Anti-Glioblastoma Potential and Phenolic Profile of Berry Juices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Berry Juice
2.3. Spectrophotometric Analysis of Total Polyphenols, Monomeric Anthocyanins and Proanthocyanidins
2.4. Evaluation of Antioxidant Activity—ABTS, DPPH, FRAP and CUPRAC Assays
2.5. Sample Preparation for High Performance Liquid Chromatography
2.6. Determination of Individual Polyphenols Using Reversed Phase HPLC
2.7. Antiproliferative Effects of Berry Fruit Juices on Glioblastoma Cells
2.8. Statistical Analysis
3. Results
3.1. Phenolic Compounds and Antioxidant Activity of Berry Juices
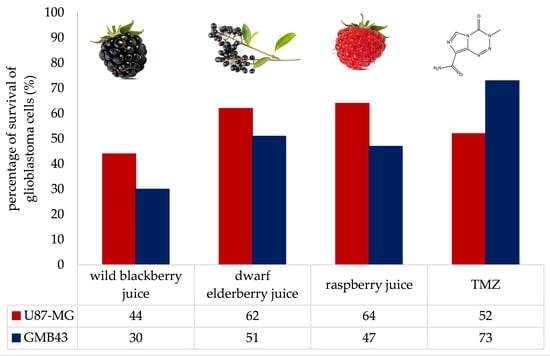
3.2. Antiproliferative Effects of Berry Juices
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haizhou, W.; Oliveira, G.; Lila, M.A. Protein-binding approaches for improving bioaccessibility and bioavailability of anthocyanins. Compr. Rev. Food Sci. Food Saf. 2023, 22, 333–354. [Google Scholar]
- Shen, Y.; Zhang, N.; Tian, J.; Xin, G.; Liu, L.; Sun, X.; Li, B. Advanced approaches for improving bioavailability and controlled release of anthocyanins. J. Control Release 2022, 341, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.A.; Sarmast, E.; Fatehi, P.; Jafari, T. Impact of dietary anthocyanins on systemic and vascular inflammation: Systematic review and meta-analysis on randomised clinical trials. Food Chem. Toxicol. 2020, 135, 110922. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Howatson, G. Dietary intake of anthocyanins and risk of cardiovascular disease: A systematic review and meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 3032–3043. [Google Scholar] [CrossRef] [PubMed]
- Krga, I.; Milenkovic, D. Anthocyanins: From sources and bioavailability to cardiovascular-health benefits and molecular mechanisms of action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling anthocyanin bioavailability for human health. Ann. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef]
- Tang, J.; Oroudjev, E.; Wilson, L.; Ayoub, G. Delphinidin and cyanidin exhibit antiproliferative and apoptotic effects in MCF7 human breast cancer cells. Integr. Cancer Sci. Therap. 2015, 2, 82–86. [Google Scholar]
- Hou, D.X.; Kai, K.; Li, J.J.; Lin, S.; Terahara, N.; Wakamatsu, M.; Fujii, M.; Young, M.R.; Colburn, N. Anthocyanidins inhibit activator protein 1 activity and cell transformation: Structure–activity relationship and molecular mechanisms. Carcinogenesis 2004, 25, 29–36. [Google Scholar] [CrossRef]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef]
- Raucher, D. Tumor targeting peptides: Novel therapeutic strategies in glioblastoma. Curr. Opin. Pharmcol. 2019, 47, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Velázquez, O.A.; Cortés-Rodríguez, M.; Milán-Carrillo, J.; Montes-Ávila, J.; Robles-Bañuelos, B.; Santamaría del Ángel, A.; Cuevas-Rodríguez, E.O.; Rangel-López, E. Anti-oxidant and anti-proliferative effect of anthocyanin enriched fractions from two Mexican wild blackberries (Rubus spp.) on HepG2 and glioma cell lines. J. Berry Res. 2020, 10, 513–529. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Rooprai, H.K.; Christidou, M.; Murray, S.A.; Davies, D.; Selway, R.; Gullan, R.W.; Pilkington, G.J. Inhibition of Invasion by Polyphenols from Citrus Fruit and Berries in Human Malignant Glioma Cells In Vitro. Anticancer Res. 2021, 41, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Expósito, M.J.; Martínez-Martos, J.M. The Delicate Equilibrium between Oxidants and Antioxidants in Brain Glioma. Curr. Neuropharmacol. 2019, 17, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Lafleur, S.; Bedard, V.; Moghrabi, A.; Barrete, S.; Gingras, D.; Béliveau, R. Anthocyanidins inhibit migration of glioblastoma cells: Structure-activity relationship and involvement of the plasmolytic system. J. Cell. Biochem. 2007, 100, 100–111. [Google Scholar] [CrossRef]
- Dragojevic, S.; Mackey, R.; Raucher, D. Evaluation of Elastin-Like Polypeptides for Tumor Targeted Delivery of Doxorubicin to Glioblastoma. Molecules 2019, 24, 3242. [Google Scholar] [CrossRef]
- Mathew, E.N.; Berry, B.C.; Yang, H.W.; Carroll, R.S.; Johnson, M.D. Delivering Therapeutics to Glioblastoma: Overcoming Biological Constraints. Int. J. Mol. Sci. 2022, 23, 1711. [Google Scholar] [CrossRef]
- Raucher, D.; Dragojevic, S.; Ryu, J. Macromolecular Drug Carriers for Targeted Glioblastoma Therapy: Preclinical Studies, Challenges, and Future Perspectives. Front. Oncol. 2018, 8, 624. [Google Scholar] [CrossRef]
- Vengoji, R.; Macha, M.A.; Batra, S.K.; Shonka, N.A. Natural products: A hope for glioblastoma patients. Oncotarget 2018, 9, 22194–22219. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Bjerke, M.; Edlund, H.; Nelander, S.; Westermark, B. Origin of the U87MG glioma cell line: Good news and bad news. Sci. Transl. Med. 2016, 8, 354re3. [Google Scholar] [CrossRef]
- Pevna, V.; Wagnières, G.; Huntosova, V. Autophagy and Apoptosis Induced in U87 MG Glioblastoma Cells by Hypericin-Mediated Photodynamic Therapy Can Be Photobiomodulated with 808 nm Light. Biomedicines 2021, 9, 1703. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, M.; Koosha, F.; Neshastehriz, A. Chemo-radiation therapy of U87-MG glioblastoma cells using SPIO@AuNP-Cisplatin-Alginate nanocomplex. Heliyon 2023, 9, e13847. [Google Scholar] [CrossRef]
- Di Cintio, F.; Dal Bo, M.; Baboci, L.; De Mattia, E.; Polano, M.; Toffoli, G. The Molecular and Microenvironmental Landscape of Glioblastomas: Implications for the Novel Treatment Choices. Front. Neurosci. 2020, 14, 603647. [Google Scholar] [CrossRef]
- Nguyen, H.-M.; Guz-Montgomery, K.; Lowe, D.B.; Saha, D. Pathogenetic Features and Current Management of Glioblastoma. Cancers 2021, 13, 856. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-Y.; Ko, H.-J.; Huang, C.-Y.F.; Lin, C.-Y.; Chiou, S.-J.; Su, Y.-F.; Lieu, A.-S.; Loh, J.-K.; Kwan, A.-L.; Chuang, T.-H.; et al. Ionizing Radiation Induces Resistant Glioblastoma Stem-Like Cells by Promoting Autophagy via the Wnt/β-Catenin Pathway. Life 2021, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotonutric acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. In Current Protocols in Food Analytical Chemistry Current Protocols; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Prior, R.L.; Fan, E.; Ji, H.; Howell, A.; Nio, C.; Payne, M.J.; Reed, J. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J. Sci. Food Agric. 2010, 90, 1473–1478. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Sci. Food Agric. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Pambianchi, E.; Hagenberg, Z.; Pecorelli, A.; Grace, M.; Therrien, J.-P.; Lila, M.A.; Valacchi, G. Alaskan bog blueberry (Vaccinium uliginosum) extract as an innovative topical approach to prevent uv-induced skin damage. Cosmetics 2021, 8, 112. [Google Scholar] [CrossRef]
- Može, Š.; Polak, T.; Gašperlin, L.; Koron, D.; Vanzo, A.; Poklar Ulrih, N.; Abram, V. Phenolics in Slovenian Bilberries (Vaccinium myrtillus L.) and Blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Polyphenols and antioxidant activity of citrus fiber/blackberry juice complexes. Molecules 2021, 26, 4400. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular proliferation and viability: Application to the proliferation and viability of murine macrophages. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Kamei, H.; Kojima, T.; Hasegawa, M.; Koide, T.; Umeda, T.; Yukawa, T.; Terabe, K. Suppression of tumor cell growth by anthocyanins in vitro. Cancer Invest. 1995, 13, 590–594. [Google Scholar] [CrossRef]
- Jing, N.; Song, J.; Liu, Z.; Wang, L.; Jiang, G. Glycosylation of anthocyanins enhances the apoptosis of colon cancer cells by handicapping energy metabolism. BMC Complement. Med. Ther. 2020, 20, 312. [Google Scholar] [CrossRef]
- Chen, P.-N.; Chu, S.-C.; Chiou, H.-L.; Kuo, W.-H.; Chiang, C.-L.; Hsieh, Y.-S. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 2006, 235, 248–259. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Reddy, M.K.; Alexander-Lindo, R.L.; Nair, M.G. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J. Agric. Food Chem. 2005, 53, 9268–9273. [Google Scholar] [CrossRef] [PubMed]
- Michaud-Levesque, J.; Bousquet-Gagnon, N.; Beliveau, R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp. Cell Res. 2012, 318, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Asl, F.R.; Barzegar, A.; Ebrahimzadeh, M.A. Evaluating Cytotoxic Potential of the Fruit and the Leaf Extracts of Sambucus ebulus (L.) on MCF7 and AGS Cell Lines. Res. Mol. Med. 2021, 9, 11–20. [Google Scholar]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Ramos, S.; Alia, M.; Bravo, L.; Goya, L. Comparative effects of food-derived polyphenols on the viability and apoptosis of a human hepatoma cell line (HepG2). J. Agric. Food Chem. 2005, 53, 1271–1280. [Google Scholar] [CrossRef]
- Heo, H.J.; Lee, C.Y. Strawberry and its anthocyanins reduce oxidative stress-induced apoptosis in PC12 cells. J. Agric. Food Chem. 2005, 53, 1984–1989. [Google Scholar] [CrossRef]
- Schnekenburger, M.; Dicato, M.; Diederich, M. Plant-derived epigenetic modulators for cancer treatment and prevention. Biotechnol. Adv. 2014, 32, 1123–1132. [Google Scholar] [CrossRef]
- Ranganathan, S.; Halagowder, D.; Sivasithambaram, N.D. Quercetin suppresses twist to induce apoptosis in MCF-7 breast cancer cells. PLoS ONE 2015, 10, e0141370. [Google Scholar] [CrossRef]
- Sang, D.P.; Li, R.J.; Lan, Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol. Sin. 2014, 35, 832–938. [Google Scholar] [CrossRef]
- Kim, H.; Moon, J.Y.; Ahn, K.S.; Cho, S.K. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid. Med. Cell. Longev. 2013, 2013, 596496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-M.; Zhao, L.; Li, H.; Xu, H.; Chen, W.-W.; Tao, L. Research progress on the anticarcinogenic actions and mechanisms of ellagic acid. Cancer Biol. Med. 2014, 11, 92–100. [Google Scholar] [PubMed]
- Hribar, U.; Poklar Ulrih, N. The Metabolism of Anthocyanins. Curr. Drug Metab. 2014, 15, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Janle, E.M.; Lila, M.A.; Grannan, M.; Wood, L.; Higgins, A.; Yousef, G.G.; Rogers, R.B.; Kim, H.; Jackson, G.S.; Weaver, C. Method for evaluating the potential of 14C labeled plant polyphenols to cross the blood-brain barrier using accelerator mass spectrometry. Nucl. Instrum. Methods Phys. Res. 2010, 268, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, R.; Anada, M.; Miyaguchi, A.; Nomi, Y.; Matsumoto, H. Evaluation of Blood-Brain Barrier Permeability of Polyphenols, Anthocyanins, and Their Metabolites. J. Agric. Food Chem. 2021, 69, 11676–11686. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood–brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef]

| Juice | TP (g/L) | MA (mg/L) | PAC (mg/L) |
|---|---|---|---|
| DE | 3.16 ± 0.02 a | 433.17 ± 2.66 b | 71.21 ± 0.81 a |
| WB | 3.21 ± 0.01 a | 759.99 ± 3.53 a | 56.21 ± 1.96 b |
| RB | 1.67 ± 0.02 b | 230.39 ± 1.10 c | 32.34 ± 0.27 c |
| DE | WB | RB | |
|---|---|---|---|
| Cyanidin-3-glucoside | nd | 389.60 ± 10.98 a | 39.11 ± 0.01 b |
| Cyanidin-3-rutinoside | nd | 269.72 ± 8.52 a | 34.28 ± 0.35 b |
| Cyanidin-3-galactoside | 261.40 ± 1.41 a | nd | nd |
| Cyanidin-3-sophoroside | nd | nd | 210.65 ± 0.23 a |
| Hyperoside | 34.19 ± 0.09 b | 73.27 ± 2.39 a | nd |
| Rutin | 109.72 ± 0.05 a | 34.37 ± 0.96 b | 14.70 ± 0.41 bc |
| Gallic acid | 4.58 ± 0.04 a | 4.56 ± 0.03 a | nd |
| Ellagic acid | nd | 5.14 ± 0.03 a | 3.41 ± 0.00 b |
| Chlorogenic acid | 160.02 ± 2.87 a | nd | nd |
| Neochlorogenic acid | 27.18 ± 1.67 a | nd | nd |
| Juice | DPPH | ABTS | FRAP | CUPRAC |
|---|---|---|---|---|
| DE | 9.86 ± 0.01 b | 26.18 ± 0.57 a | 2.11 ± 0.01 a | 107.78 ± 0.56 a |
| WB | 13.25 ± 0.06 a | 6.08 ± 0.01 c | 0.59 ± 0.01 c | 27.37 ± 0.37 c |
| RB | 6.95 ± 0.03 c | 12.59 ± 0.12 b | 1.38 ± 0.00 b | 64.00 ± 0.11 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopjar, M.; Raucher, D.; Lila, M.A.; Šimunović, J. Anti-Glioblastoma Potential and Phenolic Profile of Berry Juices. Processes 2024, 12, 242. https://doi.org/10.3390/pr12020242
Kopjar M, Raucher D, Lila MA, Šimunović J. Anti-Glioblastoma Potential and Phenolic Profile of Berry Juices. Processes. 2024; 12(2):242. https://doi.org/10.3390/pr12020242
Chicago/Turabian StyleKopjar, Mirela, Drazen Raucher, Mary Ann Lila, and Josip Šimunović. 2024. "Anti-Glioblastoma Potential and Phenolic Profile of Berry Juices" Processes 12, no. 2: 242. https://doi.org/10.3390/pr12020242
APA StyleKopjar, M., Raucher, D., Lila, M. A., & Šimunović, J. (2024). Anti-Glioblastoma Potential and Phenolic Profile of Berry Juices. Processes, 12(2), 242. https://doi.org/10.3390/pr12020242