Catalytic Aquathermolysis of Boca de Jaruco Heavy Oil with Nickel-Based Oil-Soluble Catalyst
Abstract
:1. Introduction
2. Experimental Procedures
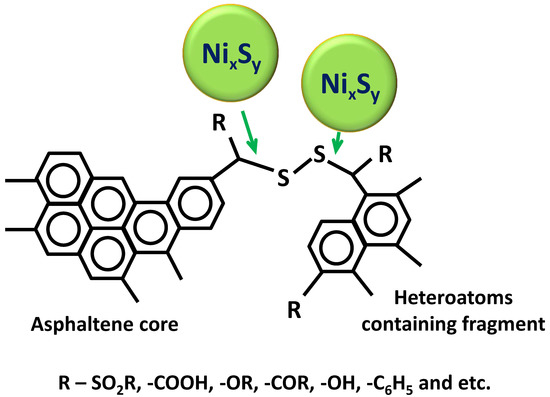
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maity, S.K.; Ancheyta, J.; Marroquín, G. Catalytic aquathermolysis used for viscosity reduction of heavy crude oils: A review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Meyer, R.F.; Attanasi, E.D.; Freeman, P.A. Heavy oil and natural bitumen resources in geological basins of the world: Map showing klemme basin classification of sedimentary provinces reporting heavy oil or natural bitumen. US Geol. Surv. Open-File Rep. 2007, 2007, 1084. [Google Scholar]
- Jiang, S.; Liu, X.; Liu, Y.; Zhong, L. In situ upgrading heavy oil by aquathermolytic treatment under steam injection conditions. In SPE International Symposium on Oilfield Chemistry; Society of Petroleum Engineers: The Woodlands, TX, USA, 2005. [Google Scholar]
- Wu, C.; Su, J.; Zhang, R.; Lei, G.; Cao, Y. The use of a nano-nickel catalyst for upgrading extra-heavy oil by an aquathermolysis treatment under steam injection conditions. Pet. Sci. Technol. 2013, 31, 2211–2218. [Google Scholar] [CrossRef]
- Elahi, S.M.; Scott, C.E.; Chen, Z.; Pereira-Almao, P. In-situ upgrading and enhanced recovery of heavy oil from carbonate reservoirs using nano-catalysts: Upgrading reactions analysis. Fuel 2019, 252, 262–271. [Google Scholar] [CrossRef]
- Kadyrov, R.; Sitnov, S.; Gareev, B.; Batalin, G. Modeling of cobalt-based catalyst use during CSS for low-temperature heavy oil upgrading. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Sitnov, S.A.; Mukhamatdinov, I.I.; Shmeleva, E.I.; Aliev, F.A.; Vakhin, A.V. Influence of nanosized iron oxides (II, III) on conversion of biodegradated oil. Pet. Sci. Technol. 2019, 37, 971–976. [Google Scholar] [CrossRef]
- Cao, Y.B.; Zhang, L.L.; Xia, D.H. Catalytic aquathermolysis of Shengli heavy crude oil with an amphiphilic cobalt catalyst. Pet. Sci. 2016, 13, 463–475. [Google Scholar] [CrossRef] [Green Version]
- Baygildin, E.R.; Sitnov, S.A.; Vakhin, A.V.; Sharifullin, A.V.; Amerkhanov, M.I.; Garifullina, E.I. Aquathermolysis of heavy oil in the presence of bimetallic catalyst that form in-situ from the mixture of oil-soluble iron and cobalt precursors. Georesursy 2019, 21, 62–67. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Sitnov, S.A.; Mukhamatdinov, I.I.; Onishchenko, Y.V.; Feoktistov, D.A. Aquathermolysis of High-Viscosity Oil in the Presence of an Oil-Soluble Iron-Based Catalyst. Chem. Technol. Fuels Oils 2017, 53, 666–674. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Foss, L.E.; Feoktistov, D.A.; Vakhin, A.V.; Petrukhina, N.N.; Romanov, G.V. Transformations of hydrocarbons of Ashal’hinskoe heavy oil under catalytic aquathermolysis conditions. Pet. Chem. 2017, 57, 657–665. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Tang, X.; Sun, Y.; Liu, H.; Deng, L.; Wei, Y. Upgrading heavy and extra-heavy crude oil for transportation by use an iron oil-soluble catalyst. Pet. Sci. Technol. 2017, 35, 1203–1208. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Khaidarova, A.R.; Zaripova, R.D.; Mukhamatdinova, R.E.; Sitnov, S.A.; Vakhin, A.V. The composition and structure of ultra-dispersed mixed oxide (Ii, iii) particles and their influence on in-situ conversion of heavy oil. Catalysts 2020, 10, 114. [Google Scholar] [CrossRef] [Green Version]
- Mukhamatdinov, I.I.; Salih, I.S.S.; Rakhmatullin, I.Z.; Sitnov, S.A.; Laikov, A.V.; Klochkov, V.V.; Vakhin, A.V. Influence of Co-based catalyst on subfractional composition of heavy oil asphaltenes during aquathermolysis. J. Pet. Sci. Eng. 2020, 186, 106721. [Google Scholar] [CrossRef]
- Sitnov, S.A.; Petrovnina, M.S.; Feoktistov, D.A.; Isakov, D.R.; Nurgaliev, D.K.; Amerkhanov, M.I. Intensification of thermal steam methods of production of heavy oil using a catalyst based on cobalt. Neft. Khozyaystvo-Oil Ind. 2016, 11, 106–108. [Google Scholar]
- Yusupova, T.N.; Ganeeva, Y.M.; Romanov, G.V.; Barskaya, E.E.; Morozov, V.I.; Okhotnikova, E.S.; Vakhin, A.V. Change in the structural-group composition of bitumen asphaltenes upon thermal bitumen recovery. Pet. Chem. 2017, 57, 198–202. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Sitnov, S.A.; Mukhamatdinov, I.I.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part I–changes in composition of saturated hydrocarbons. Pet. Sci. Technol. 2018, 36, 1829–1836. [Google Scholar] [CrossRef]
- Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Vakhin, A.V.; Sitnov, S.A.; Akhmadiayrov, A.A.; Varfolomeev, M.A.; Nurgaliev, D.K. Catalytic heavy oil upgrading by steam injection with using of transition metals catalysts. Neft. Khozyaystvo-Oil Ind. 2017, 8, 30–34. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Mukhamatdinov, I.I.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Sitnov, S.A.; Chemodanov, A.E.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part II–changes in composition of aromatic hydrocarbons. Pet. Sci. Technol. 2018, 36, 1850–1856. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Sitnov, S.A.; Mukhamatdinov, I.I.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part III—Changes in composition resins and asphaltenes. Pet. Sci. Technol. 2018, 36, 1857–1863. [Google Scholar] [CrossRef]
- Hamedi Shokrlu, Y.; Babadagli, T. Transportation and interaction of nano and micro size metal particles injected to improve thermal recovery of heavy-oil. In SPE Annual Technical Conference and Exhibition; Society of Petroleum Engineers: Denver, CO, USA, 2011. [Google Scholar]
- Hashemi, R.; Pereira, P. Experimental study of simultaneous athabasca bitumen recovery and upgrading using ultradispersed catalysts injection. In Proceedings of the Society of Petroleum Engineers—Canadian Unconventional Resources Conference, Calgary, AB, Canada, 15–17 November 2011. [Google Scholar]
- Yusuf, A.; Al-Hajri, R.S.; Al-Waheibi, Y.M.; Jibril, B.Y. In-situ upgrading of Omani heavy oil with catalyst and hydrogen donor. J. Anal. Appl. Pyrolysis 2016, 121, 102–112. [Google Scholar] [CrossRef]





| Experimental Conditions | SARA Fraction, wt% | ||||
|---|---|---|---|---|---|
| Saturates | Aromatics | Resins | Asphaltenes | ||
| Initial rock extracts | 18.5 | 29.9 | 29.6 | 22.0 | |
| Initial crude oil | 19.7 | 20.2 | 42.8 | 17.3 | |
| Autoclave experiments | |||||
| Hydrothermal treatment | 250 °C | 24.5 | 36.8 | 21.3 | 17.5 |
| in the presence of catalyst | 200 °C | 22.6 | 25.9 | 32.0 | 19.5 |
| 250 °C | 22.0 | 38.9 | 23.3 | 15.7 | |
| 300 °C | 30.4 | 32.0 | 25.0 | 12.6 | |
| in the absence of catalyst | 200 °C | 21.3 | 26.3 | 32.7 | 19.7 |
| 250 °C | 20.7 | 32.9 | 25.1 | 21.3 | |
| 300 °C | 29.3 | 30.9 | 26.4 | 13.4 | |
| Experimental Conditions | Elemental Composition, wt% | H/Cat | |||||
|---|---|---|---|---|---|---|---|
| C | H | N | S | O | |||
| Initial rock extracts | 78.1 | 10.8 | 0.4 | 4.2 | 6.4 | 1.66 | |
| Initial crude oil | 75.5 | 10.1 | 0.4 | 5.6 | 8.4 | 1.61 | |
| Autoclave experiments | |||||||
| Hydrothermal treatment | 250 | 79.0 | 11.0 | 0.4 | 4.0 | 5.6 | 1.68 |
| in the presence of catalyst | 200 | 79.9 | 10.7 | 0.4 | 3.8 | 5.2 | 1.61 |
| 250 | 80.1 | 11.2 | 0.4 | 3.3 | 4.9 | 1.68 | |
| 300 | 80.4 | 11.2 | 0.4 | 3.4 | 4.6 | 1.68 | |
| in the absence of catalyst | 200 | 80.1 | 10.7 | 0.4 | 3.8 | 5.0 | 1.61 |
| 250 | 81.2 | 10.5 | 0.4 | 3.3 | 4.6 | 1.56 | |
| 300 | 82.1 | 10.5 | 0.4 | 3.0 | 4.0 | 1.53 | |
| Spectral Coefficients | *C1 | *C2 | *C3 | *C4 | *C5 | |
|---|---|---|---|---|---|---|
| Initial rock extracts | 0.34 | 0.11 | 0.56 | 7.91 | 0.21 | |
| Hydrothermal treatment | 0.27 | 0.12 | 0.56 | 8.33 | 0.16 | |
| Hydrothermal treatment in the presence of catalyst | 200 °C | 0.48 | 0.15 | 0.57 | 5.97 | 0.15 |
| 250 °C | 0.40 | 0.15 | 0.57 | 7.17 | 0.14 | |
| 300 °C | 0.36 | 0.15 | 0.56 | 7.20 | 0.13 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakhin, A.V.; Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Sharifullin, A.V.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Catalytic Aquathermolysis of Boca de Jaruco Heavy Oil with Nickel-Based Oil-Soluble Catalyst. Processes 2020, 8, 532. https://doi.org/10.3390/pr8050532
Vakhin AV, Aliev FA, Mukhamatdinov II, Sitnov SA, Sharifullin AV, Kudryashov SI, Afanasiev IS, Petrashov OV, Nurgaliev DK. Catalytic Aquathermolysis of Boca de Jaruco Heavy Oil with Nickel-Based Oil-Soluble Catalyst. Processes. 2020; 8(5):532. https://doi.org/10.3390/pr8050532
Chicago/Turabian StyleVakhin, Alexey V., Firdavs A. Aliev, Irek I. Mukhamatdinov, Sergey A. Sitnov, Andrey V. Sharifullin, Sergey I. Kudryashov, Igor S. Afanasiev, Oleg V. Petrashov, and Danis K. Nurgaliev. 2020. "Catalytic Aquathermolysis of Boca de Jaruco Heavy Oil with Nickel-Based Oil-Soluble Catalyst" Processes 8, no. 5: 532. https://doi.org/10.3390/pr8050532
APA StyleVakhin, A. V., Aliev, F. A., Mukhamatdinov, I. I., Sitnov, S. A., Sharifullin, A. V., Kudryashov, S. I., Afanasiev, I. S., Petrashov, O. V., & Nurgaliev, D. K. (2020). Catalytic Aquathermolysis of Boca de Jaruco Heavy Oil with Nickel-Based Oil-Soluble Catalyst. Processes, 8(5), 532. https://doi.org/10.3390/pr8050532