Efficiency of Coagulation/Flocculation for the Removal of Complex Mixture of Textile Fibers from Water
Abstract
:1. Introduction
2. Materials and Methods
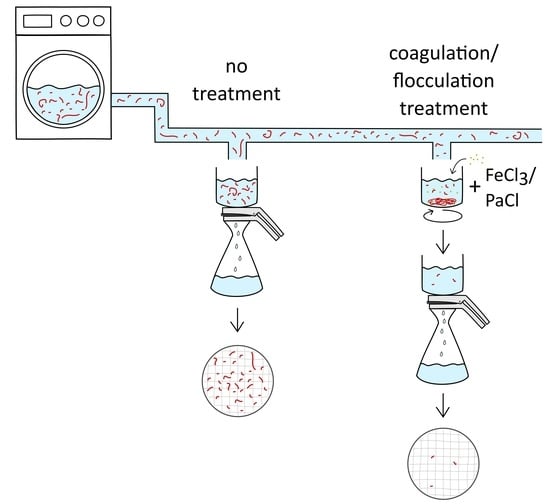
2.1. Experimental Setup
2.2. Materials
2.3. Coagulation/Flocculation Experiments
2.4. Analytical Procedures
3. Results
3.1. Water Matrix Characterization
3.2. Textile Fibers Characterization
3.2.1. SEM Characterization
3.2.2. FTIR
3.2.3. Micro-FTIR
3.3. Effects of Coagulation on Textile Fibers Removal Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radhakrishnan, S. 2—Sustainable cotton production. In Sustainable Fibres and Textiles; Woodhead Publishing: Coimbatore, India, 2017; pp. 21–67. [Google Scholar]
- Krifa, M.; Stewart, S.S. Cotton utilization in conventional and non-conventional textiles—A statistical review. Agric. Sci. 2016, 7, 747–758. [Google Scholar] [CrossRef] [Green Version]
- L’Abbate, P.; Dassisti, M.; Cappelletti, G.M.; Nicoletti, G.M.; Russo, C.; Ioppolo, G. Environmental analysis of polyester fabric for ticking. J. Clean. Prod. 2018, 172, 735–742. [Google Scholar] [CrossRef]
- Napper, I.E.; Parker-Jurd, F.N.F.; Wright, S.L.; Thompson, R.C. Examining the release of synthetic microfibres to the environment via two major pathways: Atmospheric deposition and treated wastewater effluent. Sci. Total Environ. 2023, 857, 159317. [Google Scholar] [CrossRef]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Venditti, R.A. Impact of dyes and finishes on the aquatic biodegradability of cotton textile fibers and microfibers released on laundering clothes: Correlations between enzyme adsorption and activity and biodegradation rates. Mar. Pollut. Bull. 2021, 165, 112030. [Google Scholar] [CrossRef]
- Pedrotti, M.L.; Petit, S.; Eyheraguibel, B.; Kerros, M.E.; Elineau, A.; Ghiglione, J.F. Pollution by anthropogenic microfibers in North-West Mediterranean Sea and efficiency of microfiber removal by a wastewater treatment plant. Sci. Total Environ. 2021, 758, 144195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Q.; Xing, Y.; Liu, K.; Ling, W.; Yang, J.; Yang, Q.; Wu, T.; Zhang, J.; Pei, Z.; et al. Review of research on migration, distribution, biological effects, and analytical methods of microfibers in the environment. Sci. Total Environ. 2023, 855, 158922. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; Gullo, M.P.; Gentile, G.; Di Pace, E.; Cocca, M.; Gelabert, L.; Brouta-Agnésa, M.; Rovira, A.; Escudero, R.; Villalba, R.; et al. Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ. Pollut. 2018, 236, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; Brandsma, S.H.; van Velzen, M.J.M.; Vethaak, A.D. Microplastics en route: Field measurements in the dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef]
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Çevik, C.; Güzel, E.; Kilercioğlu, S. Microplastics in municipal wastewater treatment plants in Turkey: A comparison of the influent and secondary effluent concentrations. Environ. Monit. Assess. 2018, 190, 626. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane arraybased micro-fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.A.; Arellano, J.M.; Albendín, G.; Rodríguez-Barroso, R.; Quiroga, J.M.; Coello, M.D. Microplastic pollution in wastewater treatment plants in the city of c’adiz: Abundance, removal efficiency and presence in receiving water body. Sci. Total Environ. 2021, 776, 145795. [Google Scholar] [CrossRef]
- Tian, W.; Song, P.; Zhang, H.; Duan, X.; Wei, Y.; Wang, H.; Wang, S. Microplastic materials in the environment: Problem and strategical solutions. Prog. Mater. Sci. 2023, 132, 101035. [Google Scholar] [CrossRef]
- Pivokonský, M.; Pivokonská, L.; Novotná, K.; Čermáková, L.; Klimtová, M. Occurrence and fate of microplastics at two different drinking water treatment plants within a river catchment. Sci. Total Environ. 2020, 741, 140236. [Google Scholar] [CrossRef]
- Xue, J.; Peldszus, S.; Van Dyke, M.I.; Huck, P. Removal of polystyrene microplastic spheres by alum-based coagulation-flocculation-sedimentation (CFS) treatment of surface waters. J. Chem. Eng. 2021, 422, 130023. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Novotna, K.; Čermakova, L.; Petriček, R. Jar Tests for Water Treatment Optimisation, How to Perform Jar Tests—A handbook; IWA Publishing: London, UK, 2022. [Google Scholar]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar] [CrossRef]
- Xia, Y.; Xiang, X.M.; Dong, K.Y.; Gong, Y.Y.; Li, Z.J. Surfactant stealth effect of microplastics in traditional coagulation process observed via 3-D fluorescence imaging. Sci. Total Environ. 2020, 729, 138783. [Google Scholar] [CrossRef]
- Belzagui, F.; Crespi, M.; Álvarez, A.; Gutiérrez-Bouzán, C.; Vilaseca, M. Microplastics’ emissions: Microfibers’ detachment from textile garments. Environ. Pollut. 2019, 248, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Cesa, F.S.; Turra, A.; Checon, H.H.; Leonardi, B.; Baruque-Ramos, J. Laundering and textile parameters influence fibers release in household washings. Environ. Pollut. 2020, 257, 113553. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Ding, Y.; Hu, C.; Liu, H. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J. Environ. Sci. 2019, 78, 267–275. [Google Scholar] [CrossRef]
- Skaf, D.W.; Punzi, V.L.; Rolle, J.T.; Kleinberg, K.A. Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation. J. Chem. Eng. 2019, 386, 123807. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q.; Li, J.; Li, Q.; Xu, H.; Ye, Q.; Wang, Y.; Shu, S.; Zhang, J. Removal of polystyrene and polyethylene microplastics using PAC and FeCl3 coagulation: Performance and mechanism. Sci. Total Environ. 2021, 752, 141837. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, H.; Fei, L.; Wei, B.; Zhou, T.; Zhang, H. The removal of microplastics from water by coagulation: A comprehensive review. Sci. Total Environ. 2022, 851, 158224. [Google Scholar] [CrossRef] [PubMed]
- SRPS ISO 6060:1994; Water Quality—Determination of Chemical Consumption of Oxygen. Institute for Standardization of Serbia: Belgrade, Serbia, 1994.
- SRPS ISO 9297-1:2007; Water Quality—Determination of Chloride Content—Silver-Nitrate Titration with Chromate Indicator (Mor’s Method). Institute for Standardization of Serbia: Belgrade, Serbia, 2007.
- APHA-AWWA-WEF. Standard Methods for the Examination of Water and Waste Water, 23th ed.; Eaton, A.D., Clesceri, L.S., Greenberg, A.E., Eds.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- EPA 7000 B:2007; Flame Atomic Absorption Spectrophotometry. Institute for Standardization of Serbia: Belgrade, Serbia, 2007.
- EPA 3015 A:2007; Microwave Assisted Acid Digestion of Aqueous Samples and Extracts. Institute for Standardization of Serbia: Belgrade, Serbia, 2007.
- Dalla Fontana, G.; Mossotti, R.; Montarsolo, A. Assessment of microplastics release from polyester fabrics: The impact of different washing conditions. Environ. Pollut. 2020, 264, 113960. [Google Scholar] [CrossRef]
- Dalla Fontana, G.; Mossotti, R.; Montarsolo, A. Influence of sewing on microplastic release from textiles during washing. Water Air Soil Pollut. 2021, 232, 50. [Google Scholar] [CrossRef]
- Mishra, S.; Dash, D.; Prasad Das, A. Detection, characterization and possible biofragmentation of synthetic microfibers released from domestic laundering wastewater as an emerging source of marine pollution. Mar. Pollut. Bull. 2022, 185, 114254. [Google Scholar] [CrossRef]
- Geminiani, L.; Campione, F.P.; Corti, C.; Luraschi, M.; Motella, S.; Recchia, S.; Rampazzi, L. Differentiating between Natural and Modified Cellulosic Fibres Using ATR-FTIR Spectroscopy. Heritage 2022, 5, 4114–4139. [Google Scholar] [CrossRef]
- Nuelle, M.T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, S.S.; Rocha-Santos, T.; Prata, J.C.; Duarte, A.C.; Girão, A.V.; Lopes, P.; Cristovão, T.; Pinto Da Costa, J. A straightforward method for microplastic extraction from organic-rich freshwater samples. Sci. Total Environ. 2022, 815, 152941. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Lee, M.; Choe, E.K. Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr. Polym. 2004, 58, 417–420. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Vasanthan, N.; Salem, D.R. Infrared Spectroscopic Characterization of Oriented Polyamide 66: Band Assignment and Crystallinity Measurement. J. Polym. Sci. B Polym. Phys. 2000, 38, 516–524. [Google Scholar] [CrossRef]
- Sengupta, R.; Tikku, V.K.; Somani, A.K.; Chaki, T.K.; Bhowmick, A.K. Electron beam irradiated polyamide-6,6 films—I: Characterization by wide angle X-ray scattering and infrared spectroscopy. Radiat. Phys. Chem. 2005, 72, 625–633. [Google Scholar] [CrossRef]
- Navarro-Pardo, F.; Martínez-Barrera, G.; Martínez-Hernández, A.; Castaño, V.; Rivera-Armenta, J.; Medellín-Rodríguez, F.; Velasco-Santos, C. Effects on the Thermo-Mechanical and Crystallinity Properties of Nylon 6,6 Electrospun Fibres Reinforced with One Dimensional (1D) and Two Dimensional (2D) Carbon. Materials 2013, 6, 3494–3513. [Google Scholar] [CrossRef] [Green Version]
- Tadokoro, H.; Murahashi, S.; Yamadera, R.; Kamei, T.-I. Infrared absorption spectra of polyacrylonitrile and deuterated polyacrylonitriles. J. Polym. Sci. A Gen. Pap. 1963, 1, 3029–3042. [Google Scholar] [CrossRef]
- Karacan, I.; Erdogan, G. The influence of thermal stabilization stage on the molecular structure of polyacrylonitrile fibers prior to the carbonization stage. Fibers Polym. 2012, 13, 295–302. [Google Scholar] [CrossRef]
- Barrows, A.P.W.; Cathey, S.E.; Petersen, C.W. Marine environment microfiber contamination: Global patterns and the diversity of microparticle origins. Environ. Pollut. 2018, 237, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Dagnew, M.; Ray, M.B. Effect of coagulation on microfibers in laundry wastewater. Environ. Res. 2022, 212, 113401. [Google Scholar] [CrossRef]
- Tabatabei, F.; Mafigholami, R.; Moghimi, H.; Khoramipoor, S. Effect of Fe and Al based coagulants and disinfectants on polyethylene microplastics removal in coagulation process through response surface methodology. Water Sci. Technol. 2022, 87, 99–114. [Google Scholar] [CrossRef]
- Lapointe, M.; Farner, J.M.; Hernandez, L.M.; Tufenkji, N. Understanding and improving microplastic removal during water treatment: Impact of coagulation and flocculation. Environ. Sci. Technol. 2020, 54, 8719–8727. [Google Scholar] [CrossRef] [PubMed]
- Cherniak, S.L.; Almuhtaram, H.; McKie, M.J.; Hermabessiere, L.; Yuan, C.; Rochman, C.M.; Andrews, R.C. Coventional and biological treatment for the removal of microplastics from drinking water. Chemosphere 2022, 288, 132587. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci. Total Environ. 2020, 700, 134520. [Google Scholar] [CrossRef] [PubMed]










| Parameter | MDL | Synthetic Water | Surface Water | Laundry Wastewater |
|---|---|---|---|---|
| pH | / | 7.90 ± 0.06 | 7.75 ± 0.07 | 8.13 ± 0.07 |
| Electrical conductivity 25 °C (µS/cm) | / | 303 ± 5 | 437 ± 8 | 578 ± 15 |
| Turbidity (NTU) | 0.01 | 0.3 ± 0.3 | 8.35 ± 0.15 | 149 ± 25 |
| COD (mg/L) | 15 | <15 * | <15 * | 920 ± 23 |
| Total organic carbon (mg/L) | 0.3 | <0.3 * | 2.4 ± 0.3 | 104 ± 1.1 |
| Na+ (mg/L) | 0.1 | 75.7 ± 5.7 | 5.5 ± 0.7 | 2.71 ± 0.22 |
| Mg2+ (mg/L) | 0.09 | 4.33 ± 0.41 | 10.4 ± 0.9 | 5.01 ± 0.47 |
| K+ (mg/L) | 0.11 | <0.11 * | 2.31 ± 0.17 | 6.8 ± 0.6 |
| Ca2+ (mg/L) | 0.11 | 44.0 ± 3.6 | 6.00 ± 0.54 | 7.33 ± 0.71 |
| Cl− (mg/L) | 5.0 | 52.1 ± 3.6 | 44.0 ± 1.5 | 3140 ± 150 |
| SO42− (mg/L) | 5.0 | 21.2 ± 4.9 | 25.5 ± 3.2 | 97.6 ± 27.2 |
| HCO3− (mg/L) | 18.4 | 134 ± 6 | 218 ± 43 | 252 ± 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiljević, S.; Vujić, M.; Agbaba, J.; Federici, S.; Ducoli, S.; Tomić, R.; Tubić, A. Efficiency of Coagulation/Flocculation for the Removal of Complex Mixture of Textile Fibers from Water. Processes 2023, 11, 820. https://doi.org/10.3390/pr11030820
Vasiljević S, Vujić M, Agbaba J, Federici S, Ducoli S, Tomić R, Tubić A. Efficiency of Coagulation/Flocculation for the Removal of Complex Mixture of Textile Fibers from Water. Processes. 2023; 11(3):820. https://doi.org/10.3390/pr11030820
Chicago/Turabian StyleVasiljević, Sanja, Maja Vujić, Jasmina Agbaba, Stefania Federici, Serena Ducoli, Radivoj Tomić, and Aleksandra Tubić. 2023. "Efficiency of Coagulation/Flocculation for the Removal of Complex Mixture of Textile Fibers from Water" Processes 11, no. 3: 820. https://doi.org/10.3390/pr11030820
APA StyleVasiljević, S., Vujić, M., Agbaba, J., Federici, S., Ducoli, S., Tomić, R., & Tubić, A. (2023). Efficiency of Coagulation/Flocculation for the Removal of Complex Mixture of Textile Fibers from Water. Processes, 11(3), 820. https://doi.org/10.3390/pr11030820