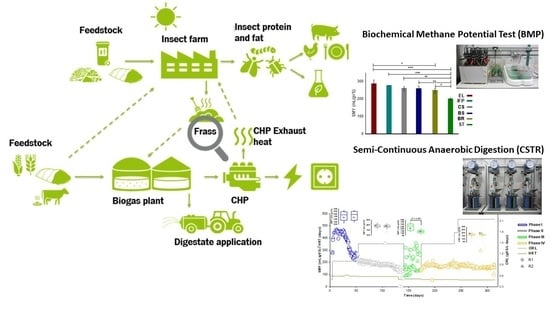
Biogas Production from Residues of Industrial Insect Protein Production from Black Soldier Fly Larvae Hermetia illucens (L.): An Evaluation of Different Insect Frass Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acquisition and Characterization of the Insect Frass Samples
2.2. Analytical Methods
2.3. Anaerobic Digestion Trials
2.3.1. Biochemical Methane Potential (BMP) Test
2.3.2. Semi-Continuous Anaerobic Digestion Tests
2.4. Kinetic Evaluation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Insect Frass Characteristics
3.2. Effect of the Six Different Insect Frass Samples on Specific Methane Yield (SMY) from the BMP Test
3.3. Effects of the Different Insect Frass Samples on Estimated Model Parameters
3.4. Results of the Semi-Continuous Anaerobic Digestion Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Future of Food and Agriculture: Alternative Pathways to 2050; FAO: Rome, Italy, 2018; ISBN 978-92-5-130989-6.
- van Huis, A.; Tomberlin, J.K. (Eds.) Insects As Food and Feed: From Production to Consumption; Wageningen Academic Publishers: Wageningen, The Netherlands, 2017; ISBN 978-90-8686-296-2. [Google Scholar]
- Stamer, A. Insect proteins—A new source for animal feed: The use of insect larvae to recycle food waste in high-quality protein for livestock and aquaculture feeds is held back largely owing to regulatory hurdles. EMBO Rep. 2015, 16, 676–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottoboni, M.; Spranghers, T.; Pinotti, L.; Baldi, A.; de Jaeghere, W.; Eeckhout, M. Inclusion of Hermetia illucens larvae or prepupae in an experimental extruded feed: Process optimisation and impact on in vitro digestibility. Ital. J. Anim. Sci. 2017, 17, 418–427. [Google Scholar] [CrossRef] [Green Version]
- Kroeckel, S.; Harjes, A.-G.E.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364–365, 345–352. [Google Scholar] [CrossRef]
- Biasato, I.; Chemello, G.; Oddon, S.B.; Ferrocino, I.; Corvaglia, M.R.; Caimi, C.; Resconi, A.; Paul, A.; van Spankeren, M.; Capucchio, M.T.; et al. Hermetia illucens meal inclusion in low-fishmeal diets for rainbow trout (Oncorhynchus mykiss): Effects on the growth performance, nutrient digestibility coefficients, selected gut health traits, and health status indices. Anim. Feed Sci. Technol. 2022, 290, 115341. [Google Scholar] [CrossRef]
- Heines, W.; Ristic, D.; Rosenberger, S.; Coudron, C.; Gai, F.; Schiavone, A.; Smetana, S. Eggs or meat?: Environmental impact and efficiency assessment of chicken protein production with potential of Hermetia illucens use in feed. Resour. Conserv. Recycl. Adv. 2022, 16, 200121. [Google Scholar] [CrossRef]
- Ipema, A.F.; Gerrits, W.J.J.; Bokkers, E.A.M.; Kemp, B.; Bolhuis, J.E. Live black soldier fly larvae (Hermetia illucens) provisioning is a promising environmental enrichment for pigs as indicated by feed- and enrichment-preference tests. Appl. Anim. Behav. Sci. 2021, 244, 105481. [Google Scholar] [CrossRef]
- Khaemba, C.N.; Kidoido, M.M.; Owuor, G.; Tanga, C.M. Consumers’ perception towards eggs from laying hens fed commercial black soldier fly (Hermetia illucens) larvae meal-based feeds. Poult. Sci. 2022, 101, 101645. [Google Scholar] [CrossRef]
- de Jong, B.; Nikolik, G. No Longer Crawling: Insect Protein to Come of Age in the 2020s. Rabo Bank Research. Available online: https://insectfeed.nl/wp-content/uploads/2021/03/Rabobank_No-Longer-Crawling-Insect-Protein-to-Come-of-Age-in-the-2020s_Feb2021-1.pdf (accessed on 11 December 2022).
- Almeida, C.; Murta, D.; Nunes, R.; Baby, A.R.; Fernandes, Â.; Barros, L.; Rijo, P.; Rosado, C. Characterization of lipid extracts from the Hermetia illucens larvae and their bioactivities for potential use as pharmaceutical and cosmetic ingredients. Heliyon 2022, 8, e09455. [Google Scholar] [CrossRef]
- Elsayed, M.; Li, W.; Abdalla, N.S.; Ai, P.; Zhang, Y.; Abomohra, A.E.-F. Innovative approach for rapeseed straw recycling using black solider fly larvae: Towards enhanced energy recovery. Renew. Energy 2022, 188, 211–222. [Google Scholar] [CrossRef]
- Ites, S.; Smetana, S.; Toepfl, S.; Heinz, V. Modularity of insect production and processing as a path to efficient and sustainable food waste treatment. J. Clean. Prod. 2020, 248, 119248. [Google Scholar] [CrossRef]
- Lopes, I.G.; Yong, J.W.; Lalander, C. Frass derived from black soldier fly larvae treatment of biodegradable wastes. A critical review and future perspectives. Waste Manag. 2022, 142, 65–76. [Google Scholar] [CrossRef]
- Seruga, P.; Krzywonos, M.; Paluszak, Z.; Urbanowska, A.; Pawlak-Kruczek, H.; Niedźwiecki, Ł.; Pińkowska, H. Pathogen Reduction Potential in Anaerobic Digestion of Organic Fraction of Municipal Solid Waste and Food Waste. Molecules 2020, 25, 275. [Google Scholar] [CrossRef] [Green Version]
- Hupfauf, S.; Winkler, A.; Wagner, A.O.; Podmirseg, S.M.; Insam, H. Biomethanation at 45 °C offers high process efficiency and supports hygienisation. Bioresour. Technol. 2020, 300, 122671. [Google Scholar] [CrossRef]
- Wöss, D.; Ortner, M.; Mensik, J.; Kirchmayr, R.; Schumergruber, A.; Pröll, T. Implementation and long term experiences with a continuous hygienisation process in food industry—A case study. Chem. Eng. Process. Process Intensif. 2019, 137, 100–107. [Google Scholar] [CrossRef]
- Levantesi, C.; Beimfohr, C.; Blanch, A.R.; Carducci, A.; Gianico, A.; Lucena, F.; Tomei, M.C.; Mininni, G. Hygienization performances of innovative sludge treatment solutions to assure safe land spreading. Environ. Sci. Pollut. Res. Int. 2015, 22, 7237–7247. [Google Scholar] [CrossRef] [Green Version]
- Pas, C.; Brodeur, D.; Deschamps, M.-H.; Lebeuf, Y.; Adjalle, K.; Barnabé, S.; Eeckhout, M.; Vandenberg, G.; Vaneeckhaute, C. Valorization of pretreated biogas digestate with black soldier fly (Hermetia illucens, L.; Diptera: Stratiomyidae) larvae. J. Environ. Manag. 2022, 319, 115529. [Google Scholar] [CrossRef]
- DIN 12880:2000; Characterization of Sludges—Determination of Dry Residue and Water Content. Deutsches Institut für Normung e. V.: Berlin, Germany, 2001.
- DIN Standard EN 12879; Characterization of Sludges—Determination of the Loss on Ignition of Dry Mass. Deutsches Institut für Normung e. V.: Berlin, Germany, 2001.
- Liebetrau, J.; Pfeiffer, D.; Thrän, D. (Eds.) Energetische Biomassenutzung: Messmethodensammlung Biogas: Methoden zur Bestimmung von Analytischen und Prozessbeschreibenden Parametern im Biogasbereich; Fischer Druck: Leipzig, Germany, 2015. [Google Scholar]
- VDI Guideline 4630; Fermentation of Organic Materials—Characterization of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. The Association of German Engineers: Berlin, Germany, 2016.
- DIN 1343:1990-01; Reference Conditions, Normal Conditions, Normal Volume; Concepts and Values. Deutsches Institut für Normung e. V.: Berlin, Germany, 1990.
- Kimura, D. Likelihood methods for the von Bertalanffy growth curve. Fish. Bull. 1980, 77, 765–776. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 5 January 2022).
- FNR. Leitfaden Biogas: Von der Gewinnung zur Nutzung, 7th ed.; Druckerei Weidner: Rostock, Germany, 2016; ISBN 3-00-014333-5. [Google Scholar]
- Bulak, P.; Proc, K.; Pawłowska, M.; Kasprzycka, A.; Berus, W.; Bieganowski, A. Biogas generation from insects breeding post production wastes. J. Clean. Prod. 2020, 244, 118777. [Google Scholar] [CrossRef]
- McEniry, J.; O’Kiely, P. Anaerobic methane production from five common grassland species at sequential stages of maturity. Bioresour. Technol. 2013, 127, 143–150. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Abduh, M.Y.; Perdana, M.P.; Bara, M.A.; Anggraeni, L.W.; Putra, R.E. Effects of aeration rate and feed on growth, productivity and nutrient composition of black soldier fly (Hermetia illucens L.) larvae. J. Asia-Pac. Entomol. 2022, 25, 101902. [Google Scholar] [CrossRef]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Weinrich, S.; Murphy, J.D. Value of Batch Tests for Biogas Potential Analysis: Method Comparison and Challenges of Substrate and Efficiency Evaluation of Biogas Plants; IEA Bioenergy: Dublin, Ireland, 2018; ISBN 978-1-910154-49-6. [Google Scholar]
- Ruffino, B.; Fiore, S.; Roati, C.; Campo, G.; Novarino, D.; Zanetti, M. Scale effect of anaerobic digestion tests in fed-batch and semi-continuous mode for the technical and economic feasibility of a full scale digester. Bioresour. Technol. 2015, 182, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Su, H.; Tan, T. Batch and semi-continuous anaerobic digestion of food waste in a dual solid-liquid system. Bioresour. Technol. 2013, 145, 10–16. [Google Scholar] [CrossRef]
- Holliger, C.; Fruteau de Laclos, H.; Hack, G. Methane Production of Full-Scale Anaerobic Digestion Plants Calculated from Substrate’s Biomethane Potentials Compares Well with the One Measured On-Site. Front. Energy Res. 2017, 5, 927. [Google Scholar] [CrossRef] [Green Version]
- Stiborova, H.; Wolfram, J.; Demnerova, K.; Macek, T.; Uhlik, O. Bacterial community structure in treated sewage sludge with mesophilic and thermophilic anaerobic digestion. Folia Microbiol. 2015, 60, 531–539. [Google Scholar] [CrossRef]


| Insect Frass | TS * | VS * | Ash * | Crude Protein | Raw Fat | Crude Fibre | Other Carbohydrates |
|---|---|---|---|---|---|---|---|
| (Feedstock) | [%FM] | [%TS] | [g/kgTS] | [g/kgTS] | [g/kgTS] | [g/kgTS] | [g/kgTS] |
| Stillage (ST) | 9.6 | 94.1 | 58.5 | 240.5 ± 1.17 | 63.1 ± 3.0 | 315.2 ± 2.7 | 322.8 ± 3.4 |
| Brewers spent grain (BS) | 2.6 | 51.2 | 487.7 | 215.2 ± 4.27 | 37.9 ± 5.0 | 47.1 ± 2.3 | 307.7 ± 2.3 |
| Corn silage (CS) | 7.3 | 81.1 | 189.0 | 230.0 ± 7.09 | 30.5 ± 4.5 | 101.7 ± 1.0 | 347.9 ± 1.4 |
| Elodea nutallii (EL) | 12.9 | 94.5 | 54.8 | 46.4 ± 3.2 | 21.2 ± 6.7 | 533.0 ± 1.8 | 344.7 ± 2.5 |
| Bran (BR) | 12.4 | 85.7 | 143.1 | 288.8 ± 2.4 | 23.3 ± 3.9 | 338.6 ± 7.8 | 206.2 ± 4.3 |
| Insect frass pilot plant (IF_PP) | 84.2 | 91.0 | 89.7 | 228.8 ± 5.1 | 33.9 ± 5.2 | 226.5 ± 0.8 | 421.1 ± 5.2 |
| Phase | Period (Day) | HRT (Days) | OLR (g VS/L·d) |
|---|---|---|---|
| Phase I | 0–52 | 80 | 1.0 |
| Phase II | 53–140 | 80 | 1.5 |
| Phase III | 141–173 | 60 | 0.7 |
| Phase IV | 174–314 | 60 | 1.5–2.2 |
| Parameters | Insect Frass Samples | |||||
|---|---|---|---|---|---|---|
| EL | CS | BS | BR | ST | IF_PP | |
| Observed SMY | 287 ± 36.8 | 262 ± 16.9 | 259 ± 26.9 | 250 ± 19.1 | 201 ± 8.6 | 277 ± 0.8 |
| First order model | ||||||
| β0 (mL/gVS) | 280 ± 4.5 | 261 ± 1.0 | 257 ± 3.8 | 259 ± 2.1 | 190 ± 2.2 | 262 ± 3.7 |
| k (1/day) | 0.13 ± 0.01 | 0.22 ± 0.00 | 0.13 ± 0.01 | 0.13 ± 0.00 | 0.38 ± 0.03 | 0.25 ± 0.02 |
| Correlation coefficient (R2) | 0.9915 | 0.9985 | 0.9932 | 0.9983 | 0.9725 | 0.9761 |
| Akaike information criterion (AIC) | 247.10 | 183.49 | 236.95 | 191.03 | 243.25 | 266.63 |
| Modified Gompertz model | ||||||
| β0 (mL/gVS) | 269 ± 3.7 | 256 ± 1.2 | 248 ± 3.7 | 248 ± 1.3 | 190 ± 2.4 | 259 ± 2.7 |
| λ (days) | −1.00 ± 0.39 | −0.33 ± 0.21 | −1.00 ± 0.45 | −0.29 ± 0.14 | −1.00 ± 0.37 | −1.00 ± 0.30 |
| βm (mL/gVS·day) | 20.35 ± 1.25 | 34.13 ± 1.93 | 18.64 ± 1.26 | 21.65 ± 0.52 | 32.75 ± 4.51 | 33.25 ± 2.73 |
| Correlation coefficient (R2) | 0.9787 | 0.9929 | 0.9825 | 0.9972 | 0.9563 | 0.9583 |
| Akaike information criterion (AIC) | 287.40 | 232.97 | 265.93 | 205.35 | 258.46 | 309.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wedwitschka, H.; Gallegos Ibanez, D.; Jáquez, D.R. Biogas Production from Residues of Industrial Insect Protein Production from Black Soldier Fly Larvae Hermetia illucens (L.): An Evaluation of Different Insect Frass Samples. Processes 2023, 11, 362. https://doi.org/10.3390/pr11020362
Wedwitschka H, Gallegos Ibanez D, Jáquez DR. Biogas Production from Residues of Industrial Insect Protein Production from Black Soldier Fly Larvae Hermetia illucens (L.): An Evaluation of Different Insect Frass Samples. Processes. 2023; 11(2):362. https://doi.org/10.3390/pr11020362
Chicago/Turabian StyleWedwitschka, Harald, Daniela Gallegos Ibanez, and Damián Reyes Jáquez. 2023. "Biogas Production from Residues of Industrial Insect Protein Production from Black Soldier Fly Larvae Hermetia illucens (L.): An Evaluation of Different Insect Frass Samples" Processes 11, no. 2: 362. https://doi.org/10.3390/pr11020362
APA StyleWedwitschka, H., Gallegos Ibanez, D., & Jáquez, D. R. (2023). Biogas Production from Residues of Industrial Insect Protein Production from Black Soldier Fly Larvae Hermetia illucens (L.): An Evaluation of Different Insect Frass Samples. Processes, 11(2), 362. https://doi.org/10.3390/pr11020362