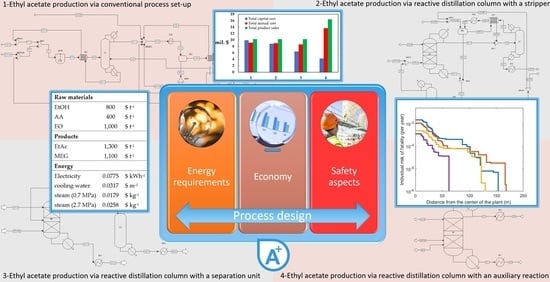
Multi-Aspect Comparison of Ethyl Acetate Production Pathways: Reactive Distillation Process Integration and Intensification via Mechanical and Chemical Approach
Abstract
:1. Introduction
1.1. Current Ethyl Acetate Production Methods
1.2. Methodology
1.3. Process Sustainability Indicators
1.4. Safety Indicators
2. Process Modeling
2.1. Thermodynamic Model
2.2. Kinetic Model


2.3. Equipment Model
2.4. Simulation Strategy and Design Specification
3. Simulation Results
3.1. Conventional Process Set-Up
3.1.1. Flowsheet Design (Figure 1)
3.1.2. Process Simulation Results & Equipment Parameters of Conventional Process Set-Up
3.2. Reactive Distillation Column with a Separation Unit
3.2.1. Flowsheet Design (Figure 2)
3.2.2. Process Simulation & Equipment Parameters of Reactive Distillation Column with a Separation Unit
3.3. Reactive Distillation Column with a Stripper
3.3.1. Flowsheet Design (Figure 3)
3.3.2. Process Simulation and Equipment Parameters of Reactive Distillation Column with a Stripper
3.4. Reactive Distillation Column with an Auxiliary Reaction
3.4.1. Flowsheet Design (Figure 4)
3.4.2. Process Simulation and Equipment Parameters of Reactive Distillation Column with Auxiliary Reaction
4. Discussion
4.1. Energy Requirements of Individual Case Studies
4.2. Economy Aspects of Individual Case Studies
4.3. Safety Aspects of Individual Case Studies
- For distillation and reactive distillation columns, the incident list contained: liquid leaks (full bore rupture and hole equivalent to 20% of diameter), vapor leaks, and complete rupture of column.
- For heat exchangers, small leaks and full rupture were used.
- For all pipelines in the simulation, the only full rupture was considered.
- continuous liquid release,
- continuous gaseous release and
- instantaneous release.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Symbols
| C | molar concentration | kmol m−3 |
| d | column internal diameter | m |
| f | feed stage position | |
| fi | frequency of incident outcome i | year−1 |
| FI | frequency of incident I | year−1 |
| H | packed section height | m |
| IRx,y | total individual risk of fatality at geographical location x, y | year−1 |
| n | total number of incident outcome case | |
| ṅ | molar flow | kmol h−1 |
| N | number of theoretical stages, total condenser, and reboiler are included | |
| NR | reactive section stages | |
| pf,i | fatality at location x, y | |
| po,i | incident outcome probability | |
| P | total pressure | kPa |
| ΔP | column section pressure drop | kPa |
| condenser duty | kW | |
| reboiler duty | kW | |
| r | reaction rate | kmol m−3 s−1 |
| R | gas constant | kJ kmol−1 K−1 |
| R | reflux ratio | |
| T | temperature | °C |
| T | thermodynamic temperature in Equations (6) and (8) | K |
| volume flow | m3 h−1 | |
| x | molar fraction in the liquid phase |
Subscripts
| AA | acetic acid |
| C | condenser |
| D | distillate |
| EtAc | ethyl acetate |
| EtOH | ethanol |
| F | feed |
| i | incident outcome case |
| I | incident |
| H2O | water |
| R | reaction occurrence |
| W | reboiler |
| x, y | geographical location |
Abbreviations
| AA | acetic acid | |
| ARD | azeotropic reactive distillation | |
| CSTR | continuous stirred tank reactor | |
| DS | design specification | |
| EQ | equilibrium stage | |
| EtAc | ethyl acetate | |
| EtOH | ethanol | |
| HETP | height equivalent of theoretical plate | |
| HOC | Hayden–O’Connell equation of state | |
| NEQ | non-equilibrium stage | |
| NRTL | non-random two liquids | |
| OEC | overall energy consumption | kW |
| RD | reactive distillation | |
| RDAR | reactive distillation with auxiliary reaction | |
| RDPV | reactive distillation with pervaporation | |
| RDS | reactive distillation with stripper | |
| RDWC | reactive distillation with a dividing wall | |
| RED | reactive extractive distillation | |
| SEC | specific energy consumption | kW t−1EtAc |
| TAC | total annual cost | $ year−1 |
| TCC | total capital cost | $ |
| TPC | total production cost | $ t−1 |
| VL | vapor–liquid | |
| VLLE | vapor–liquid–liquid phase equilibria |
Appendix A
| Conventional Path (Figure 1) | RD Column with Separation Unit (Figure 2) | RD Column with Stripper (Figure 3) | RD Column with Auxiliary Reaction (Figure 4) | |||||
|---|---|---|---|---|---|---|---|---|
| Equipment Cost | Installed Cost | Equipment Cost | Installed Cost | Equipment Cost | Installed Cost | Equipment Cost | Installed Cost | |
| Item | [103 $] | [103 $] | [103 $] | [103 $] | [103 $] | [103 $] | [103 $] | [103 $] |
| CSTR/RD | 173.7 | 334.3 | 680.5 | 1214.0 | 840.5 | 1614.0 | 790.4 | 1480.5 |
| C1 | 620.7 | 1147.2 | 610.6 | 1185.2 | 380.2 | 695.2 | - | - |
| C2 | 594.5 | 1138.1 | 148.0 | 487.8 | - | - | - | - |
| C3 | 172.6 | 564.0 | - | - | - | - | - | - |
| DEC | 16.1 | 108.9 | 16.1 | 108.9 | - | - | - | - |
| EX1 | 8.5 | 53.0 | 8.7 | 59.1 | 8.5 | 58.6 | 10.1 | 61.0 |
| EX2 | 39.3 | 120.0 | 19.3 | 85.4 | 10.5 | 60.3 | 10.5 | 60.3 |
| EX3 | 8.7 | 59.1 | 9.9 | 62.6 | - | - | - | - |
| EX4 | 8.5 | 45.7 | 14.3 | 70.7 | - | - | - | - |
| EX5 | 11.0 | 63.9 | 10.9 | 60.8 | - | - | - | - |
| EX6 | 8.5 | 45.7 | - | - | - | - | - | - |
| EX7 | 10.5 | 60.3 | - | - | - | - | - | - |
| Sum | 1672.6 | 3740.2 | 1518.3 | 3334.5 | 1239.7 | 2428.1 | 811.0 | 1601.8 |
References
- Riemenschneider, W.; Bolt, H.M. Esters, Organic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Cornils, B. Ethyl Acetate. In Catalysis from A to Z; Wiley-VCH: Weinheim, Germany, 2020; p. 2020. [Google Scholar]
- Srinivasan, R.; Nhan, N.T. A statistical approach for evaluating inherent benign-ness of chemical process routes in early design stages. Process Saf. Environ. Prot. 2008, 86, 163–174. [Google Scholar] [CrossRef]
- Li, C.; Duan, C.; Fang, J.; Li, H. Process intensification and energy saving of reactive distillation for production of ester compounds. Chin. J. Chem. Eng. 2019, 27, 1307–1323. [Google Scholar] [CrossRef]
- Toth, A.J. Comprehensive evaluation and comparison of advanced separation methods on the separation of ethyl acetate-ethanol-water highly non-ideal mixture. Sep. Purif. Technol. 2019, 224, 490–508. [Google Scholar] [CrossRef]
- Wierschem, M.; Górak, A. Reactive Distillation. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–10. [Google Scholar]
- Niesbach, A.; Kuhlmann, H.; Keller, T.; Lutze, P.; Górak, A. Optimisation of industrial-scale n-butyl acrylate production using reactive distillation. Chem. Eng. Sci. 2013, 100, 360–372. [Google Scholar] [CrossRef]
- Qasim, F.; Shin, J.S.; Park, S.J. A simulation study on selection of optimized process for azeotropic separation of methanol and benzene: Internal heat integration and economic analysis. Korean J. Chem. Eng. 2018, 35, 1185–1194. [Google Scholar] [CrossRef]
- Timoshenko, A.V.; Anokhina, E.A.; Morgunov, A.V.; Rudakov, D.G. Application of the partially thermally coupled distillation flowsheets for the extractive distillation of ternary azeotropic mixtures. Chem. Eng. Res. Des. 2015, 104, 139–155. [Google Scholar] [CrossRef]
- Staak, D.; Grützner, T. Process integration by application of an extractive dividing-wall column: An industrial case study. Chem. Eng. Res. Des. 2017, 123, 120–129. [Google Scholar] [CrossRef]
- Lv, B.; Liu, G.; Dong, X.; Wei, W.; Jin, W. Novel Reactive Distillation–Pervaporation Coupled Process for Ethyl Acetate Production with Water Removal from Reboiler and Acetic Acid Recycle. Ind. Eng. Chem. Res. 2012, 51, 8079–8086. [Google Scholar] [CrossRef]
- Harvianto, G.R.; Ahmad, F.; Lee, M. A hybrid reactive distillation process with high selectivity pervaporation for butyl acetate production via transesterification. J. Membr. Sci. 2017, 543, 49–57. [Google Scholar] [CrossRef]
- Graczová, E.; Šulgan, B.; Steltenpohl, P. Tert-Butanol–water mixture separation by extractive distillation: Application of experimental data in process simulations. Sep. Purif. Technol. 2020, 251, 116968. [Google Scholar] [CrossRef]
- Ma, S.; Shang, X.; Li, L.; Song, Y.; Pan, Q.; Sun, L. Energy-saving thermally coupled ternary extractive distillation process using ionic liquids as entrainer for separating ethyl acetate-ethanol-water ternary mixture. Sep. Purif. Technol. 2019, 226, 337–349. [Google Scholar] [CrossRef]
- Santaella, M.A.; Orjuela, A.; Narváez, P.C. Comparison of different reactive distillation schemes for ethyl acetate production using sustainability indicators. Chem. Eng. Process. Process. Intensif. 2015, 96, 1–13. [Google Scholar] [CrossRef]
- Kiss, A.A.; Jobson, M. Taking Reactive Distillation to the Next Level of Process Intensification. Chem. Eng. 2018, 69, 553–558. [Google Scholar] [CrossRef]
- Raymond, K.E.; Othmer, D.F. Encyclopedia of Chemical Technology, 8th ed.; Wiley: Weinhem, Germany, 2007. [Google Scholar]
- Segovia-Hernández, J.G.; Hernández, S.; Petriciolet, A.B. Reactive distillation: A review of optimal design using deterministic and stochastic techniques. Chem. Eng. Process. Process. Intensif. 2015, 97, 134–143. [Google Scholar] [CrossRef]
- Šulgan, B.; Labovská, Z.; Markoš, J. Production of 2-phenylethyl acetate via reactive distillation. Chem. Pap. 2020, 74, 2341–2356. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.; Omota, F.; Kiss, A. Innovative process for fatty acid esters by dual reactive distillation. Comput. Chem. Eng. 2009, 33, 743–750. [Google Scholar] [CrossRef]
- Harmsen, J. Reactive distillation: The front-runner of industrial process intensification. Chem. Eng. Process. Process. Intensif. 2007, 46, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Li, C.; Peng, F.; Dong, L.; Ma, S. Experimental and simulation of the reactive dividing wall column based on ethyl acetate synthesis. Chin. J. Chem. Eng. 2018, 26, 1468–1476. [Google Scholar] [CrossRef]
- Tavan, Y.; Behbahani, R.M.; Hosseini, S.H. A novel intensified reactive distillation process to produce pure ethyl acetate in one column—Part I: Parametric study. Chem. Eng. Process. Process. Intensif. 2013, 73, 81–86. [Google Scholar] [CrossRef]
- Huang, F.; Xu, S.; Li, T.; Zhu, N. Innovative Ethylene Glycol Diacetate synthesis process in a single reactive distillation column. Chem. Eng. Process. Process. Intensif. 2016, 109, 80–89. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, B.J.; Hou, X.Q.; Li, D.L.; Chen, Q.L. Design and simulation of an entrainer-enhanced ethyl acetate reactive distillation process. Chem. Eng. Process. Process. Intensif. 2011, 50, 1252–1265. [Google Scholar] [CrossRef]
- ASPEN Technology. Aspen Plus®: Aspen Plus User Guide, Version 10.2; Aspen Technology Inc.: Cambridge, MA, USA, 2000. [Google Scholar]
- Muthia, R.; Reijneveld, A.G.; Van Der Ham, A.G.; Kate, A.J.T.; Bargeman, G.; Kersten, S.R.; Kiss, A.A. Novel method for mapping the applicability of reactive distillation. Chem. Eng. Process. Process. Intensif. 2018, 128, 263–275. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, R.K.; Kumar, V. Simulation of a plant scale reactive distillation column for esterification of acetic acid. Comput. Chem. Eng. 2015, 73, 70–81. [Google Scholar] [CrossRef]
- Risco, A.; Plesu, V.; Heydenreich, J.A.; Bonet-Ruiz, A.-E.; Calvet, A.; Iancu, P.; Llorens, J. Pressure selection for non-reactive and reactive pressure-swing distillation. Chem. Eng. Process. Process. Intensif. 2019, 135, 9–21. [Google Scholar] [CrossRef]
- Švandová, Z.; Labovský, J.; Markoš, J.; Jelemenský, Ľ. Impact of mathematical model selection on prediction of steady state and dynamic behaviour of a reactive distillation column. Comput. Chem. Eng. 2009, 33, 788–793. [Google Scholar] [CrossRef]
- Švandová, Z.; Markoš, J.; Jelemenský, Ľ. Multiple steady states in a CSTR with total condenser: Comparison of equilibrium and nonequilibrium models. Chem. Pap. 2006, 60, 432–440. [Google Scholar] [CrossRef]
- Sun, S.; Yang, A.; Chien, I.-L.; Shen, W.; Wei, S.; Ren, J.; Zhang, X. Intensification and performance assessment for synthesis of 2-methoxy-2-methyl-heptane through the combined use of different pressure thermally coupled reactive distillation and heat integration technique. Chem. Eng. Process. Process. Intensif. 2019, 142, 107561. [Google Scholar] [CrossRef]
- Couper, J.R.; Hertz, D.W.; Smith, F.L. Process Economics. In Perry’s Chemical Engineers’ Handbook, 8th ed.; Green, D.W., Perry, R.H., Eds.; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- The Center for Chemical Process Safety. Guidelines for Chemical Process Quantitative Risk Analysis; American Institute of Chemical Engineers: New York, NY, USA, 1989. [Google Scholar]
- Trofimova, M.; Sadaev, A.; Samarov, A.; Golikova, A.; Tsvetov, N.; Toikka, M.; Toikka, A. Liquid-liquid equilibrium of acetic acid–ethanol–ethyl acetate–water quaternary system: Data review and new results at 323.15 K and 333.15 K. Fluid Phase Equilibria 2020, 503, 112321. [Google Scholar] [CrossRef]
- Wichterle, I.; Bogdanić, G. Vapour–liquid and chemical equilibria in the ethyl ethanoate+ethanol+propyl ethanoate+propanol system accompanied with transesterification reaction. Fluid Phase Equilibria 2012, 328, 61–68. [Google Scholar] [CrossRef]
- Hayden, J.G.; O’Connell, J.P. A Generalized Method for Predicting Second Virial Coefficients. Ind. Eng. Chem. Process. Des. Dev. 1975, 14, 209–216. [Google Scholar] [CrossRef]
- Altiokka, M.R.; Akyalçin, S. Kinetics of the Hydration of Ethylene Oxide in the Presence of Heterogeneous Catalyst. Ind. Eng. Chem. Res. 2009, 48, 10840–10844. [Google Scholar] [CrossRef]
- Arnikar, H.J.; Rao, T.S.; Bodhe, A.A. A gas chromatographic study of the kinetics of the uncatalysed esterification of acetic acid by ethanol. J. Chromatogr. A 1970, 47, 265–268. [Google Scholar] [CrossRef]
- Calvar, N.; González, B.; Dominguez, A. Esterification of acetic acid with ethanol: Reaction kinetics and operation in a packed bed reactive distillation column. Chem. Eng. Process. Process. Intensif. 2007, 46, 1317–1323. [Google Scholar] [CrossRef]
- Monroy-Loperena, R.; Álvarez-Ramírez, J. On the Steady-State Multiplicities for an Ethylene Glycol Reactive Distillation Column. Ind. Eng. Chem. Res. 1999, 38, 451–455. [Google Scholar] [CrossRef]
- Doherty, M.F.; Fidkowski, Z.T.; Malone, M.F.; Taylor, R. Distillation. In Perry’s Chemical Engineers’ Handbook, 8th ed.; Green, D.W., Ed.; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Bagajewicz, M.; Ji, S. Rigorous Procedure for the Design of Conventional Atmospheric Crude Fractionation Units. Part I: Targeting. Ind. Eng. Chem. Res. 2001, 40, 617–626. [Google Scholar] [CrossRef]
- Glavič, P. Integrated crude distillation design. Comput. Chem. Eng. 1995, 19. [Google Scholar] [CrossRef]
- Löser, C.; Urit, T.; Bley, T. Perspectives for the biotechnological production of ethyl acetate by yeasts. Appl. Microbiol. Biotechnol. 2014, 98, 5397–5415. [Google Scholar] [CrossRef]
- Chembid. Available online: www.chembid.com (accessed on 22 June 2020).
- Molbase. Available online: www.molbase.com (accessed on 22 June 2020).
- Vílchez, J.A.; Espejo, V.; Casal, J. Generic event trees and probabilities for the release of different types of hazardous materials. J. Loss Prev. Process. Ind. 2011, 24, 281–287. [Google Scholar] [CrossRef]







| AA0 | ETOH0 | F11 | F21 | R1 | P1 | P2 | W11 | W12 | D11 | D12 | D13 | ORG | F2 | W21 | AZ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ṅ [kmol h−1] | 10.00 | 10.00 | 22.64 | 19.88 | 42.52 | 42.52 | 42.52 | 12.65 | 12.65 | 39.86 | 187.78 | 187.78 | 41.21 | 41.21 | 20.00 | 10.00 |
| xH2O | - | - | - | 0.0869 | 0.0407 | 0.2756 | 0.2756 | - | - | 0.2940 | 0.7608 | 0.7608 | 0.1604 | 0.1604 | 0.0003 | 0.0003 |
| xAA | 1.0000 | - | 0.8325 | - | 0.4433 | 0.2083 | 0.2083 | 0.7000 | 0.7000 | - | - | - | - | - | - | - |
| xEtAc | - | - | 0.1673 | 0.1301 | 0.1499 | 0.3848 | 0.3848 | 0.2996 | 0.2996 | 0.5660 | 0.1856 | 0.1856 | 0.7672 | 0.7672 | 0.9990 | 0.9990 |
| xEtOH | - | 1.0000 | 0.0002 | 0.7830 | 0.3662 | 0.1312 | 0.1312 | 0.0004 | 0.0004 | 0.1400 | 0.0536 | 0.0536 | 0.0724 | 0.0724 | 0.0007 | 0.0007 |
| T [°C] | 25.00 | 25.00 | 39.64 | 29.38 | 36.98 | 39.01 | 54.24 | 98.20 | 49.01 | 70.02 | 48.97 | 25.00 | 25.00 | 41.79 | 76.83 | 76.83 |
| P [kPa] | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 |
| [m3 h−1] | 0.56 | 0.58 | 1.44 | 1.19 | 2.65 | 2.66 | 2.72 | 0.95 | 0.88 | 2.90 | 6.71 | 6.50 | 3.37 | 3.45 | 2.12 | 1.06 |
| W22 | W23 | D21 | D22 | H2OL | BYPASS | F31 | F32 | F33 | D31 | D32 | W31 | W32 | H2OREC | H2OIN | H2OP1 | |
| ṅ [kmol h−1] | 10.00 | 10.00 | 21.21 | 21.21 | 146.57 | 29.31 | 117.26 | 117.26 | 117.26 | 9.87 | 9.87 | 107.38 | 107.38 | 97.37 | 0.02 | 10.00 |
| xH2O | 0.0003 | 0.0003 | 0.3114 | 0.3114 | 0.9296 | 0.9296 | 0.9296 | 0.9296 | 0.9296 | 0.1749 | 0.1749 | 0.9990 | 0.9990 | 0.9990 | 1.0000 | 0.9990 |
| xAA | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| xEtAc | 0.9990 | 0.9990 | 0.5487 | 0.5487 | 0.0220 | 0.0220 | 0.0220 | 0.0220 | 0.0220 | 0.2619 | 0.2619 | - | - | - | - | - |
| xEtOH | 0.0007 | 0.0007 | 0.1399 | 0.1399 | 0.0484 | 0.0484 | 0.0484 | 0.0484 | 0.0484 | 0.5632 | 0.5632 | 0.0010 | 0.0010 | 0.0010 | - | 0.0010 |
| T [°C] | 76.83 | 25.00 | 70.02 | 35.00 | 25.00 | 25.00 | 25.00 | 30.34 | 72.79 | 71.69 | 35.00 | 99.34 | 40.34 | 40.34 | 25.00 | 40.34 |
| P [kPa] | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 |
| [m3 h−1] | 1.06 | 0.98 | 1.51 | 1.43 | 3.18 | 0.64 | 2.55 | 2.56 | 59.33 a | 0.65 | 0.61 | 2.11 | 1.98 | 1.80 | 0.00 | 0.18 |
| C1 | C2 | C3 | |
|---|---|---|---|
| N | 60 | 60 | 30 |
| fP12 | 35 | - | - |
| fAZ | 34 | - | - |
| fF2 | - | 36 | - |
| fF33 | - | - | 18 |
| DS1 | xD1(AA) = 10−5 | xW21(EtAc) = 0.999 | xW31(H2O) = 0.999 |
| DS2 | xW11(AA) = 0.70 | - | xD31(EtOH) = 0.56 |
| R | 2.00 | 5.00 | 4.00 |
| P [kPa] | 101.3 | 101.3 | 101.3 |
| Column geometry | |||
| Column section 1 | 2–40 | 2–59 | 2–29 |
| N1 | 39 | 58 | 28 |
| Packing type | RALU-RING 25 mm | RALU-RING 25 mm | RALU-RING 38 mm |
| d1 [m] | 1.0 | 1.20 | 0.60 |
| H1 [m] | 2 × 4.0 | 2 × 3.5 | 2 × 4.0 |
| HETP1 [m] | 0.21 | 0.12 | 0.29 |
| ΔP1 [kPa] | 2.0 | 1.8 | 0.8 |
| Column section 2 | 41–59 | - | - |
| N2 | 19 | - | - |
| Packing type | RALU-RING 38 mm | - | - |
| d2 [m] | 1.0 | - | - |
| H2 [m] | 4.0 | - | - |
| HETP2 [m] | 0.21 | - | - |
| ΔP2 [kPa] | 0.9 | - | - |
| AA0 | ETOH0 | ETOH | D11 | W11 | W12 | W13 a | D31 | W31 | W32 | W33 | H2OP | H2OREC | H2OIN | H2ODEC | H2OL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ṅ [kmol h−1] | 10.00 | 10.00 | 18.66 | 20.94 | 7.72 | 77.34 | 77.34 | 8.66 | 68.70 | 68.70 | 68.70 | 10.02 | 58.67 | 0.01 | 58.69 | 69.62 |
| xH2O | - | - | 0.0909 | 0.2666 | 0.7918 | 0.9096 | 0.9096 | 0.1958 | 0.9995 | 0.9995 | 0.9995 | 0.9995 | 0.9995 | 1.0000 | 0.9995 | 0.9227 |
| xAA | 1.0000 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| xEtAc | - | - | 0.0855 | 0.5536 | - | 0.0206 | 0.0206 | 0.1842 | - | - | - | - | - | - | - | 0.0229 |
| xEtOH | - | 1.0000 | 0.8236 | 0.1798 | 0.2082 | 0.0697 | 0.0698 | 0.6200 | 0.0005 | 0.0005 | 0.0005 | 0.0005 | 0.0005 | - | - | 0.0544 |
| T [°C] | 25.00 | 25.00 | 47.88 | 70.02 | 82.47 | 31.20 | 72.04 | 72.65 | 99.50 | 45.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| P [kPa] | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 |
| [m3 h−1] | 0.56 | 0.58 | 1.11 | 1.54 | 0.22 | 1.75 | 19.65a | 0.53 | 1.35 | 1.27 | 1.25 | 0.18 | 1.06 | 0.00 | 1.06 | 1.53 |
| ORG1 | ORG2 | F2 | W21 | W22 | D21 | D22 | D23 | |||||||||
| ṅ [kmol h−1] | 19.66 | 19.66 | 40.60 | 10.01 | 10.01 | 30.59 | 30.59 | 30.59 | ||||||||
| xH2O | 0.1632 | 0.1632 | 0.2165 | 0.0002 | 0.0002 | 0.2873 | 0.2873 | 0.2873 | ||||||||
| xAA | - | - | - | - | - | - | - | - | ||||||||
| xEtAc | 0.7558 | 0.7558 | 0.6515 | 0.9990 | 0.9990 | 0.5378 | 0.5378 | 0.5378 | ||||||||
| xEtOH | 0.0811 | 0.0811 | 0.1320 | 0.0008 | 0.0008 | 0.1749 | 0.1749 | 0.1749 | ||||||||
| T [°C] | 25.00 | 60.00 | 64.47 | 76.84 | 25.00 | 70.01 | 45.84 | 25.00 | ||||||||
| P [kPa] | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | ||||||||
| [m3 h−1] | 1.59 | 1.68 | 3.21 | 1.06 | 0.98 | 2.20 | 2.12 | 2.06 |
| RD | C1 | C2 | |
|---|---|---|---|
| N | 100 | 60 | 50 |
| NR | 25–100 | - | - |
| fA00 | 25 | - | - |
| fETOH0 | 70 | - | - |
| fF2 | - | 38 | - |
| fW13 | - | - | 32 |
| DS1 | xD11(AA) = 10−5 | xW21(EtAc) = 0.999 | xW31(H2O) = 0.999 |
| DS2 | xW11(AA) = 10−5 | - | xD31(EtOH) = 0.62 |
| R | 2.40 | 5.00 | 2.58 |
| P [kPa] | 101.3 | 101.3 | 101.3 |
| Column geometry | |||
| Column section 1 | 2–75 | 2–59 | 2–49 |
| N1 | 74 | 58 | 48 |
| Packing type | RALU-RING 25 mm | RALU-RING 25 mm | RALU-RING 25 mm |
| d1 [m] | 0.75 | 1.30 | 0.40 |
| H1 [m] | 3 × 3.0 | 2 × 3.5 | 2 × 3.0 |
| HETP1 [m] | 0.12 | 0.12 | 0.13 |
| ΔP1 [kPa] | 3.1 | 2.8 | 1.6 |
| Column section 2 | 76–99 | - | - |
| N2 | 24 | - | - |
| Packing type | RALU-RING 25 mm | - | - |
| d2 [m] | 0.75 | - | - |
| H2 [m] | 3.5 | - | - |
| HETP2 [m] | 0.15 | - | - |
| ΔP2 [kPa] | 0.8 | - | - |
| AA0 | ETOH0 | W11 | W12 | D1 | AZ1 a | W21 | W22 | |
|---|---|---|---|---|---|---|---|---|
| ṅ [kmol h−1] | 10.00 | 10.00 | 10.00 | 10.00 | 145.54 | 135.54 | 10.00 | 10.00 |
| xH2O | - | - | 0.9990 | 0.9990 | 0.2738 | 0.2940 | 0.0002 | 0.0002 |
| xAA | 1.0000 | - | 0.0008 | 0.0008 | - | - | - | - |
| xEtAc | - | - | - | - | 0.5902 | 0.5600 | 0.9990 | 0.9990 |
| xEtOH | - | 1.0000 | 0.0002 | 0.0002 | 0.1360 | 0.1460 | 0.0008 | 0.0008 |
| T [°C] | 25.00 | 25.00 | 99.60 | 25.00 | 70.04 | 70.04 | 76.84 | 25.00 |
| P [kPa] | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 |
| [m3 h−1] | 0.56 | 0.58 | 0.20 | 0.18 | 10.88 | 3740.18 a | 1.06 | 0.98 |
| RD | C1 | |
|---|---|---|
| N | 100 | 60 |
| NR | 30–100 | - |
| fAA0 | 30 | - |
| fAZ1 | 45 | - |
| fEtOH0 | 60 | - |
| fD1 | - | 1 |
| DS1 | xD1(AA) = 10−5 | xW21(EtAc) = 0.999 |
| DS2 | xW11(H2O) = 0.999 | - |
| R | 0.32 | - |
| P [kPa] | 101.3 | 101.3 |
| Column geometry | ||
| Column section 1 | 2–44 | 1–59 |
| N1 | 43 | 59 |
| Packing type | RALU-RING 25 mm | RALU-RING 25 mm |
| d1 [m] | 1.15 | 1.30 |
| H1 [m] | 3 × 3.4 | 3 × 3.0 |
| HETP1 [m] | 0.24 | 0.15 |
| ΔP1 [kPa] | 3.6 | 1.7 |
| Column section 2 | 45–99 | - |
| N2 | 55 | - |
| Packing type | RALU-RING 15 mm | - |
| d2 [m] | 0.65 | - |
| H2 [m] | 3 × 4.0 | - |
| HETP2 [m] | 0.22 | - |
| ΔP2 [kPa] | 2.4 | - |
| AA0 | ETOH0 | EO | D1 | D2 | W1 | W2 | |
|---|---|---|---|---|---|---|---|
| ṅ [kmol h−1] | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| xH2O | - | - | - | - | - | - | - |
| xAA | 1.0000 | - | - | - | - | 0.0004 | 0.0004 |
| xEtAc | - | - | - | 0.9993 | 0.9993 | 0.0003 | 0.0003 |
| xEtOH | - | 1.0000 | - | 0.0004 | 0.0004 | - | - |
| xEO | - | - | 1.0000 | 0.0003 | 0.0003 | - | - |
| xMEG | - | - | - | - | - | 0.9993 | 0.9993 |
| T [°C] | 25.00 | 25.00 | 25.00 | 76.81 | 25.00 | 194.63 | 25.00 |
| P [kPa] | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 | 101.3 |
| [m3 h−1] | 0.56 | 0.58 | 241.61 a | 1.06 | 0.98 | 0.65 | 0.55 |
| RD | |
|---|---|
| N | 80 |
| NR1 | 20–80 |
| NR2 | 1–80 |
| fAA0 | 20 |
| fETOH0 | 66 |
| fEO | 72 |
| DS1 | xD1(EtAc) = 0.999 |
| DS2 | xW1(MEG) = 0.999 |
| R | 3.43 |
| P [kPa] | 101.3 |
| Column geometry | |
| Column section 1 | 2–66 |
| N1 | 65 |
| Packing type | RALU-RING 25 mm |
| d1 [m] | 0.70 |
| H1 [m] | 4 × 4.0 |
| HETP1 [m] | 0.25 |
| ΔP1 [kPa] | 4.2 |
| Column section 2 | 67–79 |
| N2 | 13 |
| Packing type | RALU-RING 15 mm |
| d2 [m] | 0.40 |
| H2 [m] | 4.0 |
| HETP2 [m] | 0.31 |
| ΔP2 [kPa] | 1.1 |
| Conventional Process Set-Up (Figure 1) | RD Column with a Separation Unit (Figure 2) | RD Column with a Stripper (RDS) (Figure 3) | RD Column with an Auxiliary Reaction (RDAR) (Figure 4) | |||||
|---|---|---|---|---|---|---|---|---|
| Equipment | [kW] | [kW] | [kW] | [kW] | [kW] | [kW] | [kW] | [kW] |
| CSTR a/RD | - | - | 676.69 | 703.86 | 1816.43 | 556.42 | 396.70 | 152.57 |
| C1 | 1135.23 | 1181.19 | 1750.31 | 1759.95 | - | 1286.57 | - | - |
| C2 | 1212.97 | 1267.94 | 323.10 | 361.93 | - | - | - | - |
| C3 | 502.97 | 549.35 | - | - | - | - | - | - |
| DEC | 0.85 | - | 8.07 | - | - | - | - | - |
| EX1 | 25.01 b | - | 31.04 b | - | 15.39 | - | 25.85 | - |
| EX2 | 133.75 | - | 28.61 | - | 25.87 | - | 77.20 | - |
| EX3 | 31.04 b | - | 77.70 b | - | - | - | - | - |
| EX4 | 13.73 b | - | 27.83 | - | - | - | - | - |
| EX5 | 131.31 b | - | 25.89 | - | - | - | - | - |
| EX6 | 3.10 | - | - | - | - | - | - | - |
| EX7 | 25.85 | - | - | - | - | - | - | - |
| OEC | 3014.72 | 2998.48 | 2840.50 | 2825.74 | 1857.69 | 1842.99 | 499.76 | 152.57 |
| SEC [kW t−1EtAc] | 3426.87 | 3408.41 | 3223.86 | 3207.11 | 2110.30 | 2093.59 | 567.68 | 173.30 |
| Number of main equipment units | 5 | 4 | 2 | 1 | ||||
| Number of heat exchangers | 7 | 5 | 2 | 2 | ||||
| Raw Materials | References | ||
|---|---|---|---|
| EtOH | 800 | $ t−1 | [46,47] |
| AA | 400 | $ t−1 | [46] |
| EO | 1000 | $ t−1 | [46] |
| Products | |||
| EtAc | 1300 | $ t−1 | [45] |
| MEG | 1100 | $ t−1 | [46] |
| Energy | [26] | ||
| Electricity | 0.0775 | $ kWh−1 | |
| cooling water | 0.0317 | $ m−3 | |
| steam (0.7 MPa) | 0.0179 | $ kg−1 | |
| steam (2.7 MPa) | 0.0258 | $ kg−1 |
| Conventional Path | RD Column with a Separation Unit | RDS | RDAR | |||||
|---|---|---|---|---|---|---|---|---|
| (Figure 1) | (Figure 2) | (Figure 3) | (Figure 4) | |||||
| Rate | Price [$ h−1] | Rate | Price [$ h−1] | Rate | Price [$ h−1] | Rate | Price [$ h−1] | |
| electricity [kW] | 104.17 | 8.07 | 58.10 | 4.50 | 56.05 | 4.34 | 53.81 | 4.17 |
| cooling water [m3 h−1] | 233.11 | 7.39 | 219.36 | 6.95 | 143.87 | 4.56 | 38.71 | 1.23 |
| steam (0.7 MPa) [kg h−1] | 5219.95 | 93.68 | 4920.70 | 88.31 | 3209.30 | 57.59 | - | - |
| steam (2.7 MPa) [kg h−1] | - | - | - | - | - | - | 302.55 | 7.81 |
| Conventional Path | RD Column with a Separation Unit | RDS | RDAR | |
|---|---|---|---|---|
| (Figure 1) | (Figure 2) | (Figure 3) | (Figure 4) | |
| Total capital cost [ mil. $] | 9.91 | 8.84 | 6.43 | 4.24 |
| Total installed cost [mil. $] | 3.74 | 3.33 | 2.43 | 1.60 |
| Total annual cost [mil. $ year−1] | 9.15 | 9.04 | 8.65 | 13.72 |
| Total raw materials cost [mil. $ year−1] | 5.46 | 5.46 | 5.46 | 9.42 |
| Total product sales [mil. $ year−1] | 10.27 | 10.27 | 10.27 | 16.40 |
| Total utilities cost [mil. $ year−1] | 0.89 | 0.81 | 0.54 | 0.11 |
| Pay-back period [year] | 10.86 | 9.19 | 5.97 | 3.58 |
| Total production cost [$ t−1] | 1273.91 | 1266.14 | 1231.32 | 1018.09 a |
| Individual Risk 10−5 Year−1 | Individual Risk 10−4 Year−1 | |
|---|---|---|
| Distance from the Center [m] | Distance from the Center [m] | |
| Conventional path (Figure 1) | 122 | 95 |
| RD column with separation unit (Figure 2) | 166 | 59 |
| RDS (Figure 3) | 122 | 43 |
| RDAR (Figure 4) | 62 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šulgan, B.; Labovský, J.; Labovská, Z. Multi-Aspect Comparison of Ethyl Acetate Production Pathways: Reactive Distillation Process Integration and Intensification via Mechanical and Chemical Approach. Processes 2020, 8, 1618. https://doi.org/10.3390/pr8121618
Šulgan B, Labovský J, Labovská Z. Multi-Aspect Comparison of Ethyl Acetate Production Pathways: Reactive Distillation Process Integration and Intensification via Mechanical and Chemical Approach. Processes. 2020; 8(12):1618. https://doi.org/10.3390/pr8121618
Chicago/Turabian StyleŠulgan, Branislav, Juraj Labovský, and Zuzana Labovská. 2020. "Multi-Aspect Comparison of Ethyl Acetate Production Pathways: Reactive Distillation Process Integration and Intensification via Mechanical and Chemical Approach" Processes 8, no. 12: 1618. https://doi.org/10.3390/pr8121618
APA StyleŠulgan, B., Labovský, J., & Labovská, Z. (2020). Multi-Aspect Comparison of Ethyl Acetate Production Pathways: Reactive Distillation Process Integration and Intensification via Mechanical and Chemical Approach. Processes, 8(12), 1618. https://doi.org/10.3390/pr8121618