 12, 549. [Google Scholar] [CrossRef]
- Arraibi, A.A.; Liberal, Â.; Dias, M.I.; Alves, M.J.; Ferreira, I.C.F.R.; Barros, L.; Barreira, J.C.M. Chemical and Bioactive Characterization of Spanish and Belgian Apple Pomace for Its Potential Use as a Novel Dermocosmetic Formulation. Foods 2021, 10, 1949. [Google Scholar] [CrossRef]




| Samples | BPR | BP | Control |
|---|---|---|---|
| S. aureus ATCC BAA 1026 | 3.125 | 50 | 12.5 |
| S. aureus 1004 | 3.125 | 50 | 25 |
| E. coli ACTCC 25922 | 3.125 | ˃50 | 12.5 |
| E. coli B11 | 3.125 | ˃50 | 12.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avram, I.; Gatea, F.; Vamanu, E. Functional Compounds from Banana Peel Used to Decrease Oxidative Stress Effects. Processes 2022, 10, 248. https://doi.org/10.3390/pr10020248
Avram I, Gatea F, Vamanu E. Functional Compounds from Banana Peel Used to Decrease Oxidative Stress Effects. Processes. 2022; 10(2):248. https://doi.org/10.3390/pr10020248
Chicago/Turabian StyleAvram, Ionela, Florentina Gatea, and Emanuel Vamanu. 2022. "Functional Compounds from Banana Peel Used to Decrease Oxidative Stress Effects" Processes 10, no. 2: 248. https://doi.org/10.3390/pr10020248
APA StyleAvram, I., Gatea, F., & Vamanu, E. (2022). Functional Compounds from Banana Peel Used to Decrease Oxidative Stress Effects. Processes, 10(2), 248. https://doi.org/10.3390/pr10020248