A Green Process for the Extraction and Purification of Hesperidin from Mexican Lime Peel (Citrus aurantifolia Swingle) that is Extendible to the Citrus Genus
Abstract
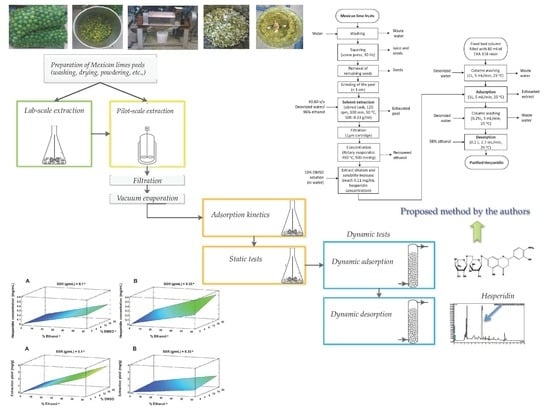
:1. Introduction
2. Materials and Methods
2.1. Hesperidin Content
2.2. Extraction
2.2.1. Lab-Scale Extraction
2.2.2. Pilot-Scale Extraction
2.3. Purification
2.3.1. Static Tests
Adsorption Kinetics
Adsorption Efficiency
2.3.2. Dynamic Tests
- First wash: The column was washed with 1 L of deionized water (5 mL/min).
- Adsorption: 1 L of diluted extract was passed through the column (5 mL/min). Samples at 25 mL, 50 mL, and 100 mL were collected at the exit of the column, until the whole volume had been treated.
- Second wash: The column was washed with 250 mL of deionized water (5 mL/min) to remove any remaining extract (water does not desorb the hesperidin from the column).
- Desorption: 1 L of ethanol 96% was passed through the column (2.7 mL/min) to recover the adsorbed hesperidin. Samples at 50 mL and 100 mL were collected until there was no volume of ethanol left in the column.
2.4. HPLC Analysis
2.5. Total Flavonoids
3. Results and Discussion
3.1. Hesperidin Content
3.2. Extraction
3.2.1. Lab-Scale Extraction
3.2.2. Pilot-Scale Extraction
3.3. Purification
3.3.1. Static Tests
Adsorption Kinetics
Adsorption Efficiency
3.3.2. Dynamic Tests
Adsorption
Desorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT (2016). Food and Agricultural Organization of the United Nations. Statistics Division. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 1 December 2018).
- Ledesma-Escobar, C.A.; Luque de Castro, M.D. Towards a comprehensive exploitation of citrus. Trends Food Sci. Technol. 2014, 39, 63–75. [Google Scholar] [CrossRef]
- Londoño-Londoño, J.; De Lima, V.R.; Lara, O.; Gil, A.; Pasa, T.B.C.; Arango, G.J.; Pineda, J.R.R. Clean recovery of antioxidant flavonoids from citrus peel: Optimizing an aqueous ultrasound-assisted extraction method. Food Chem. 2010, 119, 81–87. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Güemez, F.J.; Zapata, J.L.; González, E.; Kú, M.; Lechuga, P.; Salinas, A. Potencialidades del mercado nacional e internacional de la hesperidina de origen natural obtenida de la naranja como sustituto de antioxidantes químicos en la industria alimentaria. In Proceedings of the 5° Congreso Internacional de Sistemas de Innovación para la Competitividad, Celaya, México, 25–27 August 2010; pp. 1–22. [Google Scholar]
- Galati, E.M.; Monforte, M.T.; Kirjavainen, S.; Forestieri, A.M.; Trovato, A.; Tripodo, M.M. Biological effects of hesperidin, a citrus flavonoid. (Note I): Antiinflammatory and analgesic activity. Farmaco 1994, 40, 709–712. [Google Scholar] [PubMed]
- Bok, S.H.; Lee, S.H.; Park, Y.B.; Bae, K.H.; Son, K.H.; Jeong, T.S.; Choi, M.S. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA: cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J. Nutr. 1999, 129, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.M.; Kirjavainen, S.; Forestieri, A.M.; Rossitto, A.; Monforte, M.T. Biological effects of hesperidin, a Citrus flavonoid. (Note III): antihypertensive and diuretic activity in rat. Farmaco. 1996, 51, 219–221. [Google Scholar] [PubMed]
- Raza, S.S.; Khan, M.M.; Ahmad, A.; Ashafaq, M.; Khuwaja, G.; Tabassum, R.; Javed, H.; Siddiqui, M.S.; Safhi, M.M.; Islam, F. Hesperidin ameliorates functional and histological outcome and reduces neuroinflammation in experimental stroke. Brain Res. 2011, 1420, 93–105. [Google Scholar] [CrossRef]
- Chen, M.C.; Ye, Y.Y.; Guang, J.I.; Jian-Wen, L.I.U. Hesperidin upregulates heme oxygenase-1 to attenuate hydrogen peroxide-induced cell damage in hepatic L02 cells. J. Agric. Food Chem. 2010, 58, 3330–3335. [Google Scholar] [CrossRef]
- Dugo, G.; Di Giacomo, A. Citrus: The genus Citrus, 1st ed.; Taylor & Francis: London, UK, 2002; pp. 169–170. ISBN 0415-28491-0. [Google Scholar]
- De Oliveira, R.C.; Davantel De Barros, S.T.; Gimenes, M.L. The extraction of passion fruit oil with green solvents. J. Food Eng. 2013, 117, 458–463. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Di Mauro, A.; Fallico, B.; Passerini, A.; Maccarone, E. Waste water from citrus processing as a source of hesperidin by concentration on styrene-divinylbenzene resin. J. Agric. Food Chem. 2000, 48, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, A.; Fallico, B.; Passerini, A.; Rapisarda, P.; Maccarone, E. Recovery of hesperidin from orange peel by concentration of extracts on styrene-divinylbenzene resin. J. Agric. Food Chem. 1999, 47, 4391–4397. [Google Scholar] [CrossRef] [PubMed]
- Scordino, M.; Di Mauro, A.; Passerini, A.; Maccarone, E. Adsorption of flavonoids on resins: Hesperidin. J. Agric. Food Chem. 2003, 51, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, V.L. Extracción y caracterización de flavonoides contenidos en limón persa (Citrus Latifolia Tanaka) en un proceso a nivel piloto. Bachelor’s Thesis, Universidad de Guadalajara, Guadalajara, Mexico, 2009. [Google Scholar]
- Velásquez, M.N. Extracción y purificación de hesperidina a partir de cáscara de limón persa. Bachelor’s Thesis, Instituto Tecnológico de Durango, Durango, Mexico, 2010. [Google Scholar]
- Londoño, J.; Sierra, J. Efecto de la hesperidina sobre la captacion de hdl en células hepáticas y evaluacion de hesperidina liposomal sobre la oxidacion de LDL. Sci. Tec. 2007, 8, 63–66. [Google Scholar] [CrossRef]
- Selleckchem: Hesperidin. Available online: http://www.selleckchem.com/products/Hesperidin.html (accessed on 15 October 2016).
- Soroko, I.; Bhole, Y.; Livingston, A.G. Environmentally friendly route for the preparation of solvent resistant polyimide nanofiltration membranes. Green Chem. 2011, 13, 162–168. [Google Scholar] [CrossRef]
- McKim, A.S.; Strub, R. Dimethyl Sulfoxide USP, PhEur in Approved Pharmaceutical Products and Medical Devices. Pharm. Technol. 2008, 32, 74–85. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Escobar, M.; Hernández, H.Y.; Barragán, B.E. Extracción de compuestos fenólicos de cáscaras de cítricos producidos en México (Naranja valencia, Naranja agria, Limon mexicano, Limon real, Mandarina, Toronja y Lima). In XVII Congreso Nacional de Ingenieria Bioquímica; Colegio Mexicano de Ingenieros Bioquímicos: Acapulco, México, 2010. [Google Scholar]
- Andersen, O.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry and Applications, 1st ed.; Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 923–924. ISBN 9780849320217. [Google Scholar]
- Ladaniya, M.S. Citrus Fruit. Biology, Technology and Evaluation, 1st ed.; Academic Press: San Diego, CA, USA, 2008; ISBN 9780080556239. [Google Scholar]
- Berk, Z. Freeze drying (Lyophilization) and freeze concentration. In Food process engineering and technology, 2nd ed.; Academic Press: San Diego, CA, USA, 2013. [Google Scholar]
- Kasper, J.C.; Winter, G.; Friess, W. Recent advances and further challenges in lyophilization. Eur. J. Pharm. Biopharm. 2013, 85, 162–169. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Scordino, M.; Di Mauro, A.; Passerini, A.; Maccarone, E. Highly purified sugar concentrate from a residue of citrus pigments recovery process. LWT-Food Sci. Technol. 2007, 40, 713–721. [Google Scholar] [CrossRef]
- Silva, E.; Pompeu, D.; Larondelle, Y.; Rogez, H. Optimisation of the adsorption of polyphenols from Inga edulis leaves on macroporous resins using an experimental design methodology. Sep. Purif. Technol. 2007, 53, 274–280. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Wu, X.; Zhao, X. Macroporous Resin Adsorption for Purification of Flavonoids in Houttuynia cordata Thunb. Chin. J. Chem. Eng. 2007, 15, 872–876. [Google Scholar] [CrossRef]
- Zhao, Z.; Dong, L.; Wu, Y.; Lin, F. Preliminary separation and purification of rutin and quercetin from Euonymus alatus (Thunb.) Siebold extracts by macroporous resins. Food Bioprod. Process. 2011, 89, 266–272. [Google Scholar] [CrossRef]







| Parameter | Mean Value | Variation Coefficient (%) |
|---|---|---|
| Extract concentration (mg/mL) | 0.078 ± 0.021 | 33.4% |
| Dry peel concentration (mg/g) | 3.528 ± 0.962 | 33.4% |
| Moisture content (%) | 81.57 ± 1.92 | 2.9% |
| Fresh peel concentration (mg/g) | 0.653 ± 0.208 | 39.1% |
| Run | Solid/Solvent “RSS” (g/mL) | %Ethanol | %DMSO | Peels (g) | Hesperidin Concentration (mg/mL) | Yield (mg/g Fresh Peel) |
|---|---|---|---|---|---|---|
| 1 | 0.215 | 30 | 10 | 10.75 | 0.2191 | 1.019 |
| 2 | 0.1 | 0 | 0 | 5 | 0.0220 | 0.220 |
| 3 | 0.1 | 0 | 20 | 5 | 0.0354 | 0.354 |
| 4 | 0.33 | 60 | 0 | 16.5 | 0.2621 | 0.794 |
| 5 | 0.33 | 0 | 0 | 16.5 | 0.0165 | 0.050 |
| 6 | 0.33 | 60 | 20 | 16.5 | 0.5752 | 1.743 |
| 7 | 0.1 | 60 | 0 | 5 | 0.3410 | 3.410 |
| 8 | 0.33 | 0 | 20 | 16.5 | 0.1276 | 0.387 |
| 9 | 0.1 | 60 | 20 | 5 | 0.3631 | 3.631 |
| 10 | 0.215 | 30 | 10 | 10.75 | 0.2343 | 1.089 |
| 11 | 0.215 | 30 | 10 | 10.75 | 0.1814 | 0.843 |
| 12 | 0.1 | 0 | 0 | 5 | 0.0000 | 0.000 |
| 13 | 0.1 | 0 | 20 | 5 | 0.0000 | 0.000 |
| 14 | 0.33 | 60 | 0 | 16.5 | 0.3047 | 0.923 |
| 15 | 0.33 | 0 | 0 | 16.5 | 0.0253 | 0.076 |
| 16 | 0.33 | 60 | 20 | 16.5 | 0.4866 | 1.474 |
| 17 | 0.1 | 60 | 0 | 5 | 0.1994 | 1.994 |
| 18 | 0.33 | 0 | 20 | 16.5 | 0.1070 | 0.324 |
| 19 | 0.1 | 60 | 20 | 5 | 0.2513 | 2.513 |
| 20 | 0.215 | 30 | 10 | 10.75 | 0.1994 | 0.927 |
| Dilution | Hesperidin Concentration (mg/mL) |
|---|---|
| 0 | 0.273 |
| 1 | 0.218 |
| 2 | 0.164 |
| 3 | 0.109 |
| 4 | 0.054 |
| 5 | 0.027 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla de la Rosa, J.D.; Ruiz-Palomino, P.; Arriola-Guevara, E.; García-Fajardo, J.; Sandoval, G.; Guatemala-Morales, G.M. A Green Process for the Extraction and Purification of Hesperidin from Mexican Lime Peel (Citrus aurantifolia Swingle) that is Extendible to the Citrus Genus. Processes 2018, 6, 266. https://doi.org/10.3390/pr6120266
Padilla de la Rosa JD, Ruiz-Palomino P, Arriola-Guevara E, García-Fajardo J, Sandoval G, Guatemala-Morales GM. A Green Process for the Extraction and Purification of Hesperidin from Mexican Lime Peel (Citrus aurantifolia Swingle) that is Extendible to the Citrus Genus. Processes. 2018; 6(12):266. https://doi.org/10.3390/pr6120266
Chicago/Turabian StylePadilla de la Rosa, J. Daniel, Priscilla Ruiz-Palomino, Enrique Arriola-Guevara, Jorge García-Fajardo, Georgina Sandoval, and Guadalupe M. Guatemala-Morales. 2018. "A Green Process for the Extraction and Purification of Hesperidin from Mexican Lime Peel (Citrus aurantifolia Swingle) that is Extendible to the Citrus Genus" Processes 6, no. 12: 266. https://doi.org/10.3390/pr6120266
APA StylePadilla de la Rosa, J. D., Ruiz-Palomino, P., Arriola-Guevara, E., García-Fajardo, J., Sandoval, G., & Guatemala-Morales, G. M. (2018). A Green Process for the Extraction and Purification of Hesperidin from Mexican Lime Peel (Citrus aurantifolia Swingle) that is Extendible to the Citrus Genus. Processes, 6(12), 266. https://doi.org/10.3390/pr6120266