Catalytic-Level Identification of Prepared Pt/HY, Pt-Zn/HY, and Pt-Rh/HY Nanocatalysts on the Reforming Reactions of N-Heptane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nano Y-Zeolite
2.3. Preparation of Pt/HY, Pt-Zn/HY, and Pt-Rh/HY Nanocatalysts
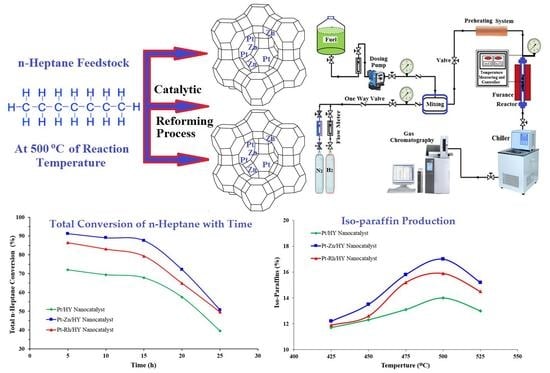
2.4. Experimental System and Procedures
3. Results and Discussion
3.1. Characterization of the Prepared Nano-Y-Zeolite
3.2. Evaluation of the Nanocatalyst Surface Area
3.3. Evaluation of the Nanocatalysts’ Activities
3.4. Evaluation of n-Heptane Conversion
3.5. Evaluation of Catalyst Selectivity
3.6. Reaction Mechanism over the Prepared Pt-Zn/HY Nanocatalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belopukhov, E.A.; Kir’yanov, D.I.; Smolikov, M.D.; Shkurenok, V.A.; Belyi, A.S.; Lavrenov, A.V.; Kleimenov, A.V.; Kondrashev, D.O. Investigation of fluorine-promoted Pt-Re/Al2O3 catalysts in reforming of n-heptane. Catal. Today 2021, 378, 113–118. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, Y.; Lin, Y.; Luo, L.; Song, N.; Lin, J.; Peng, C.; Sui, B.; Zhang, J.; Qian, G.; et al. Hierarchical pore construction of alumina microrod supports for Pt catalysts toward the enhanced performance of n-heptane reforming. Chem. Eng. Sci. 2022, 252, 117286. [Google Scholar] [CrossRef]
- Yusuf, A.Z.; Aderemi, B.O.; Patel, R.; Mujtaba, I.M. Study of industrial naphtha catalytic reforming reactions via modelling and simulation. Processes 2019, 7, 192. [Google Scholar] [CrossRef] [Green Version]
- Martínez, J.; Zúñiga-Hinojosa, M.A.; Ruiz-Martínez, R.S. A Thermodynamic Analysis of Naphtha Catalytic Reforming Reactions to Produce High-Octane Gasoline. Processes 2022, 10, 313. [Google Scholar] [CrossRef]
- Shakor, Z.M.; AbdulRazak, A.A.; Sukkar, K.A. A detailed reaction kinetic model of heavy naphtha reforming. Arab. J. Sci. Eng. 2022, 45, 7361–7370. [Google Scholar] [CrossRef]
- Saab, R.; Polychronopoulou, K.; Anjum, D.H.; Charisiou, N.D.; Goula, M.A.; Hinder, S.J.; Baker, M.A.; Schiffer, A. Effect of SiO2/Al2O3 ratio in Ni/Zeolite-Y and Ni-W/Zeolite-Y catalysts on hydrocracking of heptane. Mol. Catal. 2022, 528, 112484. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Ahmedzeki, N.S.; Al-Tabbakh, B.A.; Antwan, M.B.; Yilmaz, S. Heavy naphtha upgrading by catalytic reforming over novel bi-functional zeolite catalyst. React. Kinet. Mech. Catal. 2018, 125, 1127–1138. [Google Scholar] [CrossRef]
- Tojo, C.; Buceta, D.; López-Quintela, M.A. On metal segregation of bimetallic nanocatalysts prepared by a one-pot method in microemulsions. Catalysts 2017, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Arsentev, S.D.; Minasyan, V.T.; Grigoryan, S.L.; Tavadyan, L.A. Reforming of cyclohexane and n-heptane on nanosized Mo2C and W2C obtained by the carbidization of WO3 and MoO3 activated by vibrationally excited molecules of hydrogen. React. Kinet. Mech. Catal. 2017, 120, 219–230. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Yu, J. Advances in Catalytic Applications of Zeolite-Supported Metal Catalysts. Adv. Mater. 2021, 33, 2104442. [Google Scholar] [CrossRef]
- Tregubenko, V.Y.; Belyi, A.S. Characterization of acid-modified alumina as a support for reforming catalysts. Kinet. Catal. 2020, 61, 130–136. [Google Scholar] [CrossRef]
- Santa Cruz-Navarro, D.; Torres-Rodríguez, M.; Gutiérrez-Arzaluz, M.; Mugica-Álvarez, V.; Pergher, S.B. Comparative study of Cu/ZSM-5 catalysts synthesized by two ion-exchange methods. Crystals 2022, 12, 545. [Google Scholar] [CrossRef]
- Al-Shathr, A.; Shakor, Z.M.; Majdi, H.S.; AbdulRazak, A.A.; Albayati, T.M. Comparison between artificial neural network and rigorous aathematical model in simulation of industrial heavy naphtha reforming process. Catalysts 2021, 11, 1034. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, P.; Liu, C.; Liu, H.; Zhang, W.; Zhang, X. Regulating encapsulation of small Pt nanoparticles inside silicalite-1 zeolite with the aid of sodium ions for enhancing n-hexane reforming. Ind. Eng. Chem. Researc 2022, 61, 9249–9261. [Google Scholar] [CrossRef]
- Xu, D.; Wang, S.; Wu, B.; Zhang, B.; Qin, Y.; Huo, C.; Huang, L.; Wen, X.; Yang, Y.; Li, Y. Highly dispersed single-atom Pt and Pt clusters in the Fe-modified KL zeolite with enhanced selectivity for n-heptane aromatization. ACS Appl. Mater. Interfaces 2019, 11, 29858–29867. [Google Scholar] [CrossRef] [PubMed]
- Sukkar, K.A.; Raouf, S.R.; Hamied, R.S. Investigation and simulation of catalytic reforming reactions of Iraqi heavy naphtha using Pt-Sn/Al2O3 and Pt-Ir/Al2O3 catalysts. Eng. Technol. J. 2013, 33, 2357–2380. [Google Scholar]
- Epron, F.; Carnevillier, C.; Marécot, P. Catalytic properties in n-heptane reforming of Pt–Sn and Pt–Ir–Sn/Al2O3 catalysts prepared by surface redox reaction. Appl. Catal. A Gen. 2005, 295, 157–169. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.; Zhang, J.; Zhang, T.; Ye, M.; Liu, Z. Regeneration of catalysts deactivated by coke deposition: A review. Chin. J. Catal. 2020, 41, 1048–1061. [Google Scholar] [CrossRef]
- Almukhtar, R.; Hammoodi, S.I.; Majdi, H.S.; Sukkar, K.A. Managing Transport Processes in Thermal Cracking to Produce High-Quality Fuel from Extra-Heavy Waste Crude Oil Using a Semi-Batch Reactor. Processes 2022, 10, 2077. [Google Scholar] [CrossRef]
- Demirbas, A.; Balubaid, M.A.; Basahel, A.M.; Ahmad, W.; Sheikh, M.H. Octane rating of gasoline and octane booster additives. Petrol. Sci. Technol. 2015, 33, 1190–1197. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, Y.; Li, Z.; Liu, B.; Hu, C. Preparation, characterization and naphtha aromatization performance of the catalytic reforming catalyst Pt/MY (M = Mg, Ba or Ce). Catal. Today 2020, 353, 146–152. [Google Scholar] [CrossRef]
- Lin, C.; Pan, H.; Yang, Z.; Han, X.; Tian, P.; Li, P.; Xiao, Z.; Xu, J.; Han, Y.F. Effects of cerium doping on Pt–Sn/Al2O3 catalysts for n-heptane reforming. Ind. Eng. Chem. Res. 2020, 59, 6424–6434. [Google Scholar] [CrossRef]
- Dong, X.J.; Shen, J.N.; Ma, Z.F.; He, Y.J. Robust optimal operation of continuous catalytic reforming process under feedstock uncertainty. Int. J. Hydrog. Energy 2022, 47, 35641–35654. [Google Scholar] [CrossRef]
- Králik, M.; Gašparovičová, D.; Turáková, M.; Vallušová, Z.; Balko, J.; Major, P.; Kučera, M.; Puliš, P.; Milkovič, O. Hydrogenation of 4-chloronitrobenzenes over palladium and platinum catalysts supported on beta zeolite and γ-alumina. Chem. Pap. 2019, 73, 397–414. [Google Scholar] [CrossRef]
- Bowker, M.; Aslam, T.; Roebuck, M.; Moser, M. The effect of coke lay-down on n-heptane reforming on Pt and Pt-Sn catalysts. Appl. Catal. A Gen. 2004, 257, 57–65. [Google Scholar] [CrossRef]
- Sukkar, K.A.; Abd Al-Raheem, H.M.; Sabry, L.S.; Resym, L.A. New Development in Catalytic Reforming Process to Produce High Octane Gasoline. J. Pet. Res. Stud. 2014, 5, 223–244. [Google Scholar] [CrossRef]
- Zainullin, R.Z.; Koledina, K.F.; Akhmetov, A.F.; Gubaidullin, I.M. Kinetics of the catalytic reforming of gasoline. Kinet. Catal. 2017, 58, 279–289. [Google Scholar] [CrossRef]
- Said-Aizpuru, O.; Batista, A.T.; Bouchy, C.; Petrazzuoli, V.; Allain, F.; Diehl, F.; Farrusseng, D.; Morfin, F.; Joly, J.F.; Dandeu, A. Non monotonous product distribution dependence on Pt/γ-Al2O3-Cl catalysts formulation in n-heptane reforming. Chem. Cat. Chem. 2020, 12, 2262–2270. [Google Scholar]
- Viswanadham, N.; Saxena, S.K.; Panwar, R.; Ray, A. A single-step catalytic process for the production of high-octane molecules from normal paraffins using zeolite supported bi-functional catalysts. Chem. Eng. Process.-Process Intensif. 2022, 177, 108990. [Google Scholar] [CrossRef]
- Hanafi, S.A.; Gobara, H.M.; Elmelawy, M.S.; Abo-El-Enein, S.A.; Alkahlawy, A.A. Catalytic performance of dealuminated H–Y zeolite supported bimetallic nanocatalysts in Hydroizomerization of n-hexane and n-heptane. Egypt. J. Pet. 2014, 23, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Cerón, K.M.; Arias-Madrid, D.; Gallego, J.; Medina, O.E.; Chinchilla, L.E.; Cortés, F.B.; Franco, C.A. Catalytic Decomposition of n-C7 asphaltenes using tungsten oxides–functionalized SiO2 nanoparticles in steam/air atmospheres. Processes 2022, 10, 349. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Yu, Q.; Chen, Y.; Chai, Z.; Zhao, G.; Liu, S.; Cheong, W.C.; Pan, Y.; Zhang, Q.; et al. A general strategy for fabricating isolated single metal atomic site catalysts in Y zeolite. J. Am. Chem. Soc. 2019, 141, 9305–9311. [Google Scholar] [CrossRef]
- Tregubenko, V.Y.; Vinichenko, N.V.; Talzi, V.P.; Belyi, A.S. Catalytic properties of the platinum catalyst supported on alumina modified by oxalic acid in n-heptane reforming. Russ. Chem. Bull. 2020, 69, 1719–1723. [Google Scholar] [CrossRef]
- Xu, D.; Wu, B.; Ren, P.; Wang, S.; Huo, C.; Zhang, B.; Guo, W.; Huang, L.; Wen, X.; Qin, Y.; et al. Controllable deposition of Pt nanoparticles into a KL zeolite by atomic layer deposition for highly efficient reforming of n-heptane to aromatics. Catal. Sci. Technol. 2017, 7, 1342–1350. [Google Scholar] [CrossRef]
- Keshavarz, A.; Salabat, A. An Efficient Strategy in Microemulsion Systems to Prepare Mono-and Bimetallic Platinum-Rhenium Reforming Nanocatalyst with Remarkable Catalytic Performance. Chem. Sel. 2019, 4, 6094–6100. [Google Scholar] [CrossRef]
- Kianpoor, Z.; Falamaki, C.; Parvizi, M.R. Exceptional catalytic performance of Au–Pt/γ-Al2O3 in naphtha reforming at very low Au dosing levels. React. Kinet. Mech. Catal. 2019, 128, 427–441. [Google Scholar] [CrossRef]
- Sukkar, K.A. Evaluation of transport processes in a catalytic reforming reactor with high performance nanocatalysts. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1067, 012149. [Google Scholar] [CrossRef]
- Yan, M.; Xu, D.; Wang, S.; Wu, B.; Yang, Y.; Li, Y. Selective regulation of Pt clusters inside KY zeolite using atomic layer deposition for n-octane reforming. Fuel 2022, 330, 125671. [Google Scholar] [CrossRef]
- Daligaux, V.; Richard, R.; Manero, M.H. Deactivation and regeneration of zeolite catalysts used in pyrolysis of plastic wastes—A process and analytical review. Catalysts 2021, 11, 770. [Google Scholar] [CrossRef]
- Lutz, W. Zeolite Y: Synthesis, modification, and properties—A case revisited. Adv. Mater. Sci. Eng. 2014, 2014, 724248. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.K.; Lee, S.; Na, K. An overview on metal-related catalysts: Metal oxides, nanoporous metals and supported metal nanoparticles on metal organic frameworks and zeolites. Rare Met. 2020, 39, 751–766. [Google Scholar] [CrossRef]
- Ren, X.Y.; Cao, J.P.; Zhao, S.X.; Zhao, X.Y.; Feng, X.B.; Liu, T.L.; Li, Y.; Zhang, J.; Wei, X.Y. Insights into coke location of catalyst deactivation during in-situ catalytic reforming of lignite pyrolysis volatiles over cobalt-modified zeolites. Appl. Catal. A Gen. 2021, 613, 118018. [Google Scholar] [CrossRef]
- Talaghat, M.R.; Karimi, M.S. Operation parameters effect on yield and octane number for monometallic, bimetallic and trimetallic catalysts in naphtha reforming process. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 176–193. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, Q.; Qin, Z.; Wu, Z.; Jiao, W.; Dong, M.; Fan, W.; Wang, J. Promoting effect of alkali metal on the catalytic performance of hierarchical Pt/Beta in the aromatization of n-heptane. Microporous Mesoporous Mater. 2022, 343, 112189. [Google Scholar] [CrossRef]
- Rabe, S.; Vogel, F.; Truong, T.B.; Shimazu, T.; Wakasugi, T.; Aoki, H.; Sobukawa, H. Catalytic reforming of gasoline to hydrogen: Kinetic investigation of deactivation processes. Int. J. Hydrog. Energy 2009, 34, 8023–8033. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, W.; Jiang, A.; Huang, Q.Y.; Wang, H.; Ding, Q. Dynamic Simulation of Continuous Catalytic Reforming Process Based on Simultaneous Solution. Processes 2021, 9, 1347. [Google Scholar] [CrossRef]
- Esmaeili, E.; Rashidi, A.M.; Khodadadi, A.A.; Mortazavi, Y.; Rashidzadeh, M. Palladium–Tin nanocatalysts in high concentration acetylene hydrogenation: A novel deactivation mechanism. Fuel Process. Technol. 2014, 120, 113–122. [Google Scholar] [CrossRef]
- Crouzier, L.; Feltin, N.; Delvallée, A.; Pellegrino, F.; Maurino, V.; Cios, G.; Tokarski, T.; Salzmann, C.; Deumer, J.; Gollwitzer, C.; et al. Correlative analysis of the dimensional properties of bipyramidal titania nanoparticles by complementing electron microscopy with other methods. Nanomaterials 2021, 11, 3359. [Google Scholar] [CrossRef] [PubMed]
- Tavares, F.; Mohamed, H.O.; Kulkarni, S.R.; Morlanés, N.; Castaño, P. Decreasing the coking and deactivation of a reforming Ni-Ce/Al2O3 catalyst with intraparticle SiC in hydrogen production routes. Fuel 2023, 337, 127058. [Google Scholar] [CrossRef]










| Parameter | Uncertainty (%) |
|---|---|
| Total WHSV | ±0.04 |
| Temperature (°C) | ±0.2 |
| Pressure (bar) | ±1.4 |
| Hydrogen Flow Rate (cm3/s) | ±0.02 |
| n-Heptane Flow Rate (cm3/s) | ±0.01 |
| Operating Parameters | Value |
|---|---|
| Temperature range (°C) | 425, 450, 475, 500, and 525 |
| Pressure (bar) | 1.0 |
| Total WHSV | 4.0 |
| Hydrogen/n-Heptane ratio | 4.0 |
| Compound | Weight% |
|---|---|
| SiO2 | 56.47 |
| Al2O3 | 19.08 |
| Na2O | 24.10 |
| CaO | 0.210 |
| MgO | 0.083 |
| Fe2O3 | 0.057 |
| (Si/Al) ratio = 2.960 |
| Property | Pt/HY | Pt-Zn/HY | Pt-Rh/HY |
|---|---|---|---|
| Metal Load Wt. (%) | 0.3 Pt | 0.3% Pt and 0.31% Zn | 0.3 % Pt and 0.32 % Rh |
| Surface Area (m2/g) | 608 | 584 | 593 |
| Pore Volume (cm3/g) | 0.420 | 0.405 | 0.413 |
| Authors | Catalyst Type | Operating Temperture | Catalyst Selectivity% |
|---|---|---|---|
| Bowker et al. [26] | Pt–Sn/γ-Al2O3 Catalyst | 400 °C | 64.2 (Conv.) |
| Said-Aizpuru et al. [29] | Pt/γ-Al2O3-Cl Catalyst | 410 °C | 68.0 |
| Xu et al. [35] | Pt/KL Zeolite Nanocatalyst | 420 °C | 84.3 |
| Sukkar [38] | Pt-Ge/HY Nanocatalyst | 480 °C | 95.0 |
| Shi et al. [45] | Pt/Beta Zeolite-Rb Catalyst | 450 °C | 78.2 |
| Present Work | Pt-Zn/HY Nanocatalyst | 500 °C | 97.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamied, R.S.; Sukkar, K.A.; Majdi, H.S.; Shnain, Z.Y.; Graish, M.S.; Mahmood, L.H. Catalytic-Level Identification of Prepared Pt/HY, Pt-Zn/HY, and Pt-Rh/HY Nanocatalysts on the Reforming Reactions of N-Heptane. Processes 2023, 11, 270. https://doi.org/10.3390/pr11010270
Hamied RS, Sukkar KA, Majdi HS, Shnain ZY, Graish MS, Mahmood LH. Catalytic-Level Identification of Prepared Pt/HY, Pt-Zn/HY, and Pt-Rh/HY Nanocatalysts on the Reforming Reactions of N-Heptane. Processes. 2023; 11(1):270. https://doi.org/10.3390/pr11010270
Chicago/Turabian StyleHamied, Ramzy S., Khalid A. Sukkar, Hasan Shakir Majdi, Zainb Y. Shnain, Mohammed Shorbaz Graish, and Luma H. Mahmood. 2023. "Catalytic-Level Identification of Prepared Pt/HY, Pt-Zn/HY, and Pt-Rh/HY Nanocatalysts on the Reforming Reactions of N-Heptane" Processes 11, no. 1: 270. https://doi.org/10.3390/pr11010270
APA StyleHamied, R. S., Sukkar, K. A., Majdi, H. S., Shnain, Z. Y., Graish, M. S., & Mahmood, L. H. (2023). Catalytic-Level Identification of Prepared Pt/HY, Pt-Zn/HY, and Pt-Rh/HY Nanocatalysts on the Reforming Reactions of N-Heptane. Processes, 11(1), 270. https://doi.org/10.3390/pr11010270