Influence of Air Infiltration on Combustion Process Changes in a Rotary Tilting Furnace
Abstract
:1. Introduction
2. Experimental Device
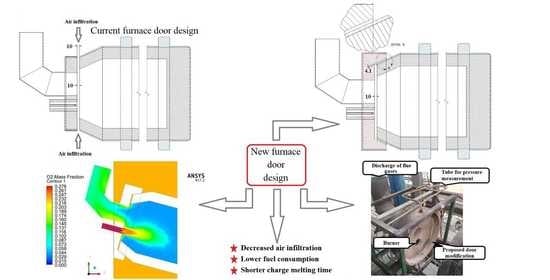
2.1. Modification of the Door
2.2. Experimental Model
2.3. Calculation of Air Infiltration
3. Results and Discussion
4. Mathematical Modeling
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nakadate, M. OECD: Organization for Economic Co-operation and Development. Jpn. J. Water Pollut. Res. 1991, 14, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Gerres, T.; Ávila, J.P.C.; Llamas, P.L.; Román, T.G.S. A review of cross-sector decarbonisation potentials in the European energy intensive industry. J. Clean. Prod. 2019, 210, 585–601. [Google Scholar] [CrossRef]
- Malinauskaite, J.; Jouhara, H.; Ahmad, L.; Milani, M.; Montorsi, L.; Venturelli, M. Energy efficiency in industry: EU and national policies in Italy and the UK. Energy 2019, 172, 255–269. [Google Scholar] [CrossRef]
- Rissman, J.; Bataille, C.; Masanet, E.; Aden, N.; Morrow, W.R.; Zhou, N.; Elliott, N.; Dell, R.; Heeren, N.; Huckestein, B.; et al. Technologies and policies to decarbonize global industry: Review and assessment of mitigation drivers through 2070. Appl. Energy 2020, 266. [Google Scholar] [CrossRef]
- Boryca, J.; Kolmasiak, C.; Wylecial, T.; Urbaniak, D. Effect of furnace efficiency on scale adhesion in steel charge heating process. Metalurgija 2020, 59, 191–194. [Google Scholar]
- Nidheesh, P.; Kumar, M.S. An overview of environmental sustainability in cement and steel production. J. Clean. Prod. 2019, 231, 856–871. [Google Scholar] [CrossRef]
- De Ras, K.; Van De Vijver, R.; Galvita, V.V.; Marin, G.B.; Van Geem, K.M. Carbon capture and utilization in the steel industry: Challenges and opportunities for chemical engineering. Curr. Opin. Chem. Eng. 2019, 26, 81–87. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Q.; Zhou, Y.; Wu, J. Material and energy flows of the iron and steel industry: Status quo, challenges and perspectives. Appl. Energy 2020, 268, 114946. [Google Scholar] [CrossRef]
- Filipponi, M.; Rossi, F.; Presciutti, A.; De Ciantis, S.; Castellani, B.; Carpinelli, A. Thermal Analysis of an Industrial Furnace. Energies 2016, 9, 833. [Google Scholar] [CrossRef] [Green Version]
- Naranjo, R.D.; Kwon, J.; Majumdar, R.; Choate, W.T. Advanced Melting Technologies: Energy Saving Concepts and Opportunities for the Metal Casting Industry, 2005. U.S. Department of Energy. pp. 19–20. Available online: https://www1.eere.energy.gov/manufacturing/resources/metalcasting/pdfs/advancedmeltingtechnologies.pdf (accessed on 25 August 2020).
- Baukal, C. Oxygen-Enhanced Combustion; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Wu, K.-K.; Chang, Y.-C.; Chen, C.-H.; Chen, Y.-D. High-efficiency combustion of natural gas with 21–30% oxygen-enriched air. Fuel 2010, 89, 2455–2462. [Google Scholar] [CrossRef]
- Jablonský, G.; Pástor, M.; Dzurňák, R. Enriching the Combustible Mixture with Oxygen in Practice (in Slovak). TU of Košice, Košice, 2015. Available online: https://uloz.to/file/ZPAXaG9LqLd5/monografia-jablonsky-spolu-pdf (accessed on 25 August 2020).
- Baukal, C.E., Jr. Industrial burners handbook, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 650–726. [Google Scholar]
- Poskart, A.; Radomiak, H.; Niegodajew, P.; Zajemska, M.; Musiał, D. The Analysis of Nitrogen Oxides Formation During Oxygen—Enriched Combustion of Natural Gas. Arch. Met. Mater. 2016, 61, 1925–1930. [Google Scholar] [CrossRef]
- Leeson, D.; Mac Dowell, N.; Shah, N.; Petit, C.; Fennell, P.S. A Techno-economic analysis and systematic review of carbon capture and storage (CCS) applied to the iron and steel, cement, oil refining and pulp and paper industries, as well as other high purity sources. Int. J. Greenh. Gas Control. 2017, 61, 71–84. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, L.; Wang, Y.; Roskilly, A. Energy saving technologies and mass-thermal network optimization for decarbonized iron and steel industry: A review. J. Clean. Prod. 2020, 274. [Google Scholar] [CrossRef]
- D’Agostini, M. High-Efficiency, High-Capacity, Low-NOx Aluminum Melting Using Oxygen-Enhanced Combustion; DOE/ID/13514; U.S. Department of Energy: Washington, DC, USA, 2000; pp. 10–35. [CrossRef] [Green Version]
- Dzurnak, R.; Varga, A.; Kizek, J.; Jablonský, G.; Lukáč, L. Influence of Burner Nozzle Parameters Analysis on the Aluminium Melting Process. Appl. Sci. 2019, 9, 1614. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Barr, P.V.; Meadowcroft, T.R. Continuous scrap melting in a short rotary furnace. Miner. Eng. 2008, 21, 178–189. [Google Scholar] [CrossRef]
- Ghadamgahi, M.; Ölund, P.; Ekman, T.; Andersson, N.; Jönsson, P. Numerical and experimental study on flameless oxy-fuel combustion in a pilot-scale and a real-size industrial furnace. Appl. Therm. Eng. 2018, 141, 788–797. [Google Scholar] [CrossRef]
- Carabalí, D.M.; Forero, C.R.; Cadavid, Y. Energy diagnosis and structuring an energy saving proposal for the metal casting industry: An experience in Colombia. Appl. Therm. Eng. 2018, 137, 767–773. [Google Scholar] [CrossRef]
- Blašková, K.; Trpčevská, J.; Kuchárová, M. Characterization of Zn-Mg-Al Based Drosses from the Continuous Galvanizing. Manuf. Technol. 2016, 16, 879–883. [Google Scholar] [CrossRef]
- Jepson, S.; Kampen, P.V. Oxygen-enhanced combustion provides advantages in Al-melting furnaces. Ind. Heat. 2005, 72, 29–35. [Google Scholar]
- Gripenberg, H.; Johansson, A.; Eichler, R.; Rangmark, L. Optimised oxyfuel melting process at sapa heat transfer ab. In Proceedings of the Light Metals 2007 at the TMS 2007 Annual Meeting & Exhibition, Orlando, FL, USA, 25 February–1 March 2007; Sorlie, M., Minerals, Metals and Materials Society, Eds.; TMS: Warrendale, PA, USA, 2007; pp. 597–601. [Google Scholar]
- Gripenberg, H.; Falk, O.; Olausson, R.; Niedermair, F. Controlled melting of secondary aluminum in rotary furnaces. In Proceedings of the Light Metals 2003 at the 132nd TMS Annual Meeting, San Diego, CA, USA, 2–6 March 2003; Crepeau, P.N., Minerals, Metals and Materials Society, Eds.; TMS: Warrendale, PA, USA, 2003; pp. 1083–1090. [Google Scholar]
- Furu, J.; Buchholz, A.; Bergstrøm, T.H.; Marthinsen, K. Heating and melting of single Al ingots in an aluminum melting furnace. In Proceedings of the Light Metals 2010 at the TMS 2010 Annual Meeting & Exhibition, Seattle, WA, USA, 14–18 February 2010; Johnson, J.A., Ed.; TMS-AIME: Warrendale, PA, USA, 2010; pp. 679–684. [Google Scholar]
- Rimar, M.; Kulikov, A. NOx formation in combustion of gaseous fuel in ejection burner. In The Application of Experimental and Numerical Methods in Fluid Mechanics and Energy, Proceedings of the XX. Anniversary of International Scientific Conference, Terchova, Slovakia, 27–29 April 2016; AIP Publishing: College Park, MD, USA, 2016; Volume 1745, p. 20051. [Google Scholar]
- Kulikov, A.; Fedák, M.; Abraham, M.; Váhovský, J. Study of the gaseous fuel combustion respect to the O2 concentration and NOx formation. Adv. Therm. Process. Energy Transform. 2018, 1, 23–26. [Google Scholar]
- Capuzzi, S.; Timelli, G. Preparation and Melting of Scrap in Aluminum Recycling: A Review. Metals 2018, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Osoba, L.O.; Owolabi, O.B.; Talabi, S.I.; Adeosun, S.O. Review on oxide formation and aluminum recovery mechanism during secondary smelting. J. Cast. Mater. Eng. 2018, 2, 45–51. [Google Scholar] [CrossRef]
- Baukal, C. The John Zink Hamworthy Combustion Handbook; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Varga, A.; Jablonský, G.; Lukáč, L.; Kizek, J. Thermal Technology for Metallurgists; TU of Košice: Košice, Slovakia, 2013. (in Slovak). Available online: https://opac.lib.tuke.sk/tukeopac/openURL?fn=*recview&uid=373383&pageId=main&full=0 (accessed on 25 August 2020).
- Baukal, C.E.; Bussman, W.R. Air infiltration effects on industrial combustion efficiency. In Fuel Efficiency, 2nd ed.; Bernard, J.K., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 101–103. [Google Scholar]
- Melting Solutions: Tilting Rotary Furnace World Class Melting Technology. Available online: http://www.meltingsolutions.co.uk/wp-content/uploads/2016/07/TRF-Brochure-new-low.pdf (accessed on 25 August 2020).
- Mass View: The Intelligent Alternative for VA Meters: Mass Flow for Gases with Flow Display. Available online: https://www.bronkhorst.com/getmedia/88dd3dac-0ddd-4dec-887f-8c3d34b28028/MASS-VIEW_EN.pdf (accessed on 25 August 2020).
- SPP-Distribucia. Available online: https://www.spp-distribucia.sk/dodavatelia/informacie/zlozenie-zemneho-plynu-a-emisny-faktor/ (accessed on 25 August 2020).
- Nieckele, A.O.; Naccache, M.F.; Gomes, M.S.P. Numerical Modeling of an Industrial Aluminum Melting Furnace. J. Energy Resour. Technol. 2004, 126, 72–81. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, Y.; Reuter, M.A.; Boin, U. Modelling of aluminium scrap melting in a rotary furnace. Miner. Eng. 2006, 19, 299–308. [Google Scholar] [CrossRef]
- Khoei, A.R.; Masters, I.; Gethin, D. Numerical modelling of the rotary furnace in aluminium recycling processes. J. Mater. Process. Technol. 2003, 139, 567–572. [Google Scholar] [CrossRef]
- Rimar, M.; Kulikov, A.; Fedak, M.; Yeromin, O.O.; Sukhyy, K.; Gupalo, O.; Belyanovskaya, E.; Berta, R.; Smajda, M.; Ratnayake, M. Mathematical Model of a Heating Furnace Implemented with Volumetric Fuel Combustion. Processes 2020, 8, 469. [Google Scholar] [CrossRef]















| MV-106, MV-306 | MV-308 | |
|---|---|---|
| Accuracy | ±2% RD for >50% of max. capacity ±(2% RD + 0.5% FS) on lower flows | |
| Repeatability | <0.2% FS typical | <0.6% FS typical |
| Operating pressure | 0–10 bar | |
| Operating temperature | 0–50 °C | |
| Response time | 2 s | |
| Model | Air, O2 (L/min) | CH4 (L/min) | |
|---|---|---|---|
| MV-106, MV-306 | range 1 | 2–200 | 1–100 |
| range 2 | 1–100 | 5–50 | |
| range 3 | 0.5–50 | 0.2–20 | |
| range 4 | 0.4–20 | 0.2–10 | |
| MV-308 | range 1 | 5–500 | 2.5–250 |
| range 2 | 2–200 | 1.25–125 | |
| range 3 | 1–100 | 0.625–62.5 | |
| range 4 | 1–50 | 0.5–25 |
| Concentration of Oxygen | (%) | 21 | 22.5 | 25 | 27.5 | 30 | 32.5 | 35 |
|---|---|---|---|---|---|---|---|---|
| Air Flow | (m3·h−1) | 14.07 | 12.88 | 11.22 | 9.86 | 8.72 | 7.77 | 6.94 |
| Oxygen Flow | (m3·h−1) | 0 | 0.25 | 0.6 | 0.88 | 1.12 | 1.32 | 1.49 |
| Fuel Consumption | (m3·h−1) | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 |
| Air Excess coefficient | (-) | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 |
| CH4 | C2H6 | C3H8 | C4H10 | C5H12 | C6H14 | CO2 | N2 |
|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | % |
| 95.171 | 2.7131 | 0.8729 | 0.2772 | 0.0486 | 0.0207 | 0.2266 | 0.6697 |
| Thermocouple | T1 | T2 | T3 | T4 |
|---|---|---|---|---|
| Distance (m) | 0.05 | 0.2 | 0.35 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzurňák, R.; Varga, A.; Jablonský, G.; Variny, M.; Atyafi, R.; Lukáč, L.; Pástor, M.; Kizek, J. Influence of Air Infiltration on Combustion Process Changes in a Rotary Tilting Furnace. Processes 2020, 8, 1292. https://doi.org/10.3390/pr8101292
Dzurňák R, Varga A, Jablonský G, Variny M, Atyafi R, Lukáč L, Pástor M, Kizek J. Influence of Air Infiltration on Combustion Process Changes in a Rotary Tilting Furnace. Processes. 2020; 8(10):1292. https://doi.org/10.3390/pr8101292
Chicago/Turabian StyleDzurňák, Róbert, Augustin Varga, Gustáv Jablonský, Miroslav Variny, Réne Atyafi, Ladislav Lukáč, Marcel Pástor, and Ján Kizek. 2020. "Influence of Air Infiltration on Combustion Process Changes in a Rotary Tilting Furnace" Processes 8, no. 10: 1292. https://doi.org/10.3390/pr8101292
APA StyleDzurňák, R., Varga, A., Jablonský, G., Variny, M., Atyafi, R., Lukáč, L., Pástor, M., & Kizek, J. (2020). Influence of Air Infiltration on Combustion Process Changes in a Rotary Tilting Furnace. Processes, 8(10), 1292. https://doi.org/10.3390/pr8101292