Mesoporous Mn-Doped Fe Nanoparticle-Modified Reduced Graphene Oxide for Ethyl Violet Elimination: Modeling and Optimization Using Artificial Intelligence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
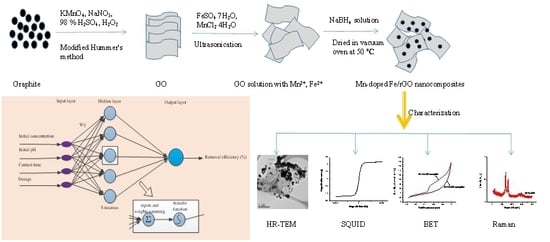
2.2. Fabrication of Fe–Mn and Mn-doped Fe/rGO
2.3. Characterization
2.4. Dye Removal Procedure
2.5. Response Surface Methodology
2.6. Artificial Neural Network (ANN)
2.7. Optimization Using ANN-GA and ANN-PSO Models
2.8. Random Forest
2.9. Radial Basis Function Neural Network
3. Results and Discussion
3.1. Surface Characterization of Fe–Mn Nanoparticles and Mn-doped Fe/rGO Nanocomposites
3.2. Central Composite Design (CCD)
3.3. Back Propagation Artificial Neural Network (BP-ANN)
3.4. Optimization for the Removal of EV by RSM, ANN-GA and ANN-PSO
3.5. Comparison of RF, RBF and BP-ANN
3.6. Comparison among Analysis of Variance, GBRT, Garson Method and RF
3.7. Adsorption Isotherms
3.8. Kinetics Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Velusamy, P.; Pitchaimuthu, S.; Rajalakshmi, S.; Kannan, N. Modification of the photocatalytic activity of TiO2 by β-Cyclodextrin in decoloration of ethyl violet dye. J. Adv. Res. 2014, 5, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, W.T.; Chang, Y.M.; Lai, C.W.; Lo, C.C. Adsorption of ethyl violet dye in aqueous solution by regenerated spent bleaching earth. J. Colloid Interface Sci. 2005, 289, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ahmad, N.; Yasar, A.; Shahid, R. Advanced oxidative decolorization of red Cl-5B: Effects of dye concentration, process optimization and reaction kinetics. Pol. J. Environ. Stud. 2010, 19, 83–92. [Google Scholar]
- Lee, W.W.; Chung, W.H.; Lu, C.S.; Lin, W.Y.; Chen, C.C. A study on the degradation efficiency and mechanisms of ethyl violet by HPLC―PDA―ESI―MS and GC―MS. Sep. Purif. Technol. 2012, 98, 488–496. [Google Scholar] [CrossRef]
- Duxbury, D.F. The photochemistry and photophysics of triphenylmethane dyes in solid and liquid media. Chem. Rev. 1993, 93, 381–433. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Li, C.; Li, Z. Fe-loaded MOF-545 (Fe): Peroxidase-like activity for dye degradation dyes and high adsorption for the removal of dyes from wastewater. Molecules 2020, 25, 168. [Google Scholar] [CrossRef] [Green Version]
- Ruan, W.; Shi, X.; Hu, J.; Hou, Y.; Fan, M.; Cao, R.; Wei, X. Modeling of malachite green removal from aqueous solutions by nanoscale zerovalent zinc using artificial neural network. Appl. Sci. 2018, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, K.; Lou, L. Photodegradation of dye pollutants on silica gel supported TiO2 particles under visible light irradiation. J. Photochem. Photobiol. A 2004, 163, 281–287. [Google Scholar] [CrossRef]
- Wang, J.Q.; Liu, Y.H.; Chen, M.W.; Xie, G.Q.; Louzguine-Luzgin, D.V.; Inoue, A.; Perepezko, J.H. Rapid degradation of azo dye by Fe-based metallic glass powder. Adv. Funct. Mater. 2012, 22, 2567–2570. [Google Scholar] [CrossRef]
- Zhang, W. Nanoscale iron particles for environmental remediation: An overview. J. Nanopart. Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Kalme, S.D.; Parshetti, G.K.; Jadhav, S.U.; Govindwar, S.P. Biodegradation of benzidine based dye direct blue-6 by pseudomonas desmolyticum NCIM 2112. Bioresour. Technol. 2007, 98, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.K. Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2009, 165, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Hmani, E.; Samet, Y.; Abdelhédi, R. Electrochemical degradation of auramine-O dye at boron-doped diamond and lead dioxide electrodes. Diam. Relat. Mater. 2012, 30, 1–8. [Google Scholar] [CrossRef]
- Basiri, F.; Ravandi, S.A.H.; Feiz, M.; Moheb, A. Recycling of direct dyes wastewater by nylon-6 nanofibrous membrane. Curr. Nanosci. 2011, 7, 633–639. [Google Scholar] [CrossRef]
- Kamal, S.; Pan, G.T.; Chong, S.; Yang, T.C.K. Ultrasonically induced sulfur-doped carbon nitride/cobalt ferrite nanocomposite for efficient sonocatalytic removal of organic dyes. Processes 2020, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Zhang, C.; Deng, J.; Jiang, Z.; Zhang, L.; Cheng, Y.; Hou, K.; Tang, L.; Zeng, G. Factors influencing degradation of trichloroethylene by sulfide-modified nanoscale zero-valent iron in aqueous solution. Water Res. 2018, 135, 1–10. [Google Scholar] [CrossRef]
- Sun, Y.P.; Li, X.Q.; Cao, J.; Zhang, W.X.; Wang, H.P. Characterization of zero-valent iron nanoparticles. Adv. Colloid Interface 2006, 120, 47–56. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, S.; Lu, X.Q.; Chen, Z.l. Removal of Pb(II) from water using synthesized kaolin supported nanoscale zero-valent iron. Chem. Eng. J. 2010, 163, 243–248. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Mehrdad, M.; Khanizadeh, M. Nitrate Removal from Water Using Synthesis Nanoscale Zero-Valent Iron (NZVI). In Proceedings of the International Conference on Applied Life Sciences (ICALS2012), Konya, Turkey, 10–12 September 2012; pp. 105–110. [Google Scholar]
- Arabi, S.; Sohrabi, M.R. Experimental design and response surface modelling for optimization of vat dye from water by nano zero valent iron (NZVI). Acta Chim. Slov. 2013, 60, 853–860. [Google Scholar]
- Huber, D.L. Synthesis, properties, and applications of iron nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef]
- Lu, H.; Wang, J.; Ferguson, S.; Wang, T.; Bao, Y.; Hao, H. Mechanism, synthesis and modification of nano zerovalent iron in water treatment. Nanoscale 2016, 8, 9962–9975. [Google Scholar] [CrossRef] [PubMed]
- Diagboya, P.N.; Olu-Owolabi, B.I.; Zhou, D.; Han, B.H. Graphene oxide–tripolyphosphate hybrid used as a potent sorbent for cationic dyes. Carbon 2014, 79, 174–182. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Gao, W.; Alemany, L.B.; Ci, L.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Katsnelson, M.I. Modeling of graphite oxide. J. Am. Chem. Soc. 2008, 130, 10697–10701. [Google Scholar] [CrossRef] [Green Version]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Tang, J.; Huang, Y.; Gong, Y.; Lyu, H.; Wang, Q.; Ma, J. Preparation of a novel graphene oxide/Fe-Mn composite and its application for aqueous Hg(II) removal. J. Hazard. Mater. 2016, 316, 151–158. [Google Scholar] [CrossRef]
- Tan, W.C.; Hofmann, M.; Hsieh, Y.P.; Lu, M.L.; Chen, Y.F. A graphene-based surface plasmon sensor. Nano Res. 2012, 5, 695–702. [Google Scholar] [CrossRef]
- Joshi, R.K.; Alwarappan, S.; Yoshimura, M.; Sahajwalla, V.; Nishina, Y. Graphene oxide: The new membrane material. Appl. Mater. Today 2015, 1, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Bai, H.; Li, C.; Shi, G. A graphene oxide/hemoglobin composite hydrogel for enzymatic catalysis in organic solvents. Chem. Commun. 2011, 47, 4962. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, Z.; Tang, Y.; Bian, H.; Ng, T.W.; Zhang, W.; Lee, C.S. Graphene-nanowall-decorated carbon felt with excellent electrochemical activity toward VO\r 2+\r /VO\r 2+\r couple for all vanadium redox flow battery. Adv. Sci. 2016, 3, 1500276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.M.; Aboutalebi, S.H.; Cardillo, D.; Liu, H.K.; Konstantinov, K.; Dou, S.X. Self-assembled multifunctional hybrids: Toward developing high-performance graphene-based architectures for energy storage devices. ACS Cent. Sci. 2015, 1, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Bissett, M.A.; Kinloch, I.A.; Dryfe, R.A.W. Characterization of MoS2-graphene composites for high-performance coin cell supercapacitors. ACS Appl. Mater. Inter. 2015, 7, 17388. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wu, S.; Yin, Z.; Zhang, H. Graphene-based electronic sensors. Chem. Sci. 2012, 3, 1764–1772. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.; Luo, T.; Wang, S.; Wang, X.; Guo, Z.; Xiong, H.; Dong, B.; Zhao, L.; Xu, Z.; Zhang, X.; et al. Pt/graphene nanocomposites with low Pt-loadings: Synthesis through one- and two-step chemical reduction methods and their use as promising counter electrodes for DSSCs. Compos. Sci. Technol. 2015, 113, 46–53. [Google Scholar] [CrossRef]
- Fan, M.; Hu, J.; Cao, R.; Ruan, W.; Wei, X. A review on experimental design for pollutants removal in water treatment with the aid of artificial intelligence. Chemosphere 2018, 200, 330. [Google Scholar] [CrossRef]
- Mocanu, D.C.; Mocanu, E.; Stone, P.; Nguyen, P.H.; Gibescu, M.; Liotta, A. Scalable training of artificial neural networks with adaptive sparse connectivity inspired by network science. Nat. Commun. 2018, 9, 2383–2395. [Google Scholar] [CrossRef] [Green Version]
- Vakili, M.; Karami, M.; Delfani, S.; Khosrojerdi, S.; Kalhor, K. Experimental investigation and modeling of thermal conductivity of CuO–water/EG nanofluid by FFBP-ANN and multiple regressions. J. Therm. Anal. Calorim. 2017, 129, 629–637. [Google Scholar] [CrossRef]
- Hua, J.; Xiao, C.; Ke, Z.; Wang, Y. Construct Drawing Man-Hour Forecasting based on GA-BP in Chemical Equipment Design Process. In Proceedings of the 2016 22nd International Conference on Automation and Computing (ICAC), Colchester, UK, 7–8 September 2016; pp. 1–6. [Google Scholar]
- Li, J.; Cheng, J.; Shi, J.; Huang, F. Brief Introduction of Back Propagation (BP) Neural Network Algorithm and its Improvement. In Advances in Computer Science and Information Engineering-Volume 2; Jin, D., Lin, S., Eds.; Vol. 169 of Advances in Intelligent and Soft Computing; Springer: Berlin/Heidelberg, Germany, 2012; pp. 553–558. [Google Scholar]
- Ahmadi Azqhandi, M.H.; Ghaedi, M.; Yousefi, F.; Jamshidi, M. Application of random forest, radial basis function neural networks and central composite design for modeling and/or optimization of the ultrasonic assisted adsorption of brilliant green on ZnS-NP-AC. J. Colloid Interface Sci. 2017, 505, 278–292. [Google Scholar] [CrossRef]
- Kayarvizhy, N.; Kanmani, S.; Rhymend, U.V. Improving fault prediction using ANN-PSO in object oriented systems. Int. J. Comput. Appl. 2014, 73, 18–25. [Google Scholar]
- Che, Z.H. PSO-based back-propagation artificial neural network for product and mold cost estimation of plastic injection molding. Comput. Ind. Eng. 2010, 58, 625–637. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Liu, Y. Signal Sorting Using Teaching-Learning-Based Optimization and Random Forest. In Proceedings of the 2018 17th International Symposium on Distributed Computing and Applications for Business Engineering and Science (DCABES), Wuxi, China, 19–23 October 2018; pp. 258–261. [Google Scholar]
- Shi, X.; Ruan, W.; Hu, J.; Fan, M.; Cao, R.; Wei, X. Optimizing the removal of Rhodamine B in aqueous solutions by reduced graphene oxide-supported nanoscale zerovalent iron (nZVI/rGO) using an artificial neural network-genetic algorithm (ANN-GA). Nanomaterials 2017, 7, 134. [Google Scholar] [CrossRef] [Green Version]
- Parsaee, Z.; Karachi, N.; Abrishamifar, S.M.; Kahkha, M.R.R.; Razavi, R. Silver-choline chloride modified graphene oxide: Novel nano-bioelectrochemical sensor for Celecoxib detection and CCD-RSM model. Ultrason. Sonochem. 2018, 45, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Liu, B.; Gao, J.X.; Zhang, X.F.; Li, S.L.; Liu, J.Q.; Tian, Z.P. Auto recognition of carbonate microfacies based on an improved back propagation neural network. J. Cent. South Univ. 2015, 22, 3521–3535. [Google Scholar] [CrossRef]
- Ghaedi, M.; Zeinali, N.; Ghaedi, A.M.; Teimuori, M.; Tashkhourian, J. Artificial neural network-genetic algorithm based optimization for the adsorption of methylene blue and brilliant green from aqueous solution by graphite oxide nanoparticle. Spectrochim. Acta A 2014, 125, 264–277. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Li, C.; Wang, C. GBRTVis: Online analysis of gradient boosting regression tree. J. Visual. 2019, 22, 125–140. [Google Scholar] [CrossRef]
- Li, X.; Bai, R. Freight Vehicle Travel Time Prediction Using Gradient Boosting Regression Tree. In Proceedings of the 2016 15th IEEE International Conference on Machine Learning and Applications (ICMLA), Anaheim, CA, USA, 18–20 December 2016; pp. 1–7. [Google Scholar]
- Montaño, J.J.; Palmer, A. Numeric sensitivity analysis applied to feedforward neural networks. Neural Comput. Appl. 2003, 12, 119–125. [Google Scholar] [CrossRef]
- Ruan, W.; Hu, J.; Qi, J.; Hou, Y.; Cao, R.; Wei, X. Removal of crystal violet by using reduced-graphene-oxide-supported bimetallic Fe/Ni nanoparticles (rGO/Fe/Ni): Application of artificial intelligence modeling for the optimization process. Materials 2018, 11, 865. [Google Scholar] [CrossRef] [Green Version]
- Dhingra, S.; Dubey, K.K.; Bhushan, G. A polymath approach for the prediction of optimized transesterification process variables of polanga biodiesel. J. Am. Oil Chem. Soc. 2014, 91, 641–653. [Google Scholar] [CrossRef]
- Gulati, T.; Chakrabarti, M.; Sing, A.; Duvuuri, M.; Banerjee, R. Comparative study of response surface methodology, artificial neural network and genetic algorithms for optimization of soybean hydration. Food Technol. Biotech. 2010, 48, 11–18. [Google Scholar]
- Sarkar, D.; Modak, J.M. Optimisation of fed-batch bioreactors using genetic algorithms. Chem. Eng. Sci. 2003, 58, 2283–2296. [Google Scholar] [CrossRef]
- Lee, K.H.; Jun, S.O.; Pak, K.H.; Lee, D.H.; Lee, K.W.; Park, J.P. Numerical optimization of site selection for offshore wind turbine installation using genetic algorithm. Curr. Appl. Phys. 2010, 10, S302–S306. [Google Scholar] [CrossRef]
- Eberhart, R.; Kennedy, J. A New Optimizer Using Particle Swarm Theory. In Proceedings of the MHS’95. Proceedings of the Sixth International Symposium on Micro Machine and Human Science, Nagoya, Japan, 4–6 October 1995; pp. 39–43. [Google Scholar]
- Mitchell, M.L.D. Davis, handbook of genetic algorithms. Artif. Intell. 1998, 100, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Bejagam, K.K.; Singh, S.; An, Y.; Berry, C.; Deshmukh, S.A. PSO assisted development of new transferable coarse-grained water models. J. Phys. Chem. B 2018, 122, 1–37. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Wu, Z.; Fu, H.; Shi, Y.; Guo, H. Scenario analysis of natural gas consumption in China based on wavelet neural network optimized by particle swarm optimization algorithm. Energies 2018, 11, 825. [Google Scholar] [CrossRef] [Green Version]
- Sami, M.; Hassanien, A.E.; Nashwa, E.B.; Berwick, R.C. Incorporating Random Forest Trees with Particle Swarm Optimization for Automatic Image Annotation. In Proceedings of the 2012 Federated Conference on Computer Science and Information Systems (FedCSIS), Wroclaw, Poland, 9–12 September 2012; pp. 791–797. [Google Scholar]
- Ming, D.; Zhou, T.; Min, W.; Tian, T. Land cover classification using random forest with genetic algorithm-based parameter optimization. J. Appl. Remote Sens. 2016, 10, 035021. [Google Scholar] [CrossRef]
- Gislason, P.O.; Benediktsson, J.A.; Sveinsson, J.R. Random forests for land cover classification. Pattern Recogn. Lett. 2006, 27, 294–300. [Google Scholar] [CrossRef]
- Li, J.; Liu, X. Melt index prediction by RBF neural network optimized with an adaptive new ant colony optimization algorithm. J. Appl. Polym. Sci. 2011, 119, 3093–3100. [Google Scholar] [CrossRef]
- Najafi-Marghmaleki, A.; Khosravi-Nikou, M.R.; Barati-Harooni, A. A new model for prediction of binary mixture of ionic liquids+water density using artificial neural network. J. Mol. Liq. 2016, 220, 232–237. [Google Scholar] [CrossRef]
- Tatar, A.; Shokrollahi, A.; Mesbah, M.; Rashid, S.; Arabloo, M.; Bahadori, A. Implementing radial basis function networks for modeling CO2-reservoir oil minimum miscibility pressure. J. Nat. Gas Sci. Eng. 2013, 15, 82–92. [Google Scholar] [CrossRef]
- Rasouli, Z.; Hassanzadeh, Z.; Ghavami, R. Application of a new version of GA-RBF neural network for simultaneous spectrophotometric determination of Zn(II), Fe(II), Co(II) and Cu(II) in real samples: An exploratory study of their complexation abilities toward MTB. Talanta 2016, 160, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Dwivedi, V.; Chi, C.; Wu, J. Graphene oxide/ferric hydroxide composites for efficient arsenate removal from drinking water. J. Hazard. Mater. 2010, 182, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Trejos, T.; Corzo, R.; Subedi, K.; Almirall, J. Characterization of toners and inkjets by laser ablation spectrochemical methods and Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy. Spectrochim. Acta B 2014, 92, 9–22. [Google Scholar] [CrossRef]
- Su, J.; Lin, S.; Chen, Z.; Megharaj, M.; Naidu, R. Dechlorination of p-chlorophenol from aqueous solution using bentonite supported Fe/Pd nanoparticles: Synthesis, characterization and kinetics. Desalination 2011, 280, 167–173. [Google Scholar] [CrossRef]
- Casiraghi, C.; Pisana, S.; Novoselov, K.S.; Geim, A.K.; Ferrari, A.C. Raman fingerprint of charged impurities in graphene. Appl. Phys. Lett. 2007, 91, 183. [Google Scholar] [CrossRef] [Green Version]
- Kotutha, I.; Swatsitang, E.; Meewassana, W.; Maensiri, S. One-pot hydrothermal synthesis, characterization, and electrochemical properties of rGO/MnFe2O4 nanocomposites. Jpn. J. Appl. Phys. 2015, 54, 06FH10. [Google Scholar] [CrossRef]
- Pimenta, M.; Dresselhaus, G.; Dresselhaus, M.; Cancado, L.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef]
- Fontana, M.D.; Bourson, P. Microstructure and defects probed by Raman spectroscopy in lithium niobate crystals and devices. Appl. Phys. Rev. 2015, 2, 040602. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Xavier, P.; Gupta, S.N.; Kar, G.P.; Bose, S.; Sood, A. Excellent electromagnetic interference shielding by graphene-MnFe2O4-multiwalled carbon nanotube hybrids at very low weight percentage in polymer matrix. ChemistrySelect 2016, 1, 5995–6003. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S.J.C. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Q.; Oyunkhand, E.; Ding, S.; Yamane, N.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Nitrogen-rich mesoporous carbon supported iron catalyst with superior activity for Fischer-Tropsch synthesis. Carbon 2018, 130, 304–314. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Lin, X.; Lv, X.; Wang, L.; Zhang, F.; Duan, L. Preparation and characterization of MnFe2O4 in the solvothermal process: Their magnetism and electrochemical properties. Mater. Res. Bull. 2013, 48, 2511–2516. [Google Scholar] [CrossRef]
- Hung, I.M.; Hung, D.T.; Fung, K.Z.; Hon, M.H. Synthesis and characterization of highly ordered mesoporous YSZ by tri-block copolymer. J. Porous Mat. 2006, 13, 225–230. [Google Scholar] [CrossRef]
- Solaymani, E.; Ghaedi, M.; Karimi, H.; Azqhandi, M.H.A.; Asfaram, A. Intensified removal of Malachite green by AgOH-AC nanoparticles combined with ultrasound: Modeling and optimization. Appl. Organomet. Chem. 2017, 31, e3857. [Google Scholar] [CrossRef]
- El-Gamal, S.; Amin, M.; Ahmed, M. Removal of methyl orange and bromophenol blue dyes from aqueous solution using Sorel’s cement nanoparticles. J. Environ. Chem. Eng. 2015, 3, 1702–1712. [Google Scholar] [CrossRef]
- Forouz, F.S.; Ravandi, S.A.H.; Allafchian, A.R. Removal of Ag and Cr heavy metals using nanofiber membranes functionalized with aminopropyltriethoxysilane (APTES). Curr. Nanosci. 2016, 12, 266–274. [Google Scholar] [CrossRef]
- Chowdhury, S.; Misra, R.; Kushwaha, P.; Das, P. Optimum sorption isotherm by linear and nonlinear methods for safranin onto alkali-treated rice husk. Bioremediat. J. 2011, 15, 77–89. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Han, M.; Su, Q.; Xia, L.; Hui, Z. Adsorption properties of magnetic magnetite nanoparticle for coexistent Cr (VI) and Cu (II) in mixed solution. Water 2020, 12, 446. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Cheng, T.; Zhang, X.; Wu, R.; Wang, Q. Synthesis of an efficient Pb adsorption nano-crystal under strong alkali hydrothermal environment using a gemini surfactant as directing agent. J. Chem. Soc. Pak. 2019, 41, 1034–1038. [Google Scholar]
- Altaher, H.; Khalil, T.E.; Abubeah, R. The effect of dye chemical structure on adsorption on activated carbon: A comparative study. Color. Technol. 2014, 130, 205–214. [Google Scholar] [CrossRef]
- Cheng, C.; Cheng, T.; Wang, Z.L.; Han, C.H. Removal of Zn2+ in aqueous solution by Linde F (K) zeolite prepared from recycled fly ash. J. Indian Chem. Soc. 2014, 91, 1–7. [Google Scholar]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Comparison of various error functions in predicting the optimum isotherm by linear and non-linear regression analysis for the sorption of basic red 9 by activated carbon. J. Hazard. Mater. 2008, 150, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Lin, X.; Xie, J.; Huang, X. Kinetic, equilibrium and thermodynamic studies for phosphate adsorption on aluminum hydroxide modified palygorskite nano-composites. RSC Adv. 2017, 7, 4492–4500. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar]
- Ho, Y.S.; Mckay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, T.; Shi, Y.; Tian, Y. Adsorption of Cu (II) from aqueous solution on fly ash based Linde F (K) zeolite. Iran. J. Chem. Chem. Eng. 2014, 33, 29–35. [Google Scholar]
- Dogan, M.; Alkan, M. Adsorption kinetics of methyl violet onto perlite. Chemosphere 2003, 50, 517–528. [Google Scholar] [CrossRef]
- Cheng, T.; Chen, C.; Tang, R.; Han, C.H.; Tian, Y. Competitive adsorption of Cu, Ni, Pb, and Cd from aqueous solution onto fly ash-based linde F(K) Zeolite. Iran. J. Chem. Chem. Eng. 2018, 37, 61–72. [Google Scholar]
- Reffas, A.; Bouguettoucha, A.; Chebli, D.; Amrane, A. Adsorption of ethyl violet dye in aqueous solution by forest wastes, wild carob. Desalin. Water Treat. 2015, 57, 9859–9870. [Google Scholar] [CrossRef]





























| Factors | Coded Variable Levels | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | α | |
| Initial concentration (mg/L) | 250 | 300 | 350 | 400 | 450 |
| Initial pH | 3 | 4 | 5 | 6 | 7 |
| Sonication time (min) | 7 | 10 | 13 | 16 | 19 |
| Dosage (mg) | 10 | 13 | 16 | 19 | 22 |
| Run No. | Initial Concentration (mg/L) | Initial pH | Sonication Time (min) | Dosage (mg) | Removal Efficiency (%) | Prediction Efficiency (%) |
|---|---|---|---|---|---|---|
| 1 | 350 | 5 | 13 | 16 | 88 | 87 |
| 2 | 300 | 6 | 10 | 13 | 76 | 75 |
| 3 | 350 | 3 | 13 | 16 | 83 | 82 |
| 4 | 300 | 4 | 10 | 19 | 80 | 82 |
| 5 | 400 | 4 | 10 | 13 | 67 | 65 |
| 6 | 300 | 4 | 16 | 19 | 85 | 88 |
| 7 | 400 | 4 | 16 | 13 | 72 | 72 |
| 8 | 350 | 5 | 13 | 16 | 86 | 87 |
| 9 | 300 | 6 | 16 | 13 | 82 | 82 |
| 10 | 300 | 4 | 16 | 13 | 80 | 80 |
| 11 | 400 | 6 | 10 | 19 | 82 | 80 |
| 12 | 350 | 5 | 13 | 22 | 87 | 85 |
| 13 | 350 | 5 | 13 | 16 | 86 | 87 |
| 14 | 400 | 4 | 10 | 19 | 75 | 75 |
| 15 | 300 | 6 | 10 | 19 | 87 | 86 |
| 16 | 400 | 6 | 16 | 19 | 85 | 86 |
| 17 | 400 | 6 | 16 | 13 | 77 | 75 |
| 18 | 350 | 5 | 13 | 16 | 90 | 87 |
| 19 | 300 | 6 | 16 | 19 | 92 | 93 |
| 20 | 450 | 5 | 13 | 16 | 71 | 74 |
| 21 | 350 | 7 | 13 | 16 | 86 | 90 |
| 22 | 350 | 5 | 19 | 16 | 80 | 78 |
| 23 | 400 | 6 | 10 | 13 | 70 | 67 |
| 24 | 400 | 4 | 16 | 19 | 80 | 80 |
| 25 | 250 | 5 | 13 | 16 | 90 | 89 |
| 26 | 350 | 5 | 13 | 16 | 87 | 87 |
| 27 | 350 | 5 | 13 | 10 | 60 | 64 |
| 28 | 300 | 4 | 10 | 13 | 74 | 73 |
| 29 | 350 | 5 | 13 | 16 | 86 | 87 |
| 30 | 350 | 5 | 7 | 16 | 61 | 66 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 1899.59 | 14 | 135.68 | 16.19 | <0.0001 | significant |
| A | 325.24 | 1 | 325.24 | 38.8 | <0.0001 | |
| B | 88.05 | 1 | 88.05 | 10.5 | 0.0055 | |
| C | 265.14 | 1 | 265.14 | 31.63 | <0.0001 | |
| D | 617.02 | 1 | 617.02 | 73.6 | <0.0001 | |
| AB | 0.24 | 1 | 0.24 | 0.028 | 0.8685 | |
| AC | 0.15 | 1 | 0.15 | 0.018 | 0.8953 | |
| AD | 0.68 | 1 | 0.68 | 0.081 | 0.7802 | |
| BC | 0.11 | 1 | 0.11 | 0.014 | 0.9087 | |
| BD | 8.63 | 1 | 8.63 | 1.03 | 0.3264 | |
| CD | 1.2 | 1 | 1.2 | 0.14 | 0.71 | |
| A2 | 51.24 | 1 | 51.24 | 6.11 | 0.0259 | |
| B2 | 3.09 | 1 | 3.09 | 0.37 | 0.553 | |
| C2 | 385.56 | 1 | 385.56 | 45.99 | <0.0001 | |
| D2 | 271.17 | 1 | 271.17 | 32.35 | <0.0001 | |
| Residual | 125.75 | 15 | 8.38 | |||
| Lack of Fit | 112.92 | 10 | 11.29 | 4.4 | 0.0578 | not significant |
| Pure Error | 12.83 | 5 | 2.57 | |||
| Cor Total | 2025.33 | 29 | ||||
| R2 = 0.9379 | ||||||
| Process Parameters | Optimization | ||
|---|---|---|---|
| ANN-GA | ANN-PSO | Response Surface Methodology (RSM) | |
| Initial concentration (mg/L) | 299.22 | 371.23 | 300 |
| Initial pH | 6.1 | 5 | 6 |
| Sonication time (min) | 13.19 | 13.95 | 14.32 |
| Dosage (mg) | 17.55 | 17.20 | 18.62 |
| Decolorization efficiency of model (%) | 92.75% | 90.11% | 94.18% |
| Experimental verification values (%) | 92.03% | 88.83% | 92.65% |
| Average values of absolute errors (%) | 0.72% | 1.28% | 1.53% |
| R2 | 0.99403 | 0.9379 | |
| MSE | 0.0005 | ||
| Number of Neurons | wi | bi Input Bias | wj Hidden Weights | bj Hidden Bias | |||
|---|---|---|---|---|---|---|---|
| Input Weights | |||||||
| A | B | C | D | ||||
| 1 | 1.901923065 | 0.366456135 | −0.653955781 | 1.420846441 | −2.489591174 | −0.930289741 | 0.149904727 |
| 2 | 1.309510183 | 1.345913921 | 0.27228768 | −1.611714128 | −1.936348691 | −0.26791453 | |
| 3 | −1.358393495 | −1.004381258 | −0.631064845 | 1.716335276 | 1.383106208 | 0.145250088 | |
| 4 | 0.835468192 | −0.017592712 | −1.268234245 | 1.972645308 | −0.829863725 | −0.328025674 | |
| 5 | 0.491800507 | −1.471722414 | 1.875969756 | 0.520544991 | −0.276621242 | −0.496795294 | |
| 6 | −0.213896428 | 1.474611835 | −1.322999207 | −1.492483021 | −0.276621242 | 0.127936272 | |
| 7 | 0.978450096 | −0.885530436 | 1.591737282 | −1.386689545 | 0.829863725 | 0.612899558 | |
| 8 | 1.812375002 | 0.731347696 | 1.323745446 | −0.791321431 | 1.383106208 | 0.861938074 | |
| 9 | −2.371460889 | −0.698563644 | −0.177167065 | 0.234218125 | −1.936348691 | 0.669754604 | |
| 10 | −1.325902143 | −0.668065352 | 1.592629016 | 1.207174065 | −2.489591174 | 0.812121353 | |
| F test | Garson | GBRT | RF | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factors | F Value | Order | Relative Influence (%) | Order | Relative Influence (%) | Order | % IncMSE | Order | IncNodePurity | Order |
| Initial concentration | 38.8 | 2 | 29.96 | 2 | 22.34 | 3 | 12.76 | 3 | 282.69 | 3 |
| Initial pH | 10.5 | 4 | 16.51 | 4 | 14.64 | 4 | 4.54 | 4 | 127.76 | 4 |
| Sonication time | 31.63 | 3 | 22.64 | 3 | 25.01 | 2 | 13.63 | 2 | 411.46 | 2 |
| Dosage | 73.6 | 1 | 30.89 | 1 | 38.00 | 1 | 21.81 | 1 | 551.58 | 1 |
| Isotherms Models | Parameters | Value of Parameters Obtained by the Linear Fitting | Value of Parameters Obtained by the Nonlinear Fitting |
|---|---|---|---|
| Freundlich | Kf (mg/g) | 705.7775 | 698.97 |
| 1/n | 0.0442 | 0.0462 | |
| R2 | 0.8503 | 0.8049 | |
| Langmuir | KL (L/mg) | 0.06211 | 0.05494 |
| qm (mg/g) | 1000.00 | 13,417.92 | |
| R2 | 0.9945 | 0.7642 | |
| RL | 0.01585−0.05093 | ||
| Temkin | KT (L/g) | 1.2 × 108 | |
| BT | 37.395 | ||
| R2 | 0.7491 | ||
| Kinetic Models | Equations | Parameters | Values of Parameters |
|---|---|---|---|
| Pseudo-first-order | k1 (1/min) | 0.2034 | |
| qe (mg/g) | 807.4209 | ||
| R2 | 0.9509 | ||
| Pseudo-second-order | k2 (g/mg/min) | 0.37 × 10−3 | |
| qe (mg/g) | 909.0909 | ||
| R2 | 0.9969 | ||
| Intraparticle diffusion | kd (mg/g/min1/2) | 115.09 | |
| C (mg/g) | 305.97 | ||
| R2 | 0.7926 | ||
| Elovich | α (mg/g/min) | 715.2812 | |
| β (g/mg) | 5.209 × 10−3 | ||
| R2 | 0.9246 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Qi, J.; Hu, J.; Xiang, Y.; Xin, L.; Wei, X. Mesoporous Mn-Doped Fe Nanoparticle-Modified Reduced Graphene Oxide for Ethyl Violet Elimination: Modeling and Optimization Using Artificial Intelligence. Processes 2020, 8, 488. https://doi.org/10.3390/pr8040488
Hou Y, Qi J, Hu J, Xiang Y, Xin L, Wei X. Mesoporous Mn-Doped Fe Nanoparticle-Modified Reduced Graphene Oxide for Ethyl Violet Elimination: Modeling and Optimization Using Artificial Intelligence. Processes. 2020; 8(4):488. https://doi.org/10.3390/pr8040488
Chicago/Turabian StyleHou, Yu, Jimei Qi, Jiwei Hu, Yiqiu Xiang, Ling Xin, and Xionghui Wei. 2020. "Mesoporous Mn-Doped Fe Nanoparticle-Modified Reduced Graphene Oxide for Ethyl Violet Elimination: Modeling and Optimization Using Artificial Intelligence" Processes 8, no. 4: 488. https://doi.org/10.3390/pr8040488
APA StyleHou, Y., Qi, J., Hu, J., Xiang, Y., Xin, L., & Wei, X. (2020). Mesoporous Mn-Doped Fe Nanoparticle-Modified Reduced Graphene Oxide for Ethyl Violet Elimination: Modeling and Optimization Using Artificial Intelligence. Processes, 8(4), 488. https://doi.org/10.3390/pr8040488