Catalytic Properties of Free-Base Porphyrin Modified Graphite Electrodes for Electrochemical Water Splitting in Alkaline Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Working Electrodes Preparation
2.3. Electrochemical Experiments
2.4. Physical-Chemical Characterizations
3. Results and Discussions
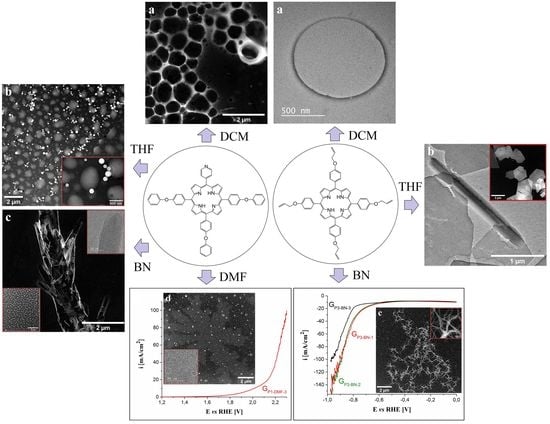
3.1. TEM/STEM Analysis of Porphyrin Specimens
3.2. OER and HER Electrocatalytic Properties of the Porphyrin Modified Electrodes
3.2.1. Studies in 0.1 M KOH Electrolyte Solution
3.2.2. OER Electrocatalytic Properties of the Porphyrin Modified Electrodes in 1 M KOH Solution
3.2.3. HER Electrocatalytic Properties of the Porphyrin Modified Electrodes in 1 M KOH Solution
3.3. SEM and Raman Characterizations
3.4. Further Considerations Regarding the GP3-BN-1 Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Agarwal, S.; Jain, A. Significance of Hydrogen as Economic and Environmentally Friendly Fuel. Energies 2021, 14, 7389. [Google Scholar] [CrossRef]
- Gao, Z.; Qi, J.; Chen, M.; Zhang, W.; Cao, R. An Electrodeposited NiSe for Electrocatalytic Hydrogen and Oxygen Evolution Reactions in Alkaline Solution. Electrochim. Acta 2017, 224, 412–418. [Google Scholar] [CrossRef]
- Li, Q.; Bao, Y.; Bai, F. Porphyrin and Macrocycle Derivatives for Electrochemical Water Splitting. MRS Bull. 2020, 45, 569–573. [Google Scholar] [CrossRef]
- Fellet, M.; Tiede, D.M. Search for Water-Splitting Catalysts for Global Usage. Mater. Res. Bull. 2017, 42, 190–191. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Mahmood, N.; Zhu, J.; Hou, Y.; Sun, S. Graphene and Its Composites with Nanoparticles for Electrochemical Energy Applications. Nano Today 2014, 9, 668–683. [Google Scholar] [CrossRef] [Green Version]
- Marwat, M.A.; Humayun, M.; Afridi, M.W.; Zhang, H.; Abdul Karim, M.R.; Ashtar, M.; Usman, M.; Waqar, S.; Ullah, H.; Wang, C.; et al. Advanced Catalysts for Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2021, 4, 12007–12031. [Google Scholar] [CrossRef]
- Mallouk, T.E. Divide and Conquer. Nat. Chem. 2013, 5, 362–363. [Google Scholar] [CrossRef]
- Kuang, Y.; Kenney, M.J.; Meng, Y.; Hung, W.H.; Liu, Y.; Huang, J.E.; Prasanna, R.; Li, P.; Li, Y.; Wang, L.; et al. Solar-Driven, Highly Sustained Splitting of Seawater into Hydrogen and Oxygen Fuels. Proc. Natl. Acad. Sci. USA 2019, 116, 6624–6629. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Dong, W.; Wang, Z.; Meng, Y.; Liu, W.; Song, P.; Liu, Z. Recent Advances in 2D Transition Metal Compounds for Electrocatalytic Full Water Splitting in Neutral Media. Mater. Today Adv. 2020, 8, 100081. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, T.; Cao, H.; Cui, S.; Du, P. Self-Supported Ni2P Nanosheets on Low-Cost Three-Dimensional Fe Foam as a Novel Electrocatalyst for Efficient Water Oxidation. J. Energy Chem. 2020, 42, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. Strongly Coupled Inorganic/Nanocarbon Hybrid Materials for Advanced Electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.K.; Liu, L.M.; et al. Platinum Single-Atom and Cluster Catalysis of the Hydrogen Evolution Reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.Y.; Zhao, B.; Wang, X. A Review on Noble-Metal-Free Bifunctional Heterogeneous Catalysts for Overall Electrochemical Water Splitting. J. Mater. Chem. A 2016, 4, 17587–17603. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, N.; Yao, Y.; Zhang, J.W.; Pan, L.; Zhang, X.; Zou, J.J. Electrocatalysts for Hydrogen Evolution in Alkaline Electrolytes: Mechanisms, Challenges, and Prospective Solutions. Adv. Sci. 2018, 5, 1700464. [Google Scholar] [CrossRef] [PubMed]
- Taranu, B.O.; Vlazan, P.; Svera, P.; Poienar, M.; Sfirloaga, P. New Functional Hybrid Materials Based on Clay Minerals for Enhanced Electrocatalytic Activity. J. Alloys Compd. 2022, 892, 162239. [Google Scholar] [CrossRef]
- Taranu, B.O.; Ivanovici, M.G.; Svera, P.; Vlazan, P.; Sfirloaga, P.; Poienar, M. Ni11□ (HPO3)8(OH)6 Multifunctional Materials: Electrodes for Oxygen Evolution Reaction and Potential Visible-Light Active Photocatalysts. J. Alloys Compd. 2020, 848, 156595. [Google Scholar] [CrossRef]
- Yasin, G.; Ibraheem, S.; Ali, S.; Arif, M.; Ibrahim, S.; Iqbal, R.; Kumar, A.; Tabish, M.; Mushtaq, M.A.; Saad, A.; et al. Defects-Engineered Tailoring of Tri-Doped Interlinked Metal-Free Bifunctional Catalyst with Lower Gibbs Free Energy of OER/HER Intermediates for Overall Water Splitting. Mater. Today Chem. 2022, 23, 100634. [Google Scholar] [CrossRef]
- Zhang, H.W.; Lu, Y.X.; Li, B.; Huang, G.F.; Zeng, F.; Li, Y.Y.; Pan, A.; Chai, Y.F.; Huang, W.Q. Acid-Induced Topological Morphology Modulation of Graphitic Carbon Nitride Homojunctions as Advanced Metal-Free Catalysts for OER and Pollutant Degradation. J. Mater. Sci. Technol. 2021, 86, 210–218. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, W.; Cao, R. Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef] [PubMed]
- Baglia, R.A.; Zaragoza, J.P.T.; Goldberg, D.P. Biomimetic Reactivity of Oxygen-Derived Manganese and Iron Porphyrinoid Complexes. Chem. Rev. 2017, 117, 13320–13352. [Google Scholar] [CrossRef] [PubMed]
- Savéant, J.-M. Molecular Catalysis of Electrochemical Reactions. Mechanistic Aspects. Chem. Rev. 2008, 108, 2348–2378. [Google Scholar] [CrossRef] [PubMed]
- Fagadar-Cosma, E.; Vlascici, D.; Fagadar-Cosma, G. Porfirinele de La Sinteză La Aplicații; Eurostampa: Timisoara, Romania, 2008; ISBN 978-973-687-680-6. [Google Scholar]
- Meng, J.; Bi, P.; Jia, J.; Sun, X.; Chen, R. Light-Assisted Catalytic Water Oxidation from Porphyrin J-Aggregate. ChemistrySelect 2017, 2, 4882–4888. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Mathias, J.P.; Seto, C.T. Molecular Self-Assembly and Nanochemistry: A Chemical Strategy for the Synthesis of Nanostructures. Science 1991, 254, 1312–1319. [Google Scholar] [CrossRef]
- Lehn, J.M. Toward Self-Organization and Complex Matter. Science 2002, 295, 2400–2403. [Google Scholar] [CrossRef] [Green Version]
- Fagadar-Cosma, E.; Fagadar-Cosma, G.; Vasile, M.; Enache, C. Synthesis, Spectroscopic and Self-Assembling Characterization of Novel Photoactive Mixed Aryl-Substituted Porphyrin. Curr. Org. Chem. 2012, 16, 931–941. [Google Scholar] [CrossRef]
- Auwärter, W.; Écija, D.; Klappenberger, F.; Barth, J.V. Porphyrins at Interfaces. Nat. Chem. 2015, 7, 105–120. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, P.; Dong, H.; Zhen, Y.; Liu, M.; Hu, W. Porphyrin Supramolecular 1D Structures via Surfactant-Assisted Self-Assembly. Adv. Mater. 2015, 27, 5379–5387. [Google Scholar] [CrossRef]
- Romanov, N.M.; Zakharova, I.B. The Composition and the Structure of Thin Films Based on Metal Porphyrin Complexes. St. Petersbg. Polytech. Univ. J. Phys. Math. 2016, 2, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Birdeanu, M.; Fagadar-Cosma, E. The Self-Assembly of Porphyrin Derivatives into 2D and 3D Architectures. In Quantum Nanosystems: Structure: Properties and Interactions; Putz, M.V., Ed.; Apple Academic Press: Toronto, ON, Canada; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781774633144. [Google Scholar]
- Seo, S.; Lee, K.; Min, M.; Cho, Y.; Kim, M.; Lee, H. A Molecular Approach to an Electrocatalytic Hydrogen Evolution Reaction on Single-Layer Graphene. Nanoscale 2017, 9, 3969–3979. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Lyu, Z.; Marcos-Hernández, M.; Villagrán, D. Free-Base Porphyrin Polymer for Bifunctional Electrochemical Water Splitting. ChemRxiv 2021, 1–7. [Google Scholar] [CrossRef]
- Patra, B.C.; Khilari, S.; Manna, R.N.; Mondal, S.; Pradhan, D.; Pradhan, A.; Bhaumik, A. A Metal-Free Covalent Organic Polymer for Electrocatalytic Hydrogen Evolution. ACS Catal. 2017, 7, 6120–6127. [Google Scholar] [CrossRef]
- Swierk, J.R.; Méndez-Hernández, D.D.; McCool, N.S.; Liddell, P.; Terazono, Y.; Pahk, I.; Tomlin, J.J.; Oster, N.V.; Moore, T.A.; Moore, A.L.; et al. Metal-Free Organic Sensitizers for Use in Water-Splitting Dye-Sensitized Photoelectrochemical Cells. Proc. Natl. Acad. Sci. USA 2015, 112, 1681–1686. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Rodríguez-López, N.; Villagrán, D. Hydrogen Gas Generation Using a Metal-Free Fluorinated Porphyrin. Chem. Sci. 2018, 9, 4689–4695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlascici, D.; Fagadar-Cosma, E.; Popa, I.; Chiriac, V.; Gil-Agusti, M. A Novel Sensor for Monitoring of Iron(III) Ions Based on Porphyrins. Sensors 2012, 12, 8193–8203. [Google Scholar] [CrossRef] [PubMed]
- Vlascici, D.; Popa, I.; Chiriac, V.A.; Fagadar-Cosma, G.; Popovici, H.; Fagadar-Cosma, E. Potentiometric Detection and Removal of Copper Using Porphyrins. Chem. Cent. J. 2013, 7, 111. [Google Scholar] [CrossRef] [Green Version]
- Taranu, B.O.; Fagadar-Cosma, E.; Popa, I.; Plesu, N.; Taranu, I. Adsorbed Functionalized Porphyrins on Polyaniline Modified Platinum Electrodes. Comparative Electrochemical Properties. Dig. J. Nanomater. Biostructures 2014, 9, 667–679. [Google Scholar]
- Popa, I.; Fagadar-Cosma, G.; Taranu, B.O.; Birdeanu, A.V.; Taranu, I.; Vlascici, D.; Birdeanu, M.; Fagadar-Cosma, E. Electrochemical Behavior of Tetra(4-Methoxyphenyl) Porphyrin Thin Films Obtained by Laser Deposition on Graphite Electrode. Dig. J. Nanomater. Biostructures 2014, 9, 1277–1287. [Google Scholar]
- Popa, I.; Fagadar-Cosma, E.; Taranu, B.-O.; Birdeanu, M.; Fagadar-Cosma, G.; Taranu, I. Corrosion Protection Efficiency of Bilayer Porphyrin-Polyaniline Film Deposited on Carbon Steel. Macromol. Symp. 2015, 352, 16–24. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Tarabukina, E.; Zakharova, N.; Birdeanu, M.; Taranu, B.; Palade, A.; Creanga, I.; Lascu, A.; Fagadar-Cosma, G. Hybrids Formed between Polyvinylpyrrolidone and an A3B Porphyrin Dye: Behaviour in Aqueous Solutions and Chemical Response to CO2 Presence. Polym. Int. 2016, 65, 200–209. [Google Scholar] [CrossRef]
- Taranu, B.-T.; Vlascici, D.; Sebarchievici, I.; Fagadar-Cosma, E. The Aggregation Behavior of an A3B Free Base Porphyrin and Its Application as Chromium(III)-Selective Membrane Sensor. Stud. Univ. Babes-Bolyai Chem. 2016, 61, 199–212. [Google Scholar]
- Taranu, B.O.; Sebarchievici, L.; Taranu, I.; Birdeanu, M.; Cosma, E.F. Electrochemical and Microscopic Characterization of Two Meso-Substituted A3B and A4 Porphyrins. Rev. Chim. 2016, 67, 892–896. [Google Scholar]
- Fagadar-Cosma, E.; Lascu, A.; Palade, A.; Creanga, I.; Birdeanu, M. Hybrid Material Based on 5-(4-Pyridyl)-10,15,20-Tris(4- Phenoxyphenyl)-Porphyrin and Gold Colloid for CO2 Detection. Dig. J. Nanomater. Biostructures 2016, 11, 419–424. [Google Scholar]
- Birdeanu, A.V.; Birdeanu, M.; Fagadar-Cosma, E. Corrosion Protection Characteristics of Ceramics, Porphyrins and Hybrid Ceramics/Porphyrins, Deposited as Single and Sandwich Layers, by Pulsed Laser Deposition (PLD). J. Alloys Compd. 2017, 706, 220–226. [Google Scholar] [CrossRef]
- Alexandrova, R.; Kalfin, R.; Tudose, R.; Fagadar-Cosma, E. Comparative Cytotoxicity Assays Performed Using a Free Porphyrin and Its Zn(II), Co(II) and Cu(II) Complexes. Influence of Optical and Aggregation Properties. Stud. Univ. Babes-Bolyai Chem. 2018, 63, 65–77. [Google Scholar] [CrossRef]
- Salageanu, L.; Muntean, D.; George, H.F.; Lascu, A.; Anghel, D.; Bagiu, I.C.; Fagadar-Cosma, E. Antimicrobial Activity of Different Substituted Meso-Porphyrin Derivatives. Rev. Rom. Med. Lab. 2020, 28, 205–216. [Google Scholar] [CrossRef]
- Anghel, D.; Lascu, A.; Epuran, C.; Fratilescu, I.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Hybrid Materials Based on Silica Matrices Impregnated with Pt-Porphyrin or PtNPs Destined for CO2 Gas Detection or for Wastewaters Color Removal. Int. J. Mol. Sci. 2020, 21, 4262. [Google Scholar] [CrossRef]
- Fagadar-cosma, E.; Enache, C.; Tudose, R.; Armeanu, I.; Mosoarca, E.; Vlascici, D.; Costisor, O. UV-VIS and Fluorescence Spectra of Meso-Tetraphenylporphyrin and Meso-Tetrakis-(4-Methoxyphenyl) Porphyrin in THF and THF-Water Systems. The Influence of PH. Rev. Chim. 2007, 58, 451–455. [Google Scholar]
- Fagadar-Cosma, E.; Enache, C.; Armeanu, I.; Dascalu, D.; Fagadar-Cosma, G.; Vasile, M.; Grozescu, I. The Influence of PH over Topography and Spectroscopic Properties of Silica Hybrid Materials Embedding Meso-Tetratolylporphyrin. Mater. Res. Bull. 2009, 44, 426–431. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Glajch, J.L. Practical HPLC Method Development, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997; Volume 3, ISBN 9780471007036. [Google Scholar]
- Menezes, P.W.; Panda, C.; Loos, S.; Bunschei-Bruns, F.; Walter, C.; Schwarze, M.; Deng, X.; Dau, H.; Driess, M. A Structurally Versatile Nickel Phosphite Acting as a Robust Bifunctional Electrocatalyst for Overall Water Splitting. Energy Environ. Sci. 2018, 11, 1287–1298. [Google Scholar] [CrossRef] [Green Version]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; ISBN 978-0-471-04372-0. [Google Scholar]
- Broaddus, E.; Brubaker, J.; Gold, S.A. Electrochemical Characterization of Platinum Nanotubules Made via Template Wetting Nanofabrication. Int. J. Electrochem. 2013, 2013, 960513. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Wu, H.; He, H.; Xu, X.; Jin, Y. Self-Standing Non-Noble Metal (Ni-Fe) Oxide Nanotube Array Anode Catalysts with Synergistic Reactivity for High-Performance Water Oxidation. J. Mater. Chem. A 2015, 3, 7179–7186. [Google Scholar] [CrossRef]
- Motoc, S.; Manea, F.; Orha, C.; Pop, A. Enhanced Electrochemical Response of Diclofenac at a Fullerene–Carbon Nanofiber Paste Electrode. Sensors 2019, 19, 1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Yang, Y.; Liu, Y.; Shen, G.; Yu, R. Platinum Nanoparticles-Doped Sol-Gel/Carbon Nanotubes Composite Electrochemical Sensors and Biosensors. Biosens. Bioelectron. 2006, 21, 1125–1131. [Google Scholar] [CrossRef]
- Taranu, B.-O. Contribuții La Caracterizarea Fizico-Chimică a Porfirinelor. Aplicații În Senzoristică Și Coroziune. Ph.D. Thesis, Institute of Chemistry Timisoara of Romanian Academy, Timisoara, Romania, 19 November 2016. [Google Scholar]
- Lensen, M.C.; Takazawa, K.; Elemans, J.A.A.W.; Jeukens, C.R.L.P.N.; Christianen, P.C.M.; Maan, J.C.; Rowan, A.E.; Nolte, R.J. Aided Self-Assembly of Porphyrin Nanoaggregates into Ring-Shaped Architectures. Chem. A Eur. J. 2004, 10, 831–839. [Google Scholar] [CrossRef]
- Jeukens, C.R.L.P.N.; Lensen, M.C.; Wijnen, F.J.P.; Elemans, J.A.A.W.; Christianen, P.C.M.; Rowan, A.E.; Gerritsen, J.W.; Nolte, R.J.M.; Maan, J.C. Polarized Absorption and Emission of Ordered Self-Assembled Porphyrin Rings. Nano Lett. 2004, 4, 1401–1406. [Google Scholar] [CrossRef]
- Mani, V.; Anantharaj, S.; Mishra, S.; Kalaiselvi, N.; Kundu, S. Iron Hydroxyphosphate and Sn-Incorporated Iron Hydroxyphosphate: Efficient and Stable Electrocatalysts for Oxygen Evolution Reaction. Catal. Sci. Technol. 2017, 7, 5092–5104. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Zhou, X.; Xia, Z.; Gao, W.; Ma, Y.; Qu, Y. Highly Efficient and Robust Nickel Phosphides as Bifunctional Electrocatalysts for Overall Water-Splitting. ACS Appl. Mater. Interfaces 2016, 8, 10826–10834. [Google Scholar] [CrossRef]
- Han, L.; Dong, S.; Wang, E. Transition-Metal (Co, Ni, and Fe)-Based Electrocatalysts for the Water Oxidation Reaction. Adv. Mater. 2016, 28, 9266–9291. [Google Scholar] [CrossRef]
- Concina, I.; Ibupoto, Z.H.; Vomiero, A. Semiconducting Metal Oxide Nanostructures for Water Splitting and Photovoltaics. Adv. Energy Mater. 2017, 7, 1700706. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chen, S.; Sun, B.; Su, D.; Huang, X.; Liu, H.; Yan, Y.; Sun, K.; Wang, G. Graphene-Co3O4 Nanocomposite as Electrocatalyst with High Performance for Oxygen Evolution Reaction. Sci. Rep. 2015, 5, 7629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebarchievici, I.; Taranu, B.O.; Birdeanu, M.; Rus, S.F.; Fagadar-Cosma, E. Electrocatalytic Behaviour and Application of Manganese Porphyrin/Gold Nanoparticle- Surface Modified Glassy Carbon Electrodes. Appl. Surf. Sci. 2016, 390, 131–140. [Google Scholar] [CrossRef]
- Li, Q.; Tang, S.; Tang, Z.; Zhang, Q.; Yang, W. Microwave-Assisted Synthesis of FeCoS2/XC-72 for Oxygen Evolution Reaction. Solid State Sci. 2019, 96, 105968. [Google Scholar] [CrossRef]
- Wang, H.; Lee, H.W.; Deng, Y.; Lu, Z.; Hsu, P.C.; Liu, Y.; Lin, D.; Cui, Y. Bifunctional Non-Noble Metal Oxide Nanoparticle Electrocatalysts through Lithium-Induced Conversion for Overall Water Splitting. Nat. Commun. 2015, 6, 7261. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [Green Version]
- Boshnakova, I.; Lefterova, E.; Slavcheva, E. Investigation of Montmorillonite as Carrier for OER. Int. J. Hydrog. Energy 2018, 43, 16897–16904. [Google Scholar] [CrossRef]
- Lahiri, A.; Li, G.; Endres, F. Highly Efficient Electrocatalytic Hydrogen Evolution Reaction on Carbonized Porous Conducting Polymers. J. Solid State Electrochem. 2020, 24, 2763–2771. [Google Scholar] [CrossRef] [Green Version]
- Ji, D.; Peng, S.; Lu, J.; Li, L.; Yang, S.; Yang, G.; Qin, X.; Srinivasan, M.; Ramakrishna, S. Design and Synthesis of Porous Channel-Rich Carbon Nanofibers for Self-Standing Oxygen Reduction Reaction and Hydrogen Evolution Reaction Bifunctional Catalysts in Alkaline Medium. J. Mater. Chem. A 2017, 5, 7507–7515. [Google Scholar] [CrossRef]
- Zou, X.; Huang, X.; Goswami, A.; Silva, R.; Sathe, B.R.; Mikmeková, E.; Asefa, T. Cobalt-Embedded Nitrogen-Rich Carbon Nanotubes Efficiently Catalyze Hydrogen Evolution Reaction at All PH Values. Angew. Chemie Int. Ed. 2014, 53, 4372–4376. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.H.; Su, H.; Lv, L.B.; Zhao, T.J.; Ge, J.M.; Wei, X.; Wang, K.X.; Li, X.H.; Chen, J.S. Nitrogen-Doped Graphene Microtubes with Opened Inner Voids: Highly Efficient Metal-Free Electrocatalysts for Alkaline Hydrogen Evolution Reaction. Nano Res. 2016, 9, 2606–2615. [Google Scholar] [CrossRef]
- Lai, J.; Li, S.; Wu, F.; Saqib, M.; Luque, R.; Xu, G. Unprecedented Metal-Free 3D Porous Carbonaceous Electrodes for Water Splitting. Energy Environ. Sci. 2016, 9, 1210–1214. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Du, A.; Gao, G.; Chen, J.; Yan, X.; Brown, C.L.; Yao, X. Defect Graphene as a Trifunctional Catalyst for Electrochemical Reactions. Adv. Mater. 2016, 28, 9532–9538. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Zheng, Y.; Jiao, Y.; Zhang, X.; Dai, S.; Qiao, S.Z. Polydopamine-Inspired, Dual Heteroatom-Doped Carbon Nanotubes for Highly Efficient Overall Water Splitting. Adv. Energy Mater. 2017, 7, 1602068. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Dai, L. Multifunctional Carbon-Based Metal-Free Electrocatalysts for Simultaneous Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution. Adv. Mater. 2017, 29, 1604942. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Solin, N.; Inganäs, O. Biocarbon Meets Carbon—Humic Acid/Graphite Electrodes Formed by Mechanochemistry. Materials 2019, 12, 4032. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xu, X.; Yang, L.; Cheng, D.; Cao, D. Single-Atom Ru Doping Induced Phase Transition of MoS2 and S Vacancy for Hydrogen Evolution Reaction. Small Methods 2019, 3, 1900653. [Google Scholar] [CrossRef]
- Putra, R.P.; Horino, H.; Rzeznicka, I.I. An Efficient Electrocatalyst for Oxygen Evolution Reaction in Alkaline Solutions Derived from a Copper Chelate Polymer via in Situ Electrochemical Transformation. Catalysts 2020, 10, 233. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.F.; Chen, Y.; Wang, S.; Gao, S.; Lou, X.W. Interfacing Manganese Oxide and Cobalt in Porous Graphitic Carbon Polyhedrons Boosts Oxygen Electrocatalysis for Zn–Air Batteries. Adv. Mater. 2019, 31, 1902339. [Google Scholar] [CrossRef]










| Electrode Code | Porphyrin | Solvent | Porphyrin Layers | Electrode Code | Porphyrin | Solvent | Porphyrin Layers |
|---|---|---|---|---|---|---|---|
| GP1-DCM-1 | P1 | DCM | 1 | GP3-DCM-1 | P3 | DCM | 1 |
| GP1-DCM-2 | 2 | GP3-DCM-2 | 2 | ||||
| GP1-DCM-3 | 3 | GP3-DCM-3 | 3 | ||||
| GP1-THF-1 | THF | 1 | GP3-THF-1 | THF | 1 | ||
| GP1-THF-2 | 2 | GP3-THF-2 | 2 | ||||
| GP1-THF-3 | 3 | GP3-THF-3 | 3 | ||||
| GP1-BN-1 | BN | 1 | GP3-BN-1 | BN | 1 | ||
| GP1-BN-2 | 2 | GP3-BN-2 | 2 | ||||
| GP1-BN-3 | 3 | GP3-BN-3 | 3 | ||||
| GP1-DMF-1 | DMF | 1 | GP4-DCM-1 | P4 | DCM | 1 | |
| GP1-DMF-2 | 2 | GP4-DCM-2 | 2 | ||||
| GP1-DMF-3 | 3 | GP4-DCM-3 | 3 | ||||
| GP2-DCM-1 | P2 | DCM | 1 | GP4-THF-1 | THF | 1 | |
| GP2-DCM-2 | 2 | GP4-THF-2 | 2 | ||||
| GP2-DCM-3 | 3 | GP4-THF-3 | 3 | ||||
| GP2-THF-1 | THF | 1 | GP4-BN-1 | BN | 1 | ||
| GP2-THF-2 | 2 | GP4-BN-2 | 2 | ||||
| GP2-THF-3 | 3 | GP4-BN-3 | 3 | ||||
| GP2-BN-1 | BN | 1 | |||||
| GP2-BN-2 | 2 | ||||||
| GP2-BN-3 | 3 | ||||||
| Electrode | ηH2 [mV] at i = −10 mA/cm2 | Tafel Slope [mV/dec] | Reference |
|---|---|---|---|
| Porous channel-rich carbon nanofibers on GC | ~690 | - | [74] |
| Multi-walled CNTs on GC | 900 | - | [75] |
| N-doped graphene microtubes on GC | 432 | 116.7 | [76] |
| Porous graphite carbon doped with N, P and O on oxidized carbon fiber cloth | 446 | 154 | [77] |
| Pristine graphene on GC | ~575 | 189 | [78] |
| N-doped graphene on GC | ~550 | 152 | [78] |
| Graphene with carbon defects on GC | 320 | 118 | [78] |
| Oxidized CNTs on GC | 670 | 187 | [79] |
| Oxidized CNTs and polydopamine hybrid material on GC | >700 | 178 | [79] |
| N-doped CNTs on GC | 620 | 187 | [79] |
| N,S-doped CNTs on GC | 450 | 133 | [79] |
| N,S co-doped graphitic sheets with stereoscopic holes on GC | ~270 | - | [80] |
| GP3-BN-1 | 500 | 190 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taranu, B.-O.; Fagadar-Cosma, E. Catalytic Properties of Free-Base Porphyrin Modified Graphite Electrodes for Electrochemical Water Splitting in Alkaline Medium. Processes 2022, 10, 611. https://doi.org/10.3390/pr10030611
Taranu B-O, Fagadar-Cosma E. Catalytic Properties of Free-Base Porphyrin Modified Graphite Electrodes for Electrochemical Water Splitting in Alkaline Medium. Processes. 2022; 10(3):611. https://doi.org/10.3390/pr10030611
Chicago/Turabian StyleTaranu, Bogdan-Ovidiu, and Eugenia Fagadar-Cosma. 2022. "Catalytic Properties of Free-Base Porphyrin Modified Graphite Electrodes for Electrochemical Water Splitting in Alkaline Medium" Processes 10, no. 3: 611. https://doi.org/10.3390/pr10030611
APA StyleTaranu, B. -O., & Fagadar-Cosma, E. (2022). Catalytic Properties of Free-Base Porphyrin Modified Graphite Electrodes for Electrochemical Water Splitting in Alkaline Medium. Processes, 10(3), 611. https://doi.org/10.3390/pr10030611