A Mechanistic Model of Mass Transfer in the Extraction of Bioactive Compounds from Intact Sorghum Pericarp
Abstract
:1. Introduction
2. Methods
2.1. Model Development
- The phytochemical compounds of sorghum (phenolic compounds, mainly condensed tannin or proanthocyanidins) are located in the pericarp of a sorghum grain [33].
- The sorghum grain is assumed to have a spherical shape with an average radius of R.
- The geometry of an intact pericarp is assumed to be a slab of thickness L that covers the spherical sorghum grain, since the pericarp’s thickness is very thin when compared to the size of the pericarp.
- P compound diffuses from the inside part of the pericarp to the surface of the pericarp, and the rate of diffusion is assumed to follow Fick’s diffusion law with an effective diffusivity of Dep.
- P compound is transferred from the surface of the pericarp to the bulk of the liquid.
- The pericarp is a homogenous medium; the position of the pericarp is between x = 0 and x = L.
- The effective diffusivity of P () is assumed to be constant.
- The solid–liquid equilibrium of P follows the coefficient distribution model with a distribution coefficient value of (Equation (2)).
- The solvent’s penetration to the pericarp is relatively fast when compared with the diffusion process, so it does not control the overall process of mass transfer of P [12].
2.2. Model Solution and Simulation
2.3. Validation
2.3.1. Pretreatment of Red Sorghum Grains
2.3.2. Extraction Process
2.3.3. Proanthocyanidins Quantification in Aqueous Extract
3. Results and Discussion
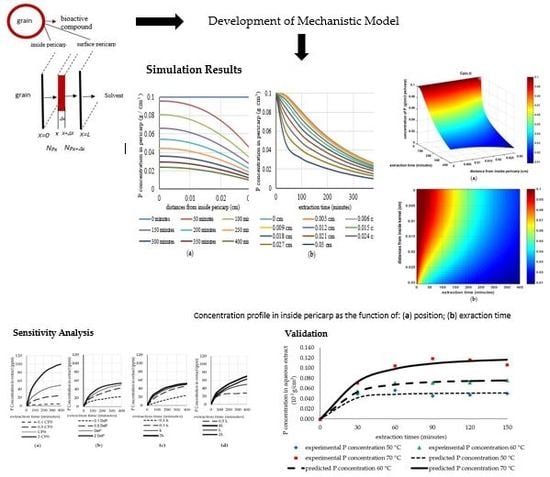
3.1. Simulation Results
3.2. Analysis of the Sensitivity of the Model to Fluctuations in Parameters
3.3. Validation of the Model Using Analytical Results
3.3.1. Validation of the Model under the Conditions of a Very Thick Slab and Very Slow Diffusivity
3.3.2. Validation of the Model under the Condition of a Constant Concentration in the Liquid Phase
3.4. Validation of the Model Using Experimental Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Homayoonfal, M.; Mousavi, S.M.; Kiani, H.; Askari, G.; Khani, M.; Rezazad Bari, M.; Alizadeh, M. The use of an innovative inverse numerical modeling method for the evaluation and parameter estimation of barberry anthocyanins ultrasound assisted extraction. Chem. Eng. Process. Process. Intensif. 2018, 133, 1–11. [Google Scholar] [CrossRef]
- Ali, A.; Lim, X.Y.; Chong, C.H.; Mah, S.H.; Chua, B.L. Ultrasound-assisted extraction of natural antioxidants from betel leaves (Piper betle): Extraction kinetics and modeling. Sep. Sci. Technol. 2018, 53, 2192–2205. [Google Scholar] [CrossRef]
- Boggia, R.; Turrini, F.; Villa, C.; Lacapra, C.; Zunin, P.; Parodi, B. Green extraction from pomegranate marcs for the production of functional foods and cosmetics. Pharmaceuticals 2016, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Tzima, K.; Kallithraka, S.; Kotseridis, Y.; Makris, D.P. Kinetic modelling for flavonoid recovery from red grape (Vitis vinifera) pomace with aqueous lactic acid. Processes 2014, 2, 901–911. [Google Scholar] [CrossRef]
- Bachtler, S.; Bart, H.J. Polyphenols from red vine leaves using alternative processing techniques. Processes 2018, 6, 262. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Ultrasound-Assisted Extraction of Pectin from Malus domestica “Fălticeni” Apple Pomace. Processes 2019, 7, 488. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Cerutti, A.K.; Beccaro, G.L. Biomolecules and natural medicine preparations: Analysis of new sources of bioactive compounds from Ribes and Rubus spp. buds. Pharmaceuticals 2016, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P. Kinetics of ultrasound-assisted flavonoid extraction from agri-food solid wastes using water/glycerol mixtures. Resources 2016, 5, 7. [Google Scholar] [CrossRef]
- Havelt, T.; Brettschneider, S.; Do, X.T.; Korte, I.; Kreyenschmidt, J.; Schmitz, M. Sustainable Extraction and Characterisation of Bioactive Compounds from Horse Chestnut Seed Coats for the Development of Bio-Based Additives. Resources 2019, 8, 114. [Google Scholar] [CrossRef]
- Bridson, J.H.; Al-Hakkak, F.; Steward, D.; Al-Hakkak, J. Preparation and morphological, rheological, and structural characterisation of proanthocyanidin coated starch granules. Ind. Crops Prod. 2019, 130, 285–291. [Google Scholar] [CrossRef]
- Luo, X.; Cui, J.; Zhang, H.; Duan, Y.; Zhang, D.; Cai, M.; Chen, G. Ultrasound assisted extraction of polyphenolic compounds from red sorghum (Sorghum bicolor L.) bran and their biological activities and polyphenolic compositions. Ind. Crops Prod. 2018, 112, 296–304. [Google Scholar] [CrossRef]
- Kurniawati, P.T.; Soetjipto, H.; Limantara, L. Antioxidant and Antibacterial Activities of Bixin Pigment from Annatto (Bixa orellana L.) Seeds. Indones. J. Chem. 2010, 7, 88–92. [Google Scholar] [CrossRef]
- Adnadjevic, B.; Koturevic, B.; Jovanovic, J. Comparative kinetic analysis of isothermal extraction of caffeine from guarana seed under conventional and microwave heating. Chem. Eng. Res. Des. 2017, 118, 61–70. [Google Scholar] [CrossRef]
- Species, F.; Doan, L.P.; Nguyen, T.T.; Pham, M.Q.; Tran, Q.T. Extraction Process, Identification of Fatty Acids, Tocopherols, Sterols and Phenolic Constituents, and Antioxidant Evaluation of Seed Oils from Five Fabaceae Species. Processes 2019, 7, 456. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Barros, L.; Carvalho, A.M.; Oliveira, M.B.P.P.; Saraiva, J.A.; Ferreira, I.C.F.R. Cold extraction of phenolic compounds from watercress by high hydrostatic pressure: Process modelling and optimization. Sep. Purif. Technol. 2018, 192, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Duan, M.H.; Yao, X.H.; Zhang, Y.H.; Zhao, C.J.; Zu, Y.G.; Fu, Y.J. Green extraction of five target phenolic acids from Lonicerae japonicae Flos with deep eutectic solvent. Sep. Purif. Technol. 2016, 157, 249–257. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep. Purif. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.G.; Xi, J. Micro-mechanism analysis of ultrahigh pressure extraction from green tea leaves by numerical simulation. Sep. Purif. Technol. 2017, 180, 51–57. [Google Scholar] [CrossRef]
- Jokić, S.; Šafranko, S.; Jakovljević, M.; Cikoš, A.-M.; Kajić, N.; Kolarević, F.; Babić, J.; Molnar, M. Sustainable Green Procedure for Extraction of Hesperidin from Selected Croatian Mandarin Peels. Processes 2019, 7, 469. [Google Scholar] [CrossRef]
- Mustapa, A.N.; Martin, A.; Gallego, J.R.; Mato, R.B.; Cocero, M.J. Microwave-assisted extraction of polyphenols from Clinacanthus nutans Lindau medicinal plant: Energy perspective and kinetics modeling. Chem. Eng. Process. Process. Intensif. 2015, 97, 66–74. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Creencia, E.C.; Nillama, J.A.P.; Librando, I.L. Microwave-assisted extraction and physicochemical evaluation of oil from Hevea brasiliensis seeds. Resources 2018, 7, 28. [Google Scholar] [CrossRef]
- Belˇ, A.; Valinger, D.; Tuˇ, A.J.; Benkovi, M. Kinetics and thermodynamics of the solid-liquid extraction process of total polyphenols, antioxidants and extraction yield from Asteraceae plants. Ind. Crop. Prod. J. 2016, 91, 205–214. [Google Scholar]
- Dias, A.L.B.; Arroio Sergio, C.S.; Santos, P.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Ultrasound-assisted extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L.): Effects on the vegetable matrix and mathematical modeling. J. Food Eng. 2017, 198, 36–44. [Google Scholar] [CrossRef]
- Kuhn, M.; Lang, S.; Bezold, F.; Minceva, M.; Briesen, H. Time-resolved extraction of caffeine and trigonelline from finely-ground espresso coffee with varying particle sizes and tamping pressures. J. Food Eng. 2017, 206, 37–47. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Brandelli, A.; Marczak, L.D.F.; Tessaro, I.C. Kinetic modeling of total polyphenol extraction from grape marc and characterization of the extracts. Sep. Purif. Technol. 2012, 100, 82–87. [Google Scholar] [CrossRef]
- Krysiak-Baltyn, K.; Martin, G.J.O.; Gras, S.L. Computational modelling of large scale phage production using a two-stage batch process. Pharmaceuticals 2018, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.S.; Barbosa, A.M.; Freitas, V.; Muniz, A.V.C.S.; Mendonça, M.C.; Calhelha, R.C.; Ferreira, I.C.F.R.; Franceschi, E.; Padilha, F.F.; Oliveira, M.B.P.P.; et al. Antiproliferative activity of neem leaf extracts obtained by a sequential pressurized liquid extraction. Pharmaceuticals 2018, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, W.; Dai, G.; Zhang, P.; Tang, K. Process optimization of reactive extraction of clorprenaline enantiomers by experiment and simulation. Chem. Eng. Process. Process. Intensif. 2018, 134, 141–152. [Google Scholar] [CrossRef]
- Pace, D.K. Modeling and simulation verification and validation challenges. Johns Hopkins APL Tech. Dig. (Appl. Phys. Lab.) 2004, 25, 163–172. [Google Scholar]
- Schmidt, A.; Strube, J. Distinct and quantitative validation method for predictive process modeling with examples of liquid-liquid extraction processes of complex feed mixtures. Processes 2019, 7, 298. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C. Modeling and kinetics study of conventional and assisted batch solvent extraction. Chem. Eng. Res. Des. 2014, 92, 1169–1186. [Google Scholar] [CrossRef]
- Cheng, S.; Sun, Y.; Halgreen, L. The Relationship perikarp and tannin content. Asian J. Crop. Sci. 2009, 1, 1–5. [Google Scholar]
- Tao, Y.; Zhang, Z.; Sun, D.W. Kinetic modeling of ultrasound-assisted extraction of phenolic compounds from grape marc: Influence of acoustic energy density and temperature. Ultrason. Sonochem. 2014, 21, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Jimenes, G.C.; Vargas-Garcia, A.; Espinoza-Pérez, D.J.; Salgado-Cervantes, M.A.; Robles-Olvera, V.J.; García-Alvarado, M.A. Mass Transfer During Vanilla Pods Solid Liquid Extraction: Effect of Extraction Method. Food Bioprocess. Technol. 2013, 6, 2640–2650. [Google Scholar] [CrossRef]
- Gessner, M.O.; Steiner, D. Acid Butanol Assay for Proanthocyanidin (Condensed Tannins). In Methods to Study Litter Decomposition; Springer: Dordrecht, The Netherlands, 2005; pp. 107–113. [Google Scholar]
- Shay, P.E.; Trofymow, J.A.; Constabel, C.P. An improved butanol-HCl assay for quantification of water-soluble, acetone: Methanol-soluble, and insoluble proanthocyanidins (condensed tannins). Plant Methods 2017, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.B.; Thomazini, M.; Echalar Barrientos, M.A.; Nalin, C.M.; Ferro-Furtado, R.; Genovese, M.I.; Favaro-Trindade, C.S. Functional properties and encapsulation of a proanthocyanidin-rich cinnamon extract (Cinnamomum zeylanicum) by complex coacervation using gelatin and different polysaccharides. Food Hydrocoll. 2018, 77, 297–306. [Google Scholar] [CrossRef]
- Kashiri, M.; Kashaninejad, M.; Aghajani, N. Modeling water absorption of sorghum during soaking. Lat. Am. Appl. Res. 2010, 40, 383–388. [Google Scholar]
- Perez, E.E.; Carelli, A.A.; Crapiste, G.H. Temperature-dependent diffusion coefficient of oil from different sunflower seeds during extraction with hexane. J. Food Eng. 2011, 105, 180–185. [Google Scholar] [CrossRef]
- Santos, D.T.; Cavalcanti, R.N.; Rostagno, M.A.; Queiroga, C.L.; Eberlin, M.N.; Meireles, M.A.A. Extraction of Polyphenols and Anthocyanins from the Jambul (Syzygium cumini) Fruit Peels. Food Public Health 2013, 3, 119–129. [Google Scholar] [CrossRef]
- Hasanzadeh, R.; Souraki, B.A. Experimental and Theoretical Investigation of Mass Transfer during Leaching of Starch and Protein from Potato. Chem. Eng. Commun. 2016, 203, 1105–1115. [Google Scholar] [CrossRef] [Green Version]
- Yang, W. A Modified Radial Basis Function Method for Predicting Debris Flow Mean Velocity. J. Eng. Technol. Sci. 2017, 49, 561. [Google Scholar] [CrossRef]
- Tan, W.N. A Low-Dimensional Model for the Maximal Amplification Factor of Bichromatic Wave Groups. J. Eng. Technol. Sci. 2003, 35, 139–153. [Google Scholar] [Green Version]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: London, UK, 1975. [Google Scholar]
- Cussler, E.L. Diffusion Mass Transfer in Fluid Systems, 3rd ed.; Cambridge University Press: Cambridge, UK, 2009; ISBN 9780521871211. [Google Scholar]
- Tsioptsias, N.; Tako, A.; Robinson, S. Model Validation and Testing in Simulation: A Literature Review. In Proceedings of the 5th Student Conference on Operational Research, Nottingham, UK, 8–10 April 2016; Volume 6, pp. 1–11. [Google Scholar]
- Mokrani, A.; Madani, K. Effect of Solvent, Time and Temperature on the Extraction of Phenolic Compounds and Antioxidant Capacity of Peach (Prunus persica L.) Fruit; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 162, ISBN 2135415065. [Google Scholar]
- Pérez-Armada, L.; Rivas, S.; González, B.; Moure, A. Extraction of phenolic compounds from hazelnut shells by green processes. J. Food Eng. 2019, 255, 1–8. [Google Scholar] [CrossRef]
- Bröhan, M.; Jerkovic, V.; Wilmotte, R.; Collin, S. Catechins and derived procyanidins in red and white sorghum: Their contributions to antioxidant activity. J. Inst. Brew. 2011, 117, 600–607. [Google Scholar] [CrossRef]
- Khoddami, A.; Truong, H.H.; Liu, S.Y.; Roberts, T.H.; Selle, P.H. Concentrations of specific phenolic compounds in six red sorghums influence nutrient utilisation in broiler chickens. Anim. Feed Sci. Technol. 2015, 210, 190–199. [Google Scholar] [CrossRef]
- Syukri, Y.; Martien, R.; Lukitaningsih, E.; Nugroho, A.E. Quantification of andrographolide isolated from andrographis paniculata nees obtained from traditional market in yogyakarta using validated HPLC. Indones. J. Chem. 2016, 16, 190–197. [Google Scholar] [CrossRef]










| Parameter | Value | Unit |
|---|---|---|
| DeP | 0.0000025 | cm2/min |
| R | 0.3 | cm |
| L | 0.03 | cm |
| H | 0.5 | - |
| V | 500 | mL |
| π | 3.14159 | - |
| Nb | 20 | grain |
| dx | 0.003 | cm |
| kc | 0.0004 | cm/min |
| CP0 | 0.1 | g Pequivalent/cm3 pericarp |
| CPf0 | 0 | g Pequivalent/cm3 solvent |
| Temperature (°C) | DeP × 107 (cm2/min) | H × 104 | kc × 102 (cm/min) | SSE × 1010 |
|---|---|---|---|---|
| 50 | 0.926 | 4.18 | 3.8 | 1.65 |
| 60 | 1.11 | 6.33 | 2.53 | 0.264 |
| 70 | 167 | 11 | 2 | 2.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susanti, D.Y.; Sediawan, W.B.; Fahrurrozi, M.; Hidayat, M. A Mechanistic Model of Mass Transfer in the Extraction of Bioactive Compounds from Intact Sorghum Pericarp. Processes 2019, 7, 837. https://doi.org/10.3390/pr7110837
Susanti DY, Sediawan WB, Fahrurrozi M, Hidayat M. A Mechanistic Model of Mass Transfer in the Extraction of Bioactive Compounds from Intact Sorghum Pericarp. Processes. 2019; 7(11):837. https://doi.org/10.3390/pr7110837
Chicago/Turabian StyleSusanti, Devi Yuni, Wahyudi Budi Sediawan, Mohammad Fahrurrozi, and Muslikhin Hidayat. 2019. "A Mechanistic Model of Mass Transfer in the Extraction of Bioactive Compounds from Intact Sorghum Pericarp" Processes 7, no. 11: 837. https://doi.org/10.3390/pr7110837
APA StyleSusanti, D. Y., Sediawan, W. B., Fahrurrozi, M., & Hidayat, M. (2019). A Mechanistic Model of Mass Transfer in the Extraction of Bioactive Compounds from Intact Sorghum Pericarp. Processes, 7(11), 837. https://doi.org/10.3390/pr7110837