Construction of a Novel Oxalic Acid Biosensor Based on the Combination of Tissue Enzyme and Peroxide Mimic Enzyme
Abstract
:1. Introduction
2. Fundamental Characteristics of Biosensors
3. Design and Performance Evaluation of Oxalic Acid Biosensor
3.1. Apparatus
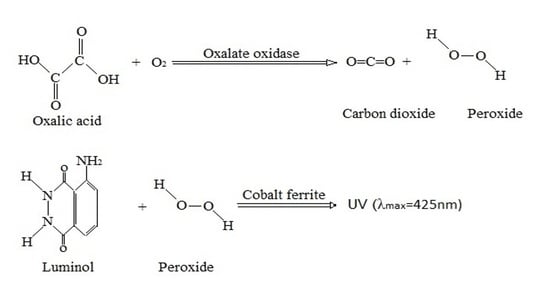
3.2. Sensor Identification Mechanisms
3.3. Influencing and Optimization Conditions of Biosensor
3.3.1. Biosensor Recognition Conditions
3.3.2. Illumination Conditions
4. Biosensors’ Limitations and Outlooks
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alizadeh, T.; Nayeri, S.; Hamidi, N. Graphitic Carbon Nitride (g-C3N4)/Graphite Nanocomposite as an Extraordinarily Sensitive Sensor for Sub-Micromolar Detection of Oxalic Acid in Biological Samples. RSC Adv. 2019, 9, 13096–13103. [Google Scholar] [CrossRef] [PubMed]
- Arguello, J.; Magosso, H.A.; Ramos, R.R.; Canevari, T.C.; Landers, R.; Pimentel, V.L.; Gushikem, Y. Structural and Electrochemical Characterization of a Cobalt Phthalocyanine Bulk-Modified SiO2/SnO2 Carbon Ceramic Electrode. Electrochim. Acta 2009, 54, 1948–1953. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Bytesnikova, Z.; Barek, J.; Richtera, L.; Adam, V. A Critical Comparison of Natural Enzymes and Nanozymes in Biosensing and Bioassays. Biosens. Bioelectron. 2021, 192, 113494. [Google Scholar] [CrossRef] [PubMed]
- Basharzad, P.F.; Farhadi, K.; Forough, M.; Molaei, R. Silver Nanoparticles as a New Colorimetric Probe for Determination of Oxalic Acid in Urine. Sens. Lett. 2016, 14, 906–912. [Google Scholar] [CrossRef]
- Boss, E.; Pegau, W.S. Relationship of Light Scattering at an Angle in the Backward Direction to the Backscattering Coefficient. Appl. Opt. 2001, 40, 5503–5507. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.R.; Frasco, M.F.; Serrano, V.; Fortunato, E.; Sales, M.G.F. Molecular Imprinting on Nanozymes for Sensing Applications. Biosensors 2021, 11, 152. [Google Scholar] [CrossRef]
- Chaibakhsh, N.; Moradi-Shoeili, Z. Enzyme Mimetic Activities of Spinel Substituted Nanoferrites (MFe2O4): A Review of Synthesis, Mechanism and Potential Applications. Mater. Sci. Eng. C 2019, 99, 1424–1447. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, S.; Bao, J.; Ju, H. Nanostruetured FeS as a Mimic Peroxidase for Biocatalysis and Biosensing. Chem. A Eur. J. 2009, 15, 4321–4326. [Google Scholar] [CrossRef]
- Fan, J.; Yin, J.-J.; Ning, B.; Wu, X.; Hu, Y.; Ferrari, M.; Anderson, G.J.; Wei, J.; Zhao, Y.; Nie, G. Direct Evidence for Catalase and Peroxidase Activities of Ferritin-Platinum Nanoparticles. Biomaterials 2011, 32, 1611–1618. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, X.; Guo, X.; Cui, B.; Wang, L. Simple and Ultrasensitive Electrochemical Sensor for Oxalic Acid Detection in Real Samples by One Step Co-Electrodeposition Strategy. Anal. Bioanal. Chem. 2020, 412, 5719–5727. [Google Scholar] [CrossRef]
- Fang, Y.; Cai, R.; Deng, J.; Deng, Z. Lettuce seed meal tissue-based membrane electrode with high biocatalytic activity for hydrogen peroxide. Electroanalysis 2004, 4, 819–822. [Google Scholar] [CrossRef]
- Fiorito, P.A.; de Torresi, S.I.C. Optimized Multilayer Oxalate Biosensor. Talanta 2004, 62, 649–654. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.O.; Singh, B. Electrochemical Biosensors for Detection of Pesticides and Heavy Metal Toxicants in Water: Recent Trends and Progress. ACS Environ. Sci. Technol. Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Hong, F.; Nilvebrant, N.-O.; Jönsson, L.J. Rapid and Convenient Determination of Oxalic Acid Employing a Novel Oxalate Biosensor Based on Oxalate Oxidase and SIRE Technology. Biosens. Bioelectron. 2003, 18, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Income, K.; Ratnarathorn, N.; Themsirimongkon, S.; Dungchai, W. An Oxalic Acid Sensor Based on Platinum/Carbon Black-Nickelreduced Graphene Oxide Nanocomposites Modified Screenprinted Carbon Electrode. J. Electrochem. Sci. Technol. 2019, 10, 416–423. [Google Scholar] [CrossRef]
- Joshi, N.; Rawat, K.; Solanki, P.R.; Bohidar, H. Biocompatible Laponite Ionogels Based Non-Enzymatic Oxalic Acid Sensor. Sens. Bio-Sens. Res. 2015, 5, 105–111. [Google Scholar] [CrossRef]
- Kaur, H.; Bhosale, A.; Shrivastav, S. Biosensors: Classification, Fundamental Characterization and New Trends: A Review. Int. J. Health Sci. Res. 2018, 8, 313–333. Available online: www.ijhsr.org (accessed on 10 April 2023).
- Lathika, K.M.; Sharma, S.; Inamdar, K.V.; Raghavan, K.G. Oxalate Depletion from Leafy Vegetables Using Alginate Entrapped Banana Oxalate Oxidase. Biotechnol. Lett. 1995, 17, 407–410. [Google Scholar] [CrossRef]
- Law, A.L.; Abdulazeez, T.; Samuel, B.A. Progress and Recent Advances in Phosphate Sensors: A Review. Talanta 2013, 114, 191–203. [Google Scholar] [CrossRef]
- Malik, P.; Katyal, V.; Malik, V.; Asatkar, A.; Inwati, G.; Mukherjee, T.K. Nanobiosensors: Concepts and Variations. ISRN Nanomaterials 2013, 2013, 327435. [Google Scholar] [CrossRef]
- Marinescu, L.G.; Bols, M. Very High Rate Enhancement of Benzyl Alcohol Oxidation by an Artificial Enzyme. Angew. Chem. 2006, 118, 4706–4709. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Gutiérrez-Sánchez, C.; García-Mendiola, T.; Lorenzo, E. Electrochemiluminescence Biosensors Using Screen-Printed Electrodes. Biosensors 2020, 10, 118. [Google Scholar] [CrossRef]
- Meunier, B. Metalloporphyrins as Versatile Catalysts for Oxidation Reactions and Oxidative DNA Cleavage. Chem. Rev. 1992, 92, 1411–1456. [Google Scholar] [CrossRef]
- Milardović, S.; Grabarić, Z.; Tkalčec, M.; Rumenjak, V. Determination of Oxalate in Urine, Using an Amperometric Biosensor with Oxalate Oxidase Immobilized on the Surface of a Chromium Hexacyanoferrate-Modified Graphite Electrode. J. AOAC Int. 2000, 83, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Nejadmansouri, M.; Majdinasab, M.; Nunes, G.S.; Marty, J.L. An Overview of Optical and Electrochemical Sensors and Biosensors for Analysis of Antioxidants in Food during the Last 5 Years. Sensors 2021, 21, 1176. [Google Scholar] [CrossRef]
- Nelson, E.M. Scientific Developments Lead To New Control Problems. Nutr. Rev. 1956, 14, 97–98. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Wei, Q.; Zhu, J.Z. Plant Tissue-Based Chemiluminescence Flow Biosensor for the Determination of Oxalate. Kao Teng Hsueh Hsiao Hua Heush Hsueh Pao/Chem. J. Chin. Univ. 2001, 22, 217. [Google Scholar]
- Piletsky, S.A.; Piletska, E.V.; Chen, B.; Karim, K.; Weston, D.; Barrett, G.; Lowe, P.; Turner, A.P.F. Chemical Grafting of Molecularly Imprinted Homopolymers to the Surface of Microplates. Application of Artificial Adrenergic Receptor in Enzyme-Linked Assay for β-Agonists Determination. Anal. Chem. 2000, 72, 4381–4385. [Google Scholar] [CrossRef]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.S.; Seal, S.; Self, W.T. Nanoceria Exhibit Redox State-Dependent Catalase Mimetic Activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef]
- Preda, G.; Bizerea, O.; Vlad-Oros, B. Sol-Gel Technology in Enzymatic Electrochemical Biosensors for Clinical Analysis. Biosens. Health Environ. Biosecurity 2011, 363–368. [Google Scholar] [CrossRef]
- Razzino, C.A.; Serafín, V.; Gamella, M.; Pedrero, M.; Montero-Calle, A.; Barderas, R.; Calero, M.; Lobo, A.O.; Yáñez-Sedeño, P.; Campuzano, S.; et al. An Electrochemical Immunosensor Using Gold Nanoparticles-PAMAM-Nanostructured Screen-Printed Carbon Electrodes for Tau Protein Determination in Plasma and Brain Tissues from Alzheimer Patients. Biosens. Bioelectron. 2020, 163, 112238. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Hernandez, P.; Salazar, V.; Castrillejo, Y.; Barrado, E. Amperometric Biosensor for Oxalate Determination in Urine Using Sequential Injection Analysis. Molecules 2012, 17, 8859–8871. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Zhao, F.; Zeng, B. Electrodeposition of PdAu Alloy Nanoparticles on Ionic Liquid Functionalized Graphene Film for the Voltammetric Determination of Oxalic Acid. Electroanalysis 2013, 25, 453–459. [Google Scholar] [CrossRef]
- Shimohigoshi, M.; Karube, I. Development of Uric Acid and Oxalic Acid Sensors Using a Bio-Thermochip. Sens. Actuators B Chem. 1996, 30, 17–21. [Google Scholar] [CrossRef]
- Su, L.; Jia, W.; Hou, C.; Lei, Y. Microbial Biosensors: A Review. Biosens. Bioelectron. 2010, 26, 1788–1799. [Google Scholar] [CrossRef]
- Thakur, M.; Bhargava, A.; Pundir, C. Determination of Urinary Oxalate with Arylamine Glass-Bound Sorghum Oxalate Oxidase and Horseradish Peroxidase. Int. J. Appl. Sci. Biotechnol. 2016, 4, 346–351. [Google Scholar] [CrossRef]
- Topçu, S.; Sezgintürk, M.K.; Dinçkaya, E. Evaluation of a New Biosensor-Based Mushroom (Agaricus Bisporus) Tissue Homogenate: Investigation of Certain Phenolic Compounds and Some Inhibitor Effects. Biosens. Bioelectron. 2004, 20, 592–597. [Google Scholar] [CrossRef]
- Wang, A.J.; Rechnitz, G.A. Prototype Transgenic Biosensor Based on Genetically Modified Plant Tissue. Anal. Chem. 1993, 65, 3067–3070. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Y.; You, Z.; Sha, H.; Gong, S.; Liu, J.; Sun, W. Sensitive Electrochemical Determination of Oxalic Acid in Spinach Samples by a Graphene-Modified Carbon Ionic Liquid Electrode. Ionics 2015, 21, 877–884. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Fe3O4 Magnetic Nanoparticles as Peroxidase Mimetics and Their Applications in H2O2 and Glucose Detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.A.; Liu, Y.; Allende, S.; Jacob, M.V. Electrochemical Sensing of Oxalic Acid Using Silver Nanoparticles Loaded Nitrogen-Doped Graphene Oxide. Carbon Trends 2022, 8, 100188. [Google Scholar] [CrossRef]
- Zeng, W.; Li, J.; Mao, Z.; Hong, Z.; Qin, S. Synthesis, Oxygenation and Catalytic Oxidation Performance of Crown Ether-Containing Schiff Base-Transition Metal Complexes. Adv. Synth. Catal. 2004, 346, 1385–1391. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, X.; Liu, J.; Yin, J.-J.; Hou, S.; Wen, T.; He, W.; Ji, Y.; Guo, Y.; Wang, Q.; et al. Formation of PdPt Alloy Nanodots on Gold Nanorods: Tuning Oxidase-like Activities via Composition. Langmuir 2011, 27, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, C.; Zhang, J.; Guo, S. Mass Transport Effect on Graphene Based Enzyme Electrochemical Biosensor for Oxalic Acid Detection. J. Electrochem. Soc. 2017, 164, B29–B33. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.; Zhu, G. A Novel Renewable Plant Tissue-Based Electrochemiluminescent Biosensor for Glycolic Acid. Sens. Actuators B Chem. 2004, 98, 115–121. [Google Scholar] [CrossRef]




| Oxalate Oxidase Source | Buffer Solution | Binding Support | Low Detection Limit | Linear Range | Optimization Conditions of Biosensor | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Element | pH | Concentration | pH | Temp °C | Concentration | |||||
| Barley seedlings | Succinic acid | 4.0 | 50 mM | Sensors based on injection of the recognition element | 20 μM | 0–5 mM | 5.0 | 45 | 25 mM | [30] |
| Barley roots | Succinate buffer | 3.5 | 20 mM | Graphene | 8 μM | 10–300 mV/s | ND | ND | 1 mM | [25] |
| Barley seedlings, lyophilized powder, bovine serum albumin and glutaraldehyde | Succinic buffer | 3.8 | 14 mM | Chromium (III) hexacyanoferrate-modified graphite electrode | <1 µM | 2.5–100 mM | ND | ND | ND | [23] |
| Potassium hydroxide | 3.8 | 4 mM | ||||||||
| Potassium chloride | 3.8 | 0.1 M | ||||||||
| EDTA | 3.8 | 5.4 mM | ||||||||
| ND | Acetate buffer solution | 4.0 | 0.1 M | Electrode modified with Fe (III)-tris(2-thiopyridone)borate complex as mediator coupled with injection of magnetic solid | 1.0 mg·L−1 | ND | ND | ND | ND | [31] |
| Barley seedlings | Succinic buffer | 3.6 | ND | Ruthenium, nickel and iron hexacyanometallate modified graphite electrode | ND | 100 μM | 3.6 | ND | 70 μM | [32] |
| ND | ND | ND | ND | Silver nanoparticles | 3.3 μM | 10–40 μM | ND | ND | ND | [33] |
| Prussian Blue, Barley seeding and self-doped polyamiline film | Succinate buffer solution | 3.8 | ND | GC electrode modified with polyprrolel hexacyano metalate | 0.08 mmol L−1 | 0.08 to 0.45 mmol L−1 | 3.8 | 35 | ND | [34] |
| Sorghum leaf | Sodium phosphate buffer | 7.0 | 0.05 M | 4-aminophnazone, phenol and immobilized peroxidase as chromogen | 0.05 mmol L−1 | 0.10–1.0 mM | 5.5 | 45 | ND | [35] |
| Spinach leaves | Sulfuric acid | ND | 0.2 M | PdAu alloy nanoparticles on ionic liquid-functionalized graphene film | 2.7 μM | 5–100 μM | ND | ND | ND | [36] |
| ND | ND | ND | ND | Cobalt phthalocyanine bulk–modified carbon ceramic composite | 7.1 × 10−6 mol L−1. | 1.6 × 10−5 –1.5 × 10−3 mol L−1 | ND | MD | ND | [37] |
| ND | Ammonium acetate | 4.5 | 50.0 mmol L−1 | Nanosized graphitic carbon nitride | 7.5 ×107 M | 1–1000 μM | ND | ND | ND | [38] |
| Spinach and tomatoes | ND | ND | ND | Platinum nanoparticles | 25 nM | 0–45 μM | ND | ND | ND | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giwa, A.S.; Maurice, N.J.; Ali, N. Construction of a Novel Oxalic Acid Biosensor Based on the Combination of Tissue Enzyme and Peroxide Mimic Enzyme. Processes 2023, 11, 3012. https://doi.org/10.3390/pr11103012
Giwa AS, Maurice NJ, Ali N. Construction of a Novel Oxalic Acid Biosensor Based on the Combination of Tissue Enzyme and Peroxide Mimic Enzyme. Processes. 2023; 11(10):3012. https://doi.org/10.3390/pr11103012
Chicago/Turabian StyleGiwa, Abdulmoseen Segun, Ndungutse Jean Maurice, and Nasir Ali. 2023. "Construction of a Novel Oxalic Acid Biosensor Based on the Combination of Tissue Enzyme and Peroxide Mimic Enzyme" Processes 11, no. 10: 3012. https://doi.org/10.3390/pr11103012
APA StyleGiwa, A. S., Maurice, N. J., & Ali, N. (2023). Construction of a Novel Oxalic Acid Biosensor Based on the Combination of Tissue Enzyme and Peroxide Mimic Enzyme. Processes, 11(10), 3012. https://doi.org/10.3390/pr11103012