Andrographis paniculata Extract Relieves Pain and Inflammation in Monosodium Iodoacetate-Induced Osteoarthritis and Acetic Acid-Induced Writhing in Animal Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of APE
2.2. High Performance Liquid Chromatography (HPLC) Analysis of APE
2.3. Animal Treatment
2.4. MIA Injection and Diet Preparation
2.5. Hind Limb Weight-Bearing Measurement
2.6. Serum Measurement of OA Induced Model
2.7. Real-Time Quantitative PCR Analysis of Knee Cartilage Tissue
2.8. Western Blot Analysis for Knee Cartilage Tissue
2.9. Measurement of Acetic Acid-Induced Writhing
2.10. Measurement of NO and Cytotoxicity
2.11. Real-Time Quantitative PCR Analysis of LPS-Stimulated RAW264.7 Cells
2.12. Statistics
3. Results
3.1. HPLC Analysis
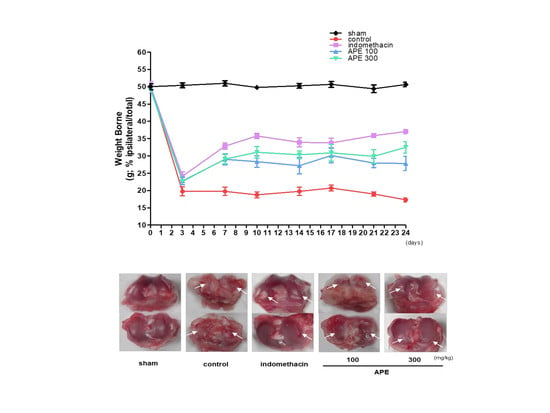
3.2. Effects on Weight-Bearing Distribution in MIA Model
3.3. Preventing Effects on Knee Joint Damage in MIA Model
3.4. Effects on Inflammatory Cytokines in MIA-Induced Rat Model
3.5. Effects on Matrix Metalloproteinases Responses in Knee Joint Cartilage Tissue
3.6. Effect on Acetic Acid-Induced Writhing Responses
3.7. Anti-Inflammatory Effects in LPS-Stimulated RAW264.7 Cells
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Xing, D.; Xu, Y.; Liu, Q.; Ke, Y.; Wang, B.; Li, Z.; Lin, J. Osteoarthritis and all-cause mortality in worldwide populations: Grading the evidence from a meta-analysis. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyreuther, B.; Callizot, N.; Stöhr, T. Research article Antinociceptive efficacy of lacosamide in the monosodium iodoacetate rat model for osteoarthritis pain. Arthritis Res. 2007, 9. [Google Scholar]
- Pharmacy, C.; Pharmacy, F.; Mulyorejo, J.; Unair, C.C. Attenuation of IL-1ß on the use of glucosamine as an adjuvant in meloxicam treatment in rat models with osteoarthritis. Basic Clin. Physiol Pharm. 2020, 1–9. [Google Scholar]
- Perrot, S. Osteoarthritis pain. Best Pract. Res. Clin. Rheumatol. 2015, 29, 90–97. [Google Scholar] [CrossRef]
- Ding, C. Do NSAIDs Affect the Progression of Osteoarthritis? Inflamm. 2002, 26. [Google Scholar]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2014, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Che Ahmad Tantowi, N.A.; Lau, S.F.; Mohamed, S. Ficus deltoidea Prevented Bone Loss in Preclinical Osteoporosis/Osteoarthritis Model by Suppressing Inflammation. Calcif. Tissue Int. 2018, 103, 388–399. [Google Scholar] [CrossRef]
- Maldonado, M.; Nam, J. The Role of Changes in Extracellular Matrix of Cartilage in the Presence of Inflammation on the Pathology of Osteoarthritis. Biomed. Res. Int. 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sebaldt, R.J.; Petrie, A.; Goldsmith, C.H.; Marentette, M.A. Appropriateness of NSAID and Coxib Prescribing for Patients with Osteoarthritis by Primary Care Physicians in Ontario: Results From the CANOAR Study. Am. J. Manag. Care. 2004, 10, 10. [Google Scholar]
- Hungin, A.P.S.; Kean, W.F. Nonsteroidal anti-inflammatory drugs: Overused or underused in osteoarthritis? Am. J. Med. 2001, 110, S8–S11. [Google Scholar] [CrossRef]
- Al-Afify, A.S.A.; El-Akabawy, G.; El-Sherif, N.M.; El-Safty, F.E.N.A.; El-Habiby, M.M. Avocado soybean unsaponifiables ameliorates cartilage and subchondral bone degeneration in mono-iodoacetate-induced knee osteoarthritis in rats. Tissue Cell 2018, 52, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, R.; Reid, A.; McDougall, J.J. Evaluation of the novel avocado/soybean unsaponifiable Arthrocen to alter joint pain and inflammation in a rat model of osteoarthritis. PLoS ONE 2018, 13, e0191906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.; Jung, I.; Hur, J.; Kim, S.H.; Lee, J.H.; Kang, J.Y.; Jung, K.C.; Kim, K.S.; Yoo, M.C.; Park, D.S.; et al. The analgesic and anti-inflammatory effect of WIN-34B, a new herbal formula for osteoarthritis composed of Lonicera japonica Thunb and Anemarrhena asphodeloides BUNGE in vivo. J. Ethnopharmacol. 2010, 131, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Roxas, M.; Jurenka, J. Colds and influenza: A review of diagnosis and conventional, botanical, and nutritional considerations. Altern. Med. Rev. 2007, 12, 25–48. [Google Scholar] [PubMed]
- TAYLOR, W.C. Cytotoxic Diterpenoid Constituents from Andrographis Paniculata Ness. Leaves. ScienceAsia 1992, 18, 187. [Google Scholar]
- Shen, Y.C.; Chen, C.F.; Chiou, W.F. Andrographolide prevents oxygen radical production by human neutrophils: Possible mechanism(s) involved in its anti-inflammatory effect. Br. J. Pharmacol. 2002, 135, 399–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheeja, K.; Shihab, P.K.; Kuttan, G. Antioxidant and anti-inflammatory activities of the plant Andrographis paniculata nees. Immunopharmacol. Immunotoxicol. 2006, 28, 129–140. [Google Scholar] [CrossRef]
- Greco, R.; Siani, F.; Demartini, C.; Zanaboni, A.; Nappi, G.; Davinelli, S.; Scapagnini, G.; Tassorelli, C. Andrographis paniculata shows anti-nociceptive effects in an animal model of sensory hypersensitivity associated with migraine. Funct. Neurol. 2016, 31, 53–60. [Google Scholar] [CrossRef]
- Basak, A.; Cooper, S.; Roberge, A.G.; Banik, U.K.; Chre, M. Inhibition of proprotein convertases-1, -7 and furin by diterpines of Andrographis paniculata and their succinoyl esters. Biochem. J. 1999, 113, 107–113. [Google Scholar] [CrossRef]
- Attla, M.A.M.; Weiss, D.W. Immunology of Spontaneous Mammary Carcinomas in Mice V. Acquired Tumor Resistance and Enhancement in Strain A Mice Infected with Mammary Tumor Virus. Cancer Res. 1966, 26, 1787–1800. [Google Scholar]
- Subramanian, R.; Asmawi, M.Z.; Sadikun, A. In vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol. 2008, 55, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koteswara Rao, Y.; Vimalamma, G.; Venkata Rao, C.; Tzeng, Y.M. Flavonoids and andrographolides from Andrographis paniculata. Phytochemistry 2004, 65, 2317–2321. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.W.; Kuo, Y.H.; Lin, B.I.F. Anti-inflammatory activity of new compounds from Andrographis paniculata by nf-κb transactivation inhibition. J. Agric. Food Chem. 2010, 58, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Wu, R.H.; Logue, M.; Blondel, C.; Lai, L.Y.W.; Stuart, B.; Flower, A.; Fei, Y.T.; Moore, M.; Shepherd, J.; et al. Andrographis paniculata (Chuān Xīn Lián) for symptomatic relief of acute respiratory tract infections in adults and children: A systematic review and meta-analysis. PLoS ONE 2017, 12, 1–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parichatikanond, W.; Suthisisang, C.; Dhepakson, P.; Herunsalee, A. Study of anti-inflammatory activities of the pure compounds from Andrographis paniculata (burm.f.) Nees and their effects on gene expression. Int. Immunopharmacol. 2010, 10, 1361–1373. [Google Scholar] [CrossRef]
- Chandrasekaran, C.V.; Gupta, A.; Agarwal, A. Effect of an extract of Andrographis paniculata leaves on inflammatory and allergic mediators in vitro. J. Ethnopharmacol. 2010, 129, 203–207. [Google Scholar] [CrossRef]
- Lin, H.C.; Li, C.C.; Yang, Y.C.; Chiu, T.H.; Liu, K.L.; Lii, C.K.; Chen, H.W. Andrographis paniculata diterpenoids and ethanolic extract inhibit TNFα-induced ICAM-1 expression in EA. hy926 cells. Phytomedicine 2019, 52, 157–167. [Google Scholar] [CrossRef]
- Hancke, J.L.; Cáceres, D.D.; Burgos, R.A. RESEARCH ARTICLE A double-blind, randomized, placebo-controlled study to assess the efficacy of Andrographis paniculata standardized extract (ParActin®) on pain reduction in subjects with knee osteoarthritis. Phytother. Res. 2019, 1469–1479. [Google Scholar] [CrossRef]
- Chien, T.; Huang, S.K.; Lee, C.; Tsai, P. Antinociceptive and Anti-Inflammatory Effects of Zerumbone against Mono-Iodoacetate-Induced Arthritis. Int. J. Mol. Sci. 2016, 17, 249. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.W.; Lee, H.H.; Kim, J.; Choi, E.O.; Hwang-Bo, H.; Kim, H.J.; Kim, M.Y.; Ahn, K.I.; Kim, G.Y.; Lee, K.W.; et al. Mori Folium water extract alleviates articular cartilage damages and inflammatory responses in monosodium iodoacetate-induced osteoarthritis rats. Mol. Med. Rep. 2017, 16, 3841–3848. [Google Scholar] [CrossRef]
- Monfort, J.; Tardif, G.; Roughley, P.; Reboul, P.; Boileau, C.; Bishop, P.N.; Ophth, F.R.C.; Barcelona, U.A.; De Mar, H.; Mar, P. Identification of opticin, a member of the small leucine-rich repeat proteoglycan family, in human articular tissues: A novel target for MMP-13 in osteoarthritis. Osteoarthr. Cartil. 2008, 16, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 8. [Google Scholar]
- Malemud, C.J. Inhibition of MMPs and ADAM / ADAMTS. Biochem. Pharmacol. 2019, 165, 33–40. [Google Scholar] [CrossRef]
- Hughes, F.M., Jr. Development of a Peptide-Derived Orally-Active Kappa-Opioid Receptor Agonist Targeting Peripheral Pain. Open Med. Chem. J. 2013, 7, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Nareika, A.; He, L.; Game, B.A.; Slate, E.H.; Sanders, J.J.; London, S.D.; Lopes-virella, M.F.; Huang, Y.; He, L.; Game, B.A.; et al. Sodium lactate increases LPS-stimulated MMP and cytokine expression in U937 histiocytes by enhancing AP-1 and NF- kB transcriptional activities. Am. J. Physiol. Endocrinol. Metab. 2020, 29403, 534–542. [Google Scholar]
- Gupta, S.; Mishra, K.P.; Singh, S.B.; Ganju, L. Inhibitory effects of andrographolide on activated macrophages and adjuvant-induced arthritis. Inflammopharmacol. 2018, 26, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tan, J.; Wang, L.; Li, Q. Andrographolide Benefits Rheumatoid Arthritis via Inhibiting MAPK Pathways. Inflammation 2017, 40, 1599–1605. [Google Scholar] [CrossRef]
- Balap, A.; Lohidasan, S.; Sinnathambi, A.; Mahadik, K. Herb-drug interaction of Andrographis paniculata (Nees) extract and andrographolide on pharmacokinetic and pharmacodynamic of naproxen in rats. J. Ethnopharmacol. 2017, 195, 214–221. [Google Scholar] [CrossRef]
- De Sousa Valente, J. The pharmacology of pain associated with the monoiodoacetate model of osteoarthritis. Front. Pharmacol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Mihara, M.; Higo, S.B.S.; Uchiyama, Y.M.S.; Tanabe, K.M.S.; Saito, K. Different effects of high molecular weight sodium hyaluronate and NSAID on the progression of the cartilage degeneration in rabbit OA model. Osteoarthr. Cartil. 2007, 15, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Uryu, N.; Okada, K.; Kawakita, K. Analgesic effects of indirect moxibustion on an experimental rat model of osteoarthritis in the knee. Acupunct. Med. 2007, 25, 175–183. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar] [PubMed]
- Alaaeddine, N.; Di Battista, J.A.; Pelletier, J.P.; Kiansa, K.; Cloutier, J.M.; Martel-Pelletier, J. Inhibition of tumor necrosis factor α-induced prostaglandin E2 production by the antiinflammatory cytokines interleukin-4, interleukin-10, and interleukin-13 in osteoarthritic synovial fibroblasts: Distinct targeting in the signaling pathways. Arthritis Rheum. 1999, 42, 710–718. [Google Scholar] [CrossRef]
- Hart, P.H.; Vitti, G.F.; Burgess, D.R.; Whitty, G.A.; Piccoli, D.S.; Hamilton, J.A. Potential antiinflammatory effects of interleukin 4: Suppression of human monocyte tumor necrosis factor α, interleukin 1, and prostagandin E2. Proc. Natl. Acad. Sci. USA 1989, 86, 3803–3807. [Google Scholar] [CrossRef] [Green Version]
- Hart, P.H.; Ahern, M.J.; Smith, M.D.; Finlay-Jones, J.J. Comparison of the suppressive effects of interleukin-10 and interleukin-4 on synovial fluid macrophages and blood monocytes from patients with inflammatory arthritis. Immunology 1995, 84, 536–542. [Google Scholar]
- Kim, J.; Xu, M.; Xo, R.; Mates, A.; Wilson, G.L.; Iv, A.W.P.; Grishko, V. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes z Department of Cell Biology and Neuroscience, University of South Alabama, College of Medicine. Osteoarthr. Cartil. 2010, 18, 424–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walakovits, L.A.; Moore, V.L.; Bhardwaj, N.; Gallick, G.S.; Lark, M.W. Detection of Stromelysin and Collagenase in Synovial Fluid From Patients with Rheumatoid Arthritis and Posttraumatic Knee Injury. Arthritis Rheum. 1992, 35, 35–42. [Google Scholar] [CrossRef]
- Woo, Y.J.; Joo, Y.B.; Jung, Y.O.; Ju, J.H.; La Cho, M.; Oh, H.J.; Jhun, J.Y.; Park, M.K.; Park, J.S.; Kang, C.M.; et al. Grape seed proanthocyanidin extract ameliorates monosodium iodoacetate induced osteoarthritis. Exp. Mol. Med. 2011, 43, 561–570. [Google Scholar] [CrossRef]
- International, S.; No, A. The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1 (IL-1), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. J. Orthop. Res. 1999, 1, 182–190. [Google Scholar]
- Sutton, S.; Clutterbuck, A.; Harris, P.; Gent, T.; Freeman, S.; Foster, N.; Barrett-jolley, R.; Mobasheri, A. The contribution of the synovium, synovial derived inflammatory cytokines and neuropeptides to the pathogenesis of osteoarthritis. Vet. J. 2009, 179, 10–24. [Google Scholar] [CrossRef]
- Manicourt, D.; Fujimoto, N.; Obata, K.E.N.I.; Thonar, E.J.A. SERUM LEVELS OF COLLAGENASE, STROMELYSIN-1, AND TIMP-1 Age- and Sex-Related Differences in Normal Subjects and Relationship to the Extent of Joint Involvement and Serum Levels of Antigenic Keratan Sulfate in Patients with Osteoarthritis. Arthritis Rheum. 1994, 37, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, A.; Vuolteenaho, K.; Nieminen, R.; Moilanen, T.; Moilanen, E. Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clin. Exp. Rheumatol. 2011, 29, 57–64. [Google Scholar] [PubMed]
- Sacco, H.L. Effect of nimesulide on the serum levels of hyaluronan and stromelysin-1 in patients with osteoarthritis: A pilot study. Int. J. Clinic. Pract. 2004, 13–19. [Google Scholar]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Mono-Iodoacetate-Induced Histologic Changes in Subchondral Bone and Articular Cartilage of Rat Femorotibial Joints: An Animal Model of Osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef]
- Hayer, S.; Bauer, G.; Willburger, M.; Sinn, K.; Alasti, F.; Plasenzotti, R.; Shvets, T.; Niederreiter, B.; Aschauer, C.; Steiner, G.; et al. Cartilage damage and bone erosion are more prominent determinants of functional impairment in longstanding experimental arthritis than synovial inflammation. Dis. Models Mech. 2016, 9. [Google Scholar] [CrossRef] [Green Version]
- Soon-sun An, K.M.D.; Dong-seok Heo, K.M.D. Effects of Kyejiinsam-tang in MIA-Induced Osteoarthritis Rats. J. Korean Med. 2013, 34, 69–85. [Google Scholar]
- Syx, D.; Tran, P.B.; Miller, R.E.; Malfait, A.M. Peripheral Mechanisms Contributing to Osteoarthritis Pain. Curr. Rheumatol. Rep. 2018, 20, 1–11. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Inflammatory Models of Pain and Hyperalgesia. ILAR J. 1999, 40, 111–118. [Google Scholar] [CrossRef]
- Deraedt, R.; Jouquey, S.; Delevallée, F.; Flahaut, M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur. J. Pharmacol. 1980, 61, 17–24. [Google Scholar] [CrossRef]
- Peana, A.T.; Aquila, P.S.D.; Chessa, M.L.; Moretti, M.D.L.; Serra, G.; Pippia, P. (-) -Linalool produces antinociception in two experimental models of pain. Eur. J. Pharm. 2003, 460, 37–41. [Google Scholar] [CrossRef]
- Ko, W.K.; Lee, S.H.; Kim, S.J.; Jo, M.J.; Kumar, H.; Han, I.B.; Sohn, S. Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages. PLoS ONE 2017, 12, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carretta, M.D.; Alarcón, P.; Jara, E.; Solis, L.; Hancke, J.L.; Concha, I.I.; Hidalgo, M.A.; Burgos, R.A. Andrographolide reduces IL-2 production in T-cells by interfering with NFAT and MAPK activation. Eur. J. Pharmacol. 2009, 602, 413–421. [Google Scholar] [CrossRef]
- Low, M.; Khoo, C.S.; Münch, G.; Govindaraghavan, S.; Sucher, N.J. An in vitro study of anti-inflammatory activity of standardised Andrographis paniculata extracts and pure andrographolide. BMC Complement Altern. Med. 2015, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, A.R.; Dave, M. COX-2, NO, and Cartilage Damage and Repair. Curr. Rheumatol. Rep. 2000, 2, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Rifas, L.; Avioli, L. V A Novel T Cell Cytokine Stimulates Interleukin-6 in Human Osteoblastic Cells. J. Bone Min. Res. 1999, 14, 1096–1103. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, S.; Peng, H. The Effect of Hyaluronic Acid on IL-1 b -Induced Chondrocyte Apoptosis in a Rat Model of Osteoarthritis. J. Orthop. Res. 2008, 26, 1643–1648. [Google Scholar] [CrossRef]
- Lopez-Armada, M.J.; Carames, B.; Lires-Dean, M.; Cillero-Pastor, B.; Ruiz-Romero, C.; Galdo, F.; Blanco, F.J. Cytokines, tumor necrosis factor-α and interleukin-1β, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes 1. Osteoarthr. Cartil. 2006, 14, 660–669. [Google Scholar] [CrossRef] [Green Version]
- Suebsasana, S.; Pongnaratorn, P.; Sattayasai, J.; Arkaravichien, T.; Tiamkao, S.; Aromdee, C. Analgesic, antipyretic, anti-inflammatory and toxic effects of andrographolide derivatives in experimental animals. Arch. Pharm. Res. 2009, 32, 1191–1200. [Google Scholar] [CrossRef]
- Chao, W.-W.; Lin, B.-F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, Z.-T.; Ji, L.-L. In Vivo and In Vitro Anti-inflammatory Activities of Neoandrographolide. Am. J. Chin. Med. 2007, 35, 317–328. [Google Scholar] [CrossRef]
- Cheung, H.Y.; Cheung, C.S.; Kong, C.K. Determination of bioactive diterpenoids from Andrographis paniculata by micellar electrokinetic chromatography. J. Chromatogr. A. 2001, 930, 171–176. [Google Scholar] [CrossRef]









| MMP-1 | F | 5′-GCTTAGCCTTCCTTTGCTGTTGC-3′ |
| R | 5′-GACGTCTTCACCCAAGTTGTAGTAG-3′ | |
| MMP-3 | F | 5′-CTGGGCTATCCGAGGTCATG-3′ |
| R | 5′-TGGACGGTTTCAGGGAGGC-3′ | |
| MMP-8 | F | 5′-ACGCTCAAGTCGCTGAACAACC-3′ |
| R | 5′-ATCCAGTAGTCTCCGCTCTTCCAC-3′ | |
| MMP-13 | F | 5′-TGGTCCAGGAGATGAAGACC -3′ |
| R | 5′-GTGCAGACGCCAGAAGAATC-3′ | |
| β-actin | F | 5′-CACCCGCGAGTACAACCTTC-3′ |
| R | 5′-CATCACACCCTGGTGCCTAGG-3′ |
| IL-6 | F | 5′-ACCAGAGGAAATTTTCAATAGG-3′ |
| R | 5′-TGATGCACTTGCAGAAAACA-3′ | |
| COX-2 | F | 5′-AACCGCATTGCCTCTGAAT-3′ |
| R | 5′-CATGTTCCAGGAGGATGGAG-3′ | |
| TNF-α | F | 5′-ATGGGCTTTCCGAATTCAC-3′ |
| R | 5′-GAGGCAACCTGACCACTCTC-3′ | |
| IL-1β | F | 5′-CCTAAAGTATGGGCTGGACTGT-3′ |
| R | 5′-GACTAAGGAGTCCCCTGGAGAT-3′ | |
| iNOS | F | 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ |
| R | 5′-GGCTGTCAGAGCCTCGTGGCTTTGG-3′ | |
| GAPDH | F | 5′-TGGCCTCCAAGGAGTAAGAAAC-3′ |
| R | 5′-CAGCAACTGAGGGCCTCTCT-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Baek, C.Y.; Hwang, J.H.; Kim, M.-Y. Andrographis paniculata Extract Relieves Pain and Inflammation in Monosodium Iodoacetate-Induced Osteoarthritis and Acetic Acid-Induced Writhing in Animal Models. Processes 2020, 8, 873. https://doi.org/10.3390/pr8070873
Lee D, Baek CY, Hwang JH, Kim M-Y. Andrographis paniculata Extract Relieves Pain and Inflammation in Monosodium Iodoacetate-Induced Osteoarthritis and Acetic Acid-Induced Writhing in Animal Models. Processes. 2020; 8(7):873. https://doi.org/10.3390/pr8070873
Chicago/Turabian StyleLee, Donghun, Chae Yun Baek, Ji Hong Hwang, and Mi-Yeon Kim. 2020. "Andrographis paniculata Extract Relieves Pain and Inflammation in Monosodium Iodoacetate-Induced Osteoarthritis and Acetic Acid-Induced Writhing in Animal Models" Processes 8, no. 7: 873. https://doi.org/10.3390/pr8070873
APA StyleLee, D., Baek, C. Y., Hwang, J. H., & Kim, M. -Y. (2020). Andrographis paniculata Extract Relieves Pain and Inflammation in Monosodium Iodoacetate-Induced Osteoarthritis and Acetic Acid-Induced Writhing in Animal Models. Processes, 8(7), 873. https://doi.org/10.3390/pr8070873