Anodic Behavior of Hafnium in Anhydrous Electrodissolution-Coupled Hafnium Alkoxide Synthesis
Abstract
:1. Introduction
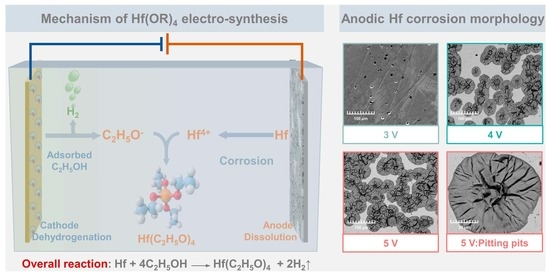
2. Theoretical Mechanism of Electro-Synthesis in the EHS System
3. Experimental Section
3.1. Materials
3.2. Electrochemical Measurement
3.3. Numerical Analysis
3.3.1. Determination of Anodic Corrosion Kinetic Parameters
3.3.2. Linear Polarization Resistance Measurements
4. Results and Discussion
4.1. Anodic Behavior of Hf in Et4NCl-Based Anhydrous Ethanol System
4.2. Anodic Behavior of Hf in Et4NHSO4-Based Anhydrous Ethanol System
4.3. Comparison between Hf Corrosion in Et4NCl and Et4NHSO4 Systems by Polarization Resistance Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, R.; Taoka, N.; Yokoyama, M.; Lee, S.; Kim, S.; Hoshii, T.; Yasuda, T.; Jevasuwan, W.; Maeda, T.; Ichikawa, O.; et al. 1-nm-capacitance-equivalent-thickness HfO2/Al2O3/InGaAs metal-oxide-semiconductor structure with low interface trap density and low gate leakage current density. Appl. Phys. Lett. 2012, 100, 132906. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, C.; Cho, M.-H.; Chung, K.; An, C.-H.; Kim, H.; Lee, H.; Kim, C.; Lee, T. Thickness dependence on crystalline structure and interfacial reactions in HfO2 films on InP (001) grown by atomic layer deposition. Appl. Phys. Lett. 2010, 97, 172108. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, C.; Cho, M.; An, C.; Kim, H.; Seo, J.; Kim, C.; Lee, T.; Ko, D. Interfacial Reactions between HfO2 Films Prepared by Atomic-Layer-Deposition and an InP Substrate Using Postnitridation with NH3 Vapor. Electrochem. Solid-State Lett. 2012, 15, G9. [Google Scholar] [CrossRef]
- Zhang, S.; Northrup, J. Chemical potential dependence of defect formation energies in GaAs: Application to Ga self-diffusion. Phys. Rev. Lett. 1991, 67, 2339–2342. [Google Scholar] [CrossRef] [PubMed]
- Timm, R.; Head, A.R.; Yngman, S.; Knutsson, J.V.; Hjort, M.; McKibbin, S.R.; Troian, A.; Persson, O.; Urpelainen, S.; Knudsen, J.; et al. Self-cleaning and surface chemical reactions during hafnium dioxide atomic layer deposition on indium arsenide. Nat. Commun. 2018, 9, 1412. [Google Scholar] [CrossRef]
- Gordon, R.G.; Becker, J.; Hausmann, D.; Suh, S. Vapor Deposition of Metal Oxides and Silicates: Possible Gate Insulators for Future Microelectronics. Chem. Mater. 2001, 13, 2463–2464. [Google Scholar] [CrossRef]
- Hausmann, D.M.; Kim, E.; Becker, J.; Gordon, R.G. Atomic Layer Deposition of Hafnium and Zirconium Oxides Using Metal Amide Precursors. Chem. Mater 2002, 14, 4350–4358. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys 2013, 113, 021301. [Google Scholar] [CrossRef]
- Mui, C.; Musgrave, C.B. Atomic Layer Deposition of HfO2 Using Alkoxides as Precursors. J. Phys. Chem. B 2004, 108, 15150–15164. [Google Scholar] [CrossRef]
- Bradley, D.; Mehrotra, R.C.; Rothwell, I.; Singh, A. Alkoxo and Aryloxo Derivatives of Metals; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef]
- Peng, R. Metallurgy of Heavy Metals; Central South University Press: Changsha, China, 2004. [Google Scholar]
- Li, Y.G.; Liu, S.S.; Wang, C.H.; Luo, T.; Xiang, C.L.; Li, S.; Chang, C.; Yang, S.H.; Wang, H.H.; Chen, Y.M. Electro-Deposition Behavior in Methanesulfonic-Acid-Based Lead Electro-Refining. J. Sustain. Metall 2021, 7, 1910–1916. [Google Scholar] [CrossRef]
- Turner, J.A. A Realizable Renewable Energy Future. Science 1999, 285, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Glenk, G.; Reichelstein, S. Economics of converting renewable power to hydrogen. Nat. Energy 2019, 4, 216–222. [Google Scholar] [CrossRef]
- Sharifian, R.; Wagterveld, R.M.; Digdaya, I.A.; Xiang, C.; Vermaas, D.A. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 2021, 14, 781–814. [Google Scholar] [CrossRef]
- Li, S.; Yang, S.; Wang, C. Electrochemical Behavior of Tetraethylammonium-Hydrogen Sulfate-Based Electrodissolution-Coupled Hafnium Alkoxide Synthesis. JOM 2022, 74, 3548–3556. [Google Scholar] [CrossRef]
- Li, S.; Yang, S.; Li, K.; Lai, Y.; Deng, C.; Wang, C. Electrodissolution-Coupled Hafnium Alkoxide Synthesis with High Environmental and Economic Benefits. ChemSusChem 2022, 15, e202200474. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons, Inc.: New York, NY, USA, 1980. [Google Scholar]
- Stern, M.; Geary, A.L. Electrochemical Polarization: I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56. [Google Scholar] [CrossRef]
- Bockris, J.O.M.; Reddy, A.K.N. Modern Electrochemistry; Springer: Boston, MA, USA, 1970; Volume 2. [Google Scholar]
- Tafel, J. Über die Polarisation bei kathodischer Wasserstoffentwicklung. Z. Phys. Chem. 1905, 50, 641–712. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A. Uniform and pitting corrosion events induced by SCN− anions on Al alloys surfaces and the effect of UV light. Electrochim. Acta 2011, 56, 2518–2531. [Google Scholar] [CrossRef]
- Lin, L.F.; Chao, C.Y.; Macdonald, D.D. A Point Defect Model for Anodic Passive Films: II. Chemical Breakdown and Pit Initiation. J. Electrochem. Soc. 1981, 128, 1194–1198. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.; Yuan, Y.; Chen, Y.; Wang, B.; He, J.; Tang, C. Corrosion behavior of hafnium in anhydrous isopropanol and acetonitrile solutions containing bromide ions. Trans. Nonferrous Met. Soc. China 2017, 27, 1896–1906. [Google Scholar] [CrossRef]
- Li, S.; Yang, S.; Chen, Y.; Tang, C.; Lai, Y.; Deng, C.; Wang, C. Electrochemical mechanism and kinetics of electrodissolution-coupled hafnium alkoxide synthesis in a tetraethylammonium-chloride-based anhydrous system. Trans. Nonferrous Met. Soc. China, 2023; submitted for publication. [Google Scholar]
- Wang, C.H.; Yang, S.H.; Chen, Y.M.; Wang, B.; He, J.; Tang, C.B. Effect of bromide ions on the corrosion behavior of hafnium in anhydrous ethanol. RSC Adv. 2015, 5, 34580–34587. [Google Scholar] [CrossRef]
- Wang, C.H.; Yang, S.H.; Chen, Y.M.; Yang, X.Y.; Wang, B.; He, J.; Tang, C.B. Electrochemical Behaviour of Hafnium in Anhydrous n-butanol Containing Tetraethylammonium Bromide. Int. J. Electrochem. Sci. 2017, 12, 545–560. [Google Scholar] [CrossRef]
- Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, K.; Yu, H.; Yang, S.; Li, K. Copper electrowinning-coupled CO2 capture in solvent based post-combustion capture. Appl. Energy 2022, 316, 119086. [Google Scholar] [CrossRef]
- Amin, M.A. A newly synthesized glycine derivative to control uniform and pitting corrosion processes of Al induced by SCN—anions—Chemical, electrochemical and morphological studies. Corros. Sci. 2010, 52, 3243–3257. [Google Scholar] [CrossRef]
- Wang, C.H.; Jiang, K.Q.; Jones, T.W.; Yang, S.H.; Yu, H.; Feron, P.; Li, K.K. Electrowinning-coupled CO2 capture with energy-efficient absorbent regeneration: Towards practical application. Chem. Eng. J. 2022, 427, 131981. [Google Scholar] [CrossRef]
- Scully, J.R. Polarization Resistance Method for Determination of Instantaneous Corrosion Rates. Corrosion 2000, 56, 3548–3556. [Google Scholar] [CrossRef]
- Wang, C.; Li, K.; Yu, H.; Yang, S.; Jiang, K. Electrochemical behavior of Cu-mediated electrowinning-coupled CO2 capture. Electrochim. Acta 2022, 422, 140571. [Google Scholar] [CrossRef]
- Xiang, C.; Zhu, S.; Song, J.; Li, Y.; Luo, T.; Chang, C.; Qu, J.; Yang, S.; Wang, C.; Chen, Y. Green Electrorefining of Crude Lead with High-Quality Deposits in an Additive-Assisted Methanesulfonic Acid System. ACS Sustain. Chem. Eng. 2022, 10, 11223–11231. [Google Scholar] [CrossRef]







| (a) T/°C | ba (V dec−1) | vcorr (μm y−1) | (b) C/M | ba (V dec−1) | vcorr (μm y−1) |
|---|---|---|---|---|---|
| 25.0 | 0.547 | 6.38 | 0.02 | 0.468 | 5.56 |
| 27.5 | 0.465 | 6.93 | 0.04 | 0.415 | 6.52 |
| 30.0 | 0.397 | 7.45 | 0.06 | 0.397 | 7.45 |
| 32.5 | 0.363 | 7.98 | 0.08 | 0.322 | 8.25 |
| 35.0 | 0.273 | 8.55 | 0.10 | 0.296 | 8.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yang, S.; Zhao, P.; Chen, Y.; Tang, C.; Lai, Y.; Deng, C.; Wang, C. Anodic Behavior of Hafnium in Anhydrous Electrodissolution-Coupled Hafnium Alkoxide Synthesis. Processes 2023, 11, 564. https://doi.org/10.3390/pr11020564
Li S, Yang S, Zhao P, Chen Y, Tang C, Lai Y, Deng C, Wang C. Anodic Behavior of Hafnium in Anhydrous Electrodissolution-Coupled Hafnium Alkoxide Synthesis. Processes. 2023; 11(2):564. https://doi.org/10.3390/pr11020564
Chicago/Turabian StyleLi, Shuai, Shenghai Yang, Pengfei Zhao, Yongming Chen, Chaobo Tang, Yanqing Lai, Chaoyong Deng, and Changhong Wang. 2023. "Anodic Behavior of Hafnium in Anhydrous Electrodissolution-Coupled Hafnium Alkoxide Synthesis" Processes 11, no. 2: 564. https://doi.org/10.3390/pr11020564
APA StyleLi, S., Yang, S., Zhao, P., Chen, Y., Tang, C., Lai, Y., Deng, C., & Wang, C. (2023). Anodic Behavior of Hafnium in Anhydrous Electrodissolution-Coupled Hafnium Alkoxide Synthesis. Processes, 11(2), 564. https://doi.org/10.3390/pr11020564