Correlations between a Friabilin Content Indicator and Selected Physicochemical and Mechanical Properties of Wheat Grain for Processing Suitability Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Approximate Composition, Vitreousness, and Hardness
2.3. Geometric Properties, Mass, and Mechanical Properties
2.4. SDS-PAGE Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Properties, Vitreousness, and the Hardness Index
3.2. Geometric Properties and Mass
3.3. Mechanical Properties
3.4. Friabilin Content Indicator
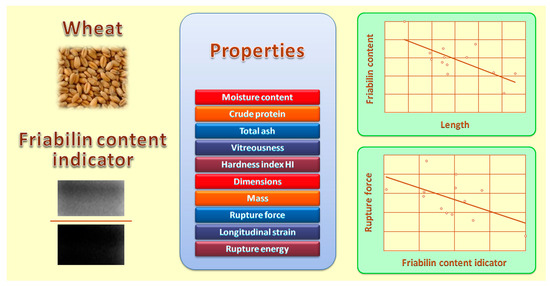
3.5. Correlations between the Fiabilin Content Indicator and the Physicochemical Properties of Grain
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.-H.; Kim, J.-Y. Understanding wheat starch metabolism in properties, environmental stress condition, and molecular approaches for value-added utilization. Plants 2021, 10, 2282. [Google Scholar] [CrossRef]
- Gooding, M.J.; Shewry, P.R. Wheat: Environment, Food and Health; Wiley Online Library: John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Biel, W.; Maciorowski, L. Assessing nutritional value of grains of selected wheat cultivars. Zywn.-Nauk. Technol. Ja. 2012, 2, 45–55. [Google Scholar] [CrossRef]
- Groos, C.; Bervas, E.; Charmet, G. Genetic analysis of grain protein content, grain hardness and dough rheology in a hard × hard bread wheat progeny. J. Cereal Sci. 2004, 40, 93–100. [Google Scholar] [CrossRef]
- Różyło, R.; Laskowski, J. Study of relationships between physical ad technological properties of spring wheat grain. Acta Agroph. 2007, 9, 459–470. [Google Scholar]
- Pasha, I.; Anjum, F.M.; Morris, C.F. Grain hardness: A major determinant of wheat quality. Food Sci. Technol. Int. 2010, 16, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Başlar, M.; Kalkan, F.; Kara, M.; Ertugay, M.F. Correlation between the protein content and mechanical properties of wheat. Turk. J. Agric. For. 2012, 36, 601–607. [Google Scholar] [CrossRef]
- Kasraei, M.; Nejadi, J.; Shafiei, S. Relationship between grain physicochemical and mechanical properties of some Iranian wheat cultivars. J. Agric. Sci. Technol. 2015, 17, 635–647. [Google Scholar]
- Kaliniewicz, Z.; Markowska-Mendik, A.; Warechowska, M. An evaluation of selected engineering properties of polish durum wheat grain. J. Cereal Sci. 2022, 104, 103401. [Google Scholar] [CrossRef]
- Hogg, A.C.; Sripo, T.; Beecher, B.; Martin, J.M.; Giroux, M.J. Wheat puroindolines interact to form friabilin and control wheat grain hardness. Theor. Appl. Genet. 2004, 108, 1089–1097. [Google Scholar] [CrossRef]
- Turnbull, K.-M.; Rahman, S. Endosperm texture in wheat. J. Cereal Sci. 2002, 36, 327–337. [Google Scholar] [CrossRef]
- Laskowski, J.; Różyło, R. Influence of gluten content and wheat glassiness on the wheat grain grinding energy. Acta Agroph. 2003, 2, 589–596. [Google Scholar]
- Morris, C.F. Puroindolines: The molecular genetic basis of wheat grain hardness. Plant Mol. Biol. 2002, 48, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Mikulíková, D. The effect of friabilin on wheat grain hardness. Czech J. Genet. Plant Breed. 2007, 43, 35–43. [Google Scholar] [CrossRef]
- Bhave, M.; Morris, C.F. Molecular genetics of puroindolines and related genes: Regulation of expression, membrane binding properties and applications. Plant Mol. Biol. 2008, 66, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Lullien-Pellerin, V.; Haraszi, R.; Anderssen, R.S.; Morris, C.F. Understanding the mechanics of wheat grain fractionation and the impact of puroindolines on milling and product quality. In Wheat Quality for Improving Processing and Human Health; Igrejas, G., Ikeda, T., Guzmán, C., Eds.; Springer: Cham, Switzerland, 2020; pp. 369–385. [Google Scholar] [CrossRef]
- Giroux, M.J.; Morris, C.F. Wheat grain hardness results from highly conserved mutations in the friabilin components puroindoline a and b. Proc. Natl. Acad. Sci. USA 1998, 95, 6262–6266. [Google Scholar] [CrossRef]
- Gazza, L.; Nocente, F.; Ng, P.K.W.; Pogna, N.E. Genetic and biochemical analysis of common wheat cultivars lacking puroindoline a. Theor. Appl. Genet. 2005, 110, 470–478. [Google Scholar] [CrossRef]
- Ni, D.; Yang, F.; Lin, L.; Sun, C.; Ye, X.; Wang, L.; Kong, X. Interrelating grain hardness index of wheat with physicochemical and structural properties of starch extracted therefrom. Foods 2022, 11, 1087. [Google Scholar] [CrossRef] [PubMed]
- Tranquilli, G.; Heaton, J.; Chicaiza, O.; Dubcovsky, J. Substitutions and deletions of genes related to grain hardness in wheat and their effect on grain texture. Crop Sci. 2002, 42, 1812–1817. [Google Scholar] [CrossRef]
- Gasparis, S.; Nadolska-Orczyk, A. Genetic background of wheat and triticale grain hardness. Biul. Inst. Hod. i Aklim. Roślin 2013, 267, 17–29. (In Polish) [Google Scholar]
- Nucia, A.; Okoń, S.; Tomczyńska-Mleko, M.; Nawrocka, A. Molecular and physical characterization of grain hardness in European spring common wheat (Triticum aestivum L.). 3 Biotech 2021, 11, 345. [Google Scholar] [CrossRef]
- Capparelli, R.; Amoroso, M.G.; Palumbo, D.; Iannaccone, M.; Faleri, C.; Cresti, M. Two plant puroindolines colocalize in wheat seed and in vitro synergistically fight against pathogens. Plant Mol. Biol. 2005, 58, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Wiley, P.R.; Tosi, P.; Evrard, A.; Lovergrove, A.; Jones, H.D.; Shewry, P.R. Promoter analysis and immunolocalisation show that puroindoline genes are exclusively expressed in starchy endosperm cells of wheat grain. Plant Mol. Biol. 2007, 64, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Feiz, L.; Wanjugi, H.W.; Melnyk, C.W.; Altossar, I.; Martin, J.M.; Giroux, M.J. Puroindolines co-localize to the starch granule surface and increase seed bound polar lipid content. J. Cereal Sci. 2009, 50, 91–98. [Google Scholar] [CrossRef]
- Tu, M.; Li, Y. Toward the genetic basis and multiple QTLs of kernel hardness in wheat. Plants 2020, 9, 1631. [Google Scholar] [CrossRef] [PubMed]
- Beecher, B.; Bettge, A.; Smidansky, E.; Giroux, M.J. Expression of wild-type PinB sequence in transgenic wheat complements a hard phenotype. Theor. Appl. Genet. 2002, 105, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wilkinson, M.; Tosi, P.; He, G.; Shewry, P. Novel puroindoline and grain softness protein alleles in Aegilops species with the C, D, S, M and U genomes. Theor. Appl. Genet. 2005, 111, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Preston, K.; Krokhin, O.; Mellish, J.; Ens, W. Characterization of wheat gluten proteins by HPLC and MALDI TOF mass spectrometry. J. Am. Soc. Mass Spectrom. 2008, 19, 1542–1550. [Google Scholar] [CrossRef]
- Obuchowski, W.; Salmanowicz, B.; Banaszak, Z.; Adamski, T.; Surma, M.; Kaczmarek, Z.; Majcher, M.; Ługowska, B.; Kuczyńska, A.; Krystkowiak, K. Grain hardness of wheat bred in Poland and its relationship to starch damage during milling. Int. Agrophys. 2010, 24, 69–74. [Google Scholar]
- Zhang, Y.; Hu, X.; Juhasz, A.; Islam, S.; Yu, Z.; Zhao, Y.; Li, G.; Ding, W.; Ma, W. Characterising avenin-like proteins (ALPs) from albumin/globulin fraction of wheat grains by RP-HPLC, SDS-PAGE, and MS/MS peptides sequencing. BMC Plant Biol. 2020, 20, 45. [Google Scholar] [CrossRef]
- Martin, J.M.; Meyer, F.D.; Smidansky, E.D.; Wanjugi, H.; Blechl, A.E.; Giroux, M.J. Complementation of the pina (null) allele with the wild type Pina sequence restores a soft phenotype in transgenic wheat. Theor. Appl. Genet. 2006, 113, 1563–1570. [Google Scholar] [CrossRef]
- Li, G.; He, Z.; Peňa, R.J.; Xia, X.; Lillemo, M.; Qixin, S. Identification of novel secaloindoline-a and secaloindoline-b alleles in CIMMYT hexaploid triticale lines. J. Cereal Sci. 2006, 43, 378–386. [Google Scholar] [CrossRef]
- Chantret, N.; Salse, J.; Sabot, F.; Rahman, S.; Bellec, A.; Laubin, B.; Dubois, I.; Dossat, C.; Sourdille, P.; Joudrier, P.; et al. Molecular basis of evolutionary events that shaped the Hardness locus in diploid and polyploid wheat species (Triticum and Aegilops). Plant Cell 2005, 17, 1033–1045. [Google Scholar] [CrossRef]
- Wanjugi, H.W.; Hogg, A.C.; Martin, J.M.; Giroux, M.J. The role of puroindoline A and B individually and in combination on grain hardness and starch association. Crop Sci. 2007, 47, 67–76. [Google Scholar] [CrossRef]
- ISO 20483:2013; Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/59162.html (accessed on 16 January 2023).
- ISO 2171:2007; Cereals, Pulses and By-Products—Determination of Ash Yield by Incineration. International Organization for Standardization: Geneva, Switzerland, 2007. Available online: https://www.iso.org/standard/37264.html (accessed on 16 January 2023).
- Symons, S.J.; Van Schepdael, L.; Dexter, J.E. Measurement of hard vitreous kernels in durum wheat by machine vision. Cereal Chem. 2003, 80, 511–517. [Google Scholar] [CrossRef]
- AACC method 55-31.01. Single-kernel characterization system for wheat kernel texture. In AACC Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: Eagan, MN, USA, 2010. [CrossRef]
- Martin, C.R.; Rousser, R.; Brabec, D.L. Development of a single-kernel wheat characterization system. Trans. ASAE 1993, 36, 1399–1404. [Google Scholar] [CrossRef]
- ISO 24333:2009; Cereals and Cereal Products—Sampling. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/42165.html (accessed on 16 January 2023).
- Dziki, D.; Cacak-Pietrzak, G.; Miś, A.; Jończyk, K.; Gawlik-Dziki, U. Influence of wheat kernel physical properties on the pulverizing process. J. Food Sci. Technol. 2014, 51, 2648–2655. [Google Scholar] [CrossRef] [PubMed]
- Babić, L.; Babić, M.; Turan, J.; Matić-Kekić, S.; Radojčin, M.; Mehandžić-Stanišić, S.; Pavkova, I.; Zoranović, M. Physical and stress-strain properties of wheat (Triticum aestivum) kernel. J. Sci. Food Agric. 2011, 91, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.F.; Bhave, M. Reconciliation of D-genome puroindoline allele designations with current DNA sequence data. J. Cereal Sci. 2008, 48, 277–287. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Liu, D.; Yang, W.; Wang, D.; Tong, Y.; Zhang, A. Analysis of Pina and Pinb alleles in the micro-core collections of Chinese wheat germplasm by ecotilling and identification of a novel Pinb allele. J. Cereal Sci. 2008, 48, 836–842. [Google Scholar] [CrossRef]
- Feiz, L.; Martin, J.M.; Giroux, M.J. Creation and functional analysis of new puroindoline alleles in Triticum aestivum. Theor. Appl. Genet. 2009, 118, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Bettge, A.D.; Morris, C.F.; Greenblatt, G.A. Assessing genotypic softness in single wheat kernels using starch granule-associated friabilin as a biochemical marker. Euphytica 1995, 86, 65–72. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, H.P.; Xu, J.; Li, W.H.; Liu, G.T.; You, M.S.; Li, B.Y. Identification of allelic variations of puroindoline genes controlling grain hardness in wheat using a modified denaturing PAGE. Euphytica 2006, 152, 225–234. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Gromova, I.; Celis, J.E. Chapter 27—Protein detection in gels by silver staining: A procedure compatible with mass spectrometry. In Cell Biology. A Laboratory Handbook, 3rd ed.; Celis, J.E., Carter, N., Simons, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 4, pp. 219–223. [Google Scholar] [CrossRef]
- Gasparis, S. Analysis of Pina and Pinb Genes Determining Grain Hardness in Hexaploid Wheat and Their Orthologs in Triticale; Instytut Hodowli i Aklimatyzacji Roślin—Państwowy Instytut Badawczy: Radzików, Poland, 2012. (In Polish) [Google Scholar]
- Greffeuille, V.; Abecassis, J.; Rousset, M.; Oury, F.-X.; Faye, A.; L’Helgouac’h, C.B.; Lullien-Pellerin, V. Grain characterization and milling behaviour of near-isogenic lines differing by hardness. Theor. Appl. Genet. 2006, 114, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hrušková, M.; Švec, I. Wheat hardness in relation to other quality factors. Czech J. Food Sci. 2009, 27, 240–248. [Google Scholar] [CrossRef]
- Oury, F.X.; Lasme, P.; Michelet, C.; Rousset, M.; Abecassis, J.; Lullien-Pellerin, V. Relationships between wheat grain physical characteristics studied through near-isogenic lines with distinct puroindoline-b allele. Theor. Appl. Genet. 2015, 128, 913–929. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Varga, A.C.; Jolánkai, M.; Safranyik, F. Applying infrared technique as a nondestructive method to assess wheat grain hardness. Int. J. Sci. Qual. Anal. 2018, 4, 100–107. Available online: https://article.sciencepublishinggroup.com/pdf/10.11648.j.ijsqa.20180403.16.pdf (accessed on 16 January 2023).
- Manai-Djebali, H.; Oueslati, I.; Nouairi, I.; Taamalli, A.; Nait-Mohamed, S.; Mliki, A.; Ghorbel, A. Chemical composition of durum wheat kernels: Impact of the growing location. Euro-Mediterr. J. Environ. Integr. 2021, 6, 26. [Google Scholar] [CrossRef]
- El-Khayat, G.H.; Samaan, J.; Manthey, F.A.; Fuller, M.P.; Brennan, C.S. Durum wheat quality I: Some physical and chemical characteristics of Syrian durum wheat genotypes. Int. J. Food Sci. Technol. 2006, 41, 22–29. [Google Scholar] [CrossRef]
- Boz, H.; Gerçekaslan, K.E.; Karaoğlu, M.M.; Kotancilar, H.G. Differences in some physical and chemical properties of wheat grains from different parts within the spike. Turk. J. Agric. For. 2012, 36, 309–316. [Google Scholar] [CrossRef]
- Korkut, K.Z.; Bílgín, O.; Başer, Í.; Sağlam, N. Stability of grain vitreousness in durum wheat (Triticum durum L. Desf.) genotypes in the north-western region of Turkey. Turk. J. Agric. For. 2007, 31, 313–318. [Google Scholar]
- Manley, M.; du Toit, G.; Geladi, P. Tracking diffusion of conditioning water in single wheat kernels of different hardnesses by near infrared hyperspectral imaging. Anal. Chim. Acta 2011, 686, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.X.; Wang, K.; Dupuis, B.; Taylor, D.; Nam, S. Kernel vitreousness and protein content: Relationship, interaction and synergistic effects on durum wheat quality. J. Cereal Sci. 2017, 78, 2–9. [Google Scholar] [CrossRef]
- Woźniak, A.; Gontarz, D. Evaluation of selected quality features of grain of durum wheat cv. Floradur depending on tillage and nitrogen fertilisation. Acta Agroph. 2011, 18, 481–489. [Google Scholar]
- Mahesh, S.; Jayas, D.S.; Paliwal, J.; White, N.D.G. Comparison of partial least squares regression (PLSR) and principal components regression (PCR) methods for protein and hardness predictions using the near-infrared (NIR) hyperspectral images of bulk samples of Canadian wheat. Food Bioproc. Tech. 2015, 8, 31–40. [Google Scholar] [CrossRef]
- Dobraszczyk, B.J.; Withworth, M.B.; Vincent, J.F.V.; Khan, A.A. Single kernel wheat hardness and fracture properties in relation to density and the modeling of fracture in wheat endosperm. J. Cereal Sci. 2002, 35, 245–263. [Google Scholar] [CrossRef]
- Anderssen, R.; Haraszi, R. Characterizing and exploiting the rheology of wheat hardness. Eur. Food Res. Technol. 2009, 229, 159–174. [Google Scholar] [CrossRef]
- Erkinbaev, C.; Derksen, K.; Paliwal, J. Single kernel wheat hardness estimation using near infrared hyperspectral imaging. Infrared Phys. Technol. 2019, 98, 250–255. [Google Scholar] [CrossRef]
- Misailidis, N.; Campbell, G. Interpreting crush response profiles from the single kernel characterization system. J. Cereal Sci. 2013, 57, 222–229. [Google Scholar] [CrossRef]
- Dziki, D.; Laskowski, J. Wheat kernel physical properties and milling process. Acta Agroph. 2005, 6, 59–71. [Google Scholar]
- Yücel, C.; Baloch, F.S.; Özkan, H. Genetic analysis of some physical properties of bread wheat grain (Triticum aestivum L. em Thell). Turk. J. Agric. For. 2009, 33, 525–535. [Google Scholar] [CrossRef]
- Boac, J.M.; Casada, M.E.; Maghirang, R.G.; Harner, J.P., III. Material and interaction properties of selected grains and oilseeds for modeling discrete particles. Trans. ASABE 2010, 53, 1201–1216. [Google Scholar] [CrossRef]
- Kalkan, F.; Kara, M. Handling, frictional and technological properties of wheat as affected by moisture content and cultivar. Powder Technol. 2011, 213, 116–122. [Google Scholar] [CrossRef]
- Markowski, M.; Żuk-Gołaszewska, K.; Kwiatkowski, D. Influence of variety on selected physical and mechanical properties of wheat. Ind. Crops Prod. 2013, 47, 113–117. [Google Scholar] [CrossRef]
- Gorji, A.; Rajabipour, A.; Tawakoli, H. Fracture resistance of wheat grain as a function of moisture content, loading rate and grain orientation. Aust. J. Crop Sci. 2010, 4, 448–452. [Google Scholar]
- Voicu, G.; Tudosie (Stefan), E.M.; Ungureanu, N.; Constantin, G.A. Some mechanical characteristics of wheat seeds obtained by uniaxial compression tests. UPB Sci. Bull. Ser. D 2013, 75, 265–278. [Google Scholar]
- Langer, M.; Salmanowicz, B.P. Identification of puroindoline alleles in Polish wheat cultivars by molecular marker. Biul. Inst. Hod. Aklim. Rośl. 2009, 253, 93–101. (In Polish) [Google Scholar]
- Anjum, F.M.; Walker, C.E. Review on the significance of starch and protein to wheat kernel hardness. J. Sci. Food Agric. 2006, 56, 1–13. [Google Scholar] [CrossRef]
- Geneix, N.; Dalgalarrondo, M.; Tassy, C.; Nadaud, I.; Barret, P.; Bakan, B.; Elmorjani, K.; Marion, D. Relationships between puroindoline A-prolamin interactions and wheat grain hardness. PLoS ONE 2020, 15, e0225293. [Google Scholar] [CrossRef]




| Cultivar | Moisture [% DB] | Crude Protein [% DM] | Total Ash [% DM] | Vitreousness [%] | Hardness Index HI [–] |
|---|---|---|---|---|---|
| Chinese Spring | 12.63 ± 0.32 de | 12.69 ± 0.03 bc | 2.01 ± 0.01 j | 0 a | 39.74 ± 11.56 a |
| Türkis | 12.75 ± 0.39 fg | 14.17 ± 0.01 g | 1.55 ± 0.01 a | 2.60 ± 0.55 a | 48.16 ± 16.36 b |
| Astoria | 12.83 ± 0.39 g | 14.82 ± 0.02 k | 1.68 ± 0.02 d | 54.00 ± 4.74 c | 53.22 ± 13.79 c |
| Julius | 12.99 ± 0.40 h | 13.66 ± 0.03 f | 1.64 ± 0.02 cd | 10.20 ± 2.39 b | 57.81 ± 16.83 d |
| Fregata | 12.57 ± 0.36 cd | 15.36 ± 0.10 l | 1.68 ± 0.02 d | 72.20 ± 1.92 de | 66.08 ± 12.82 e |
| Radunia | 12.60 ± 0.41 de | 14.52 ± 0.08 hi | 2.02 ± 0.01 j | 54.60 ± 1.95 c | 66.64 ± 14.09 e |
| Parabola | 12.62 ± 0.40 de | 14.78 ± 0.05 jk | 2.01 ± 0.01 j | 72.80 ± 3.08 de | 67.26 ± 13.80 e |
| Cytra | 12.80 ± 0.43 fg | 14.59 ± 0.06 ij | 1.85 ± 0.03 fg | 49.00 ± 1.23 c | 68.30 ± 14.66 e |
| Tonacja | 12.58 ± 0.43 de | 13.42 ± 0.12 e | 1.73 ± 0.02 e | 80.20 ± 1.48 ef | 70.35 ± 12.93 ef |
| Askalon | 13.08 ± 0.35 h | 14.11 ± 0.14 g | 1.62 ± 0.01 bc | 86.80 ± 2.77 gh | 73.89 ± 15.16 fg |
| Magic | 12.81 ± 0.43 fg | 14.58 ± 0.03 ij | 1.57 ± 0.01 ab | 61.60 ± 2.19 d | 76.76 ± 14.99 gh |
| Nutka | 12.70 ± 0.37 ef | 13.14 ± 0.05 d | 1.82 ± 0.01 f | 82.60 ± 2.41 fg | 77.87 ± 13.08 g–i |
| Bamberka | 13.07 ± 0.33 h | 14.31 ± 0.08 gh | 1.65 ± 0.01 cd | 89.40 ± 0.89 h | 80.56 ± 12.36 h–j |
| SMH196 | 12.52 ± 0.48 cd | 12.72 ± 0.08 bc | 1.87 ± 0.01 gh | 77.20 ± 1.48 e | 81.89 ± 18.49 ij |
| SMH87 | 12.34 ± 0.47 b | 13.52 ± 0.09 ef | 1.96 ± 0.01 i | 84.80 ± 1.64 f–h | 83.66 ± 16.84 j |
| Ceres | 12.16 ± 0.43 a | 12.85 ± 0.05 c | 1.93 ± 0.02 hi | 87.80 ± 2.27 h | 85.96 ± 17.40 k |
| SMH200 | 12.46 ± 0.41 c | 12.52 ± 0.05 b | 1.84 ± 0.01 fg | 85.40 ± 1.67 gh | 88.17 ± 17.88 k |
| SMH214 | 12.81 ± 0.44 g | 12.20 ± 0.02 a | 1.90 ± 0.01 hi | 81.20 ± 2.09 e–g | 88.62 ± 20.05 k |
| Cultivar | Length [mm] | Width [mm] | Thickness [mm] | Mass [mg] | Aspect Ratio [%] | Sphericity [%] |
|---|---|---|---|---|---|---|
| Chinese Spring | 5.41 ± 0.23 a | 2.76 ± 0.27 a | 2.46 ± 0.17 a | 26.960 ± 5.58 a | 50.91 ± 3.90 cd | 61.33 ± 2.56 gh |
| Türkis | 6.22 ± 0.39 c–e | 3.61 ± 0.39 ef | 2.96 ± 0.24 d–f | 47.38 ± 9.90 d–f | 58.03 ± 5.26 h | 65.05 ± 2.95 k |
| Astoria | 6.33 ± 0.37 e | 3.48 ± 0.32 c–f | 2.87 ± 0.29 b–e | 45.04 ± 10.33 c–e | 55.09 ± 4.15 e–g | 62.97 ± 3.04 h–j |
| Julius | 6.24 ± 0.40 de | 3.43 ± 0.33 b–f | 2.94 ± 0.23 d–f | 46.07 ± 9.49 c–f | 55.07 ± 4.96 e–g | 63.70 ± 2.76 i–k |
| Fregata | 6.23 ± 0.37 de | 3.52 ± 0.25 d-f | 3.04 ± 0.27 f | 47.97 ± 8.54 d–f | 56.55 ± 2.83 f–h | 65.10 ± 1.91 k |
| Radunia | 6.34 ± 0.38 e | 3.31 ± 0.35 bc | 2.95 ± 0.29 d–f | 42.86 ± 9.99 b–d | 52.20 ± 4.71 cd | 62.31 ± 3.16 g–i |
| Parabola | 6.96 ± 0.56 fg | 3.36 ± 0.35 b–d | 3.02 ± 0.32 ef | 49.06 ± 10.96 ef | 48.38 ± 5.20 bc | 59.36 ± 3.44 ef |
| Cytra | 5.78 ± 0.41 b | 3.29 ± 0.36 bc | 2.75 ± 0.24 bc | 37.46 ± 8.68 b | 56.97 ± 5.45 f–h | 64.68 ± 3.24 jk |
| Tonacja | 6.74 ± 0.40 f | 3.62 ± 0.25 f | 2.97 ± 0.23 d–f | 51.62 ± 8.16 f | 53.73 ± 3.16 de | 61.81 ± 2.12 gh |
| Askalon | 5.99 ± 0.26 b-d | 3.30 ± 0.31 bc | 2.73 ± 0.21 b | 40.51 ± 8.35 bc | 55.02 ± 4.06 e–g | 62.98 ± 2.51 h–j |
| Magic | 5.96 ± 0.38 bc | 3.26 ± 0.29 b | 2.82 ± 0.22 b–d | 40.89 ± 8.54 bc | 54.63 ± 3.61 d–f | 63.67 ± 2.18 i–k |
| Nutka | 6.93 ± 0.37 f | 3.63 ± 0.23 f | 2.94 ± 0.16 d–f | 51.50 ± 6.54 f | 52.41 ± 2.85 c–e | 60.57 ± 1.89 fg |
| Bamberka | 6.26 ± 0.31 e | 3.59 ± 0.30 ef | 3.05 ± 0.27 f | 49.77 ± 9.03 ef | 57.41 ± 3.94 gh | 65.35 ± 3.07 k |
| SMH196 | 7.78 ± 0.50 jk | 3.27 ± 0.29 b | 3.03 ± 0.22 ef | 51.54 ± 10.10 f | 42.13 ± 3.84 a | 54.71 ± 2.80 ab |
| SMH87 | 7.54 ± 0.41 ij | 3.24 ± 0.24 b | 3.01 ± 0.26 ef | 50.49 ± 9.01 ef | 43.06 ± 2.79 a | 55.58 ± 2.39 bc |
| Ceres | 7.99 ± 0.40 k | 3.24 ± 0.27 b | 3.00 ± 0.24 ef | 52.02 ± 10.40 f | 40.57 ± 3.30 a | 53.36 ± 2.65 a |
| SMH200 | 7.32 ± 0.47 hi | 3.37 ± 0.28 b–d | 2.90 ± 0.20 c–f | 48.10 ± 8.38 d-f | 46.22 ± 4.16 b | 56.76 ± 2.83 cd |
| SMH214 | 7.20 ± 0.46 gh | 3.42 ± 0.26 b–e | 3.01 ± 0.21 ef | 48.88 ± 9.11 ef | 47.60 ± 3.57 b | 58.37 ± 2.74 de |
| Cultivar | Rupture Force [N] | Longitudinal Strain [mm] | Relative Strain [%] | Rupture Energy [mJ] |
|---|---|---|---|---|
| Chinese Spring | 76.10 ± 13.22 a | 0.46 ± 0.05 a | 18.57 ± 1.75 fg | 11.55 ± 3.57 a |
| Türkis | 100.26 ± 18.70 b–d | 0.46 ± 0.04 a | 15.58 ± 1.63 ab | 15.51 ± 4.50 a–d |
| Astoria | 105.00 ± 18.93 b–e | 0.50 ± 0.05 bc | 17.50 ± 1.93 c–f | 18.69 ± 5.93 c–f |
| Julius | 92.43 ± 22.10 ab | 0.45 ± 0.05 a | 15.28 ± 1.83 a | 13.83 ± 5.07 ab |
| Fregata | 113.05 ± 22.06 c–f | 0.51 ± 0.06 b–e | 16.93 ± 2.16 b–d | 20.49 ± 7.60 ef |
| Radunia | 117.50 ± 27.90 d–f | 0.50 ± 0.06 b–d | 17.10 ± 1.73 c–e | 19.74 ± 6.83 d–f |
| Parabola | 111.96 ± 27.79 c–f | 0.52 ± 0.10 c–e | 17.40 ± 3.56 c–f | 20.49 ± 8.25 ef |
| Cytra | 102.76 ± 17.69 b–d | 0.46 ± 0.04 a | 16.79 ± 1.49 bc | 15.93 ± 4.04 a–d |
| Tonacja | 140.75 ± 19.93 gh | 0.54 ± 0.06 d–f | 18.29 ± 2.03 ef | 25.08 ± 7.14 gh |
| Askalon | 98.51 ± 21.39 bc | 0.46 ± 0.04 a | 16.93 ± 1.40 b–d | 14.81 ± 4.62 a–c |
| Magic | 112.32 ± 23.57 c–f | 0.48 ± 0.04 ab | 16.93 ± 1.51 b–d | 17.73 ± 5.47 b–e |
| Nutka | 123.70 ± 26.47 fg | 0.64 ± 0.07 h | 21.84 ± 2.13 j | 22.65 ± 7.63 fg |
| Bamberka | 126.10 ± 17.83 fg | 0.52 ± 0.05 c–e | 16.98 ± 1.17 c–e | 20.95 ± 4.49 e–g |
| SMH196 | 122.41 ± 36.36 ef | 0.55 ± 0.07 ef | 18.22 ± 2.72 d–f | 21.32 ± 9.60 e–g |
| SMH87 | 124.80 ± 28.39 fg | 0.60 ± 0.06 f | 19.91 ± 2.30 gh | 21.95 ± 7.59 e–g |
| Ceres | 154.87 ± 40.96 h | 0.64 ± 0.06 h | 21.27 ± 2.09 ij | 28.43 ± 10.34 h |
| SMH200 | 154.35 ± 28.40 h | 0.58 ± 0.05 fg | 19.98 ± 1.85 hi | 27.69 ± 7.76 h |
| SMH214 | 152.77 ± 37.99 h | 0.59 ± 0.06 g | 19.82 ± 1.97 gh | 28.89 ± 9.97 h |
| Property | Correlation with Friabilin Content |
|---|---|
| Moisture | 0.368 |
| Crude protein | 0.109 |
| Total ash | −0.083 |
| Vitreousness | −0.492 |
| Hardness index, HI | −0.600 |
| Length | −0.621 |
| Width | −0.153 |
| Thickness | −0.584 |
| Mass | −0.552 |
| Aspect ratio | 0.436 |
| Sphericity | 0.445 |
| Rupture force | −0.592 |
| Longitudinal strain | −0.435 |
| Relative strain | −0.213 |
| Rupture energy | −0.552 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaliniewicz, Z.; Markowska-Mendik, A.; Warechowska, M.; Lipiński, S.; Gasparis, S. Correlations between a Friabilin Content Indicator and Selected Physicochemical and Mechanical Properties of Wheat Grain for Processing Suitability Assessment. Processes 2024, 12, 398. https://doi.org/10.3390/pr12020398
Kaliniewicz Z, Markowska-Mendik A, Warechowska M, Lipiński S, Gasparis S. Correlations between a Friabilin Content Indicator and Selected Physicochemical and Mechanical Properties of Wheat Grain for Processing Suitability Assessment. Processes. 2024; 12(2):398. https://doi.org/10.3390/pr12020398
Chicago/Turabian StyleKaliniewicz, Zdzisław, Agnieszka Markowska-Mendik, Małgorzata Warechowska, Seweryn Lipiński, and Sebastian Gasparis. 2024. "Correlations between a Friabilin Content Indicator and Selected Physicochemical and Mechanical Properties of Wheat Grain for Processing Suitability Assessment" Processes 12, no. 2: 398. https://doi.org/10.3390/pr12020398
APA StyleKaliniewicz, Z., Markowska-Mendik, A., Warechowska, M., Lipiński, S., & Gasparis, S. (2024). Correlations between a Friabilin Content Indicator and Selected Physicochemical and Mechanical Properties of Wheat Grain for Processing Suitability Assessment. Processes, 12(2), 398. https://doi.org/10.3390/pr12020398