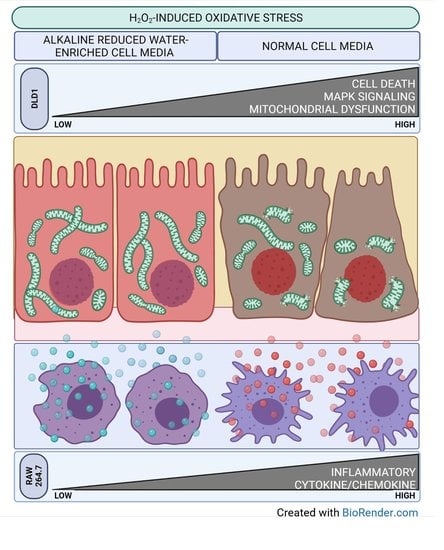
Alkaline Reduced Water Attenuates Oxidative Stress-Induced Mitochondrial Dysfunction and Innate Immune Response Triggered by Intestinal Epithelial Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Media Preparation and Cell Cultures
2.2. Scratch Wound Healing Assay
2.3. Western Blot Analysis
2.4. Catalase Assay
2.5. Multiplex Assay
2.6. TMRE Mitochondrial Membrane Potential Measurement
2.7. Mitochondrial Morphology
2.8. Data Management and Statistical Analysis
3. Results
3.1. ARW Promotes Cytoprotective and Promigratory Effecs in DLD1 Cells under Oxidative Stress Condition
3.2. ARW Attenuated Overactivation of ROS-Regulated Signal Pathways
3.3. ARW Protects Mitochondria from H2O2-Induced Oxidative Damage in DLD1 Cells
3.4. ARW Counteracts Activation of Innate Immune Response Triggered by Intestinal Dysfunction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schoultz, I.; Keita, Å.V. The intestinal barrier and current techniques for the assessment of gut permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef]
- Cavin, J.-B.; Cuddihey, H.; MacNaughton, W.K.; Sharkey, K.A.J. Acute regulation of intestinal ion transport and permeability in response to luminal nutrients: The role of the enteric nervous system. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G254–G264. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H.J. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E.J. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoo, L.; Noti, M.; Krebs, P. Keep calm: The intestinal barrier at the interface of peace and war. Cell Death Dis. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidinger, A.; Kozlov, A.V. Biological activities of reactive oxygen and nitrogen species: Oxidative stress versus signal transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ros-induced ros release: An update and review. Biochim. Biophys. Acta 2006, 1757, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Jones, D. Reactive oxygen species (ros) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Kim, K.-Y.; Park, K.I.; Kim, S.-H.; Park, S.-G.; Yu, S.-N.; Kim, Y.-W.; Kim, D.S.; Chung, K.T.; Ahn, S.-C.J.M.; et al. Dual role of reactive oxygen species in autophagy and apoptosis induced by compound pn in prostate cancer cells. Mol. Cell Toxicol. 2021, 17, 41–50. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and detrimental effects of reactive oxygen species on lifespan: A comprehensive review of comparative and experimental studies. Front. Cell Dev. Biol. 2021, 9, 181. [Google Scholar] [CrossRef]
- Ballway, J.W.; Song, B.-J.J. Translational approaches with antioxidant phytochemicals against alcohol-mediated oxidative stress, gut dysbiosis, intestinal barrier dysfunction and fatty liver disease. Antioxidants 2021, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Deledda, A.; Annunziata, G.; Tenore, G.C.; Palmas, V.; Manzin, A.; Velluzzi, F.J. Diet-derived antioxidants and their role in inflammation, obesity and gut microbiota modulation. Antioxidants 2021, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- He, L.-X.; Wang, J.-B.; Sun, B.; Zhao, J.; Li, L.; Xu, T.; Li, H.; Sun, J.-Q.; Ren, J.; Liu, R.J. Suppression of tnf-α and free radicals reduces systematic inflammatory and metabolic disorders: Radioprotective effects of ginseng oligopeptides on intestinal barrier function and antioxidant defense. J. Nutr. Biochem. 2017, 40, 53–61. [Google Scholar] [CrossRef]
- Hu, Q.; Ren, J.; Li, G.; Wu, J.; Wu, X.; Wang, G.; Gu, G.; Ren, H.; Hong, Z.; Li, J.J. The mitochondrially targeted antioxidant mitoq protects the intestinal barrier by ameliorating mitochondrial DNA damage via the nrf2/are signaling pathway. Cell Death Dis. 2018, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, K.; Chen, X.; Xu, J. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef]
- Liu, W.-C.; Guo, Y.; Zhihui, Z.; Jha, R.; Balasubramanian, B. Algae-derived polysaccharides promote growth performance by improving antioxidant capacity and intestinal barrier function in broiler chickens. Front. Vet. Sci. 2020, 7, 990. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K.J. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.-J.; Kim, J.-R.; Ryang, Y.-S.; Kim, D.-H.; Deung, Y.-K.; Park, S.-K.; Lee, K.-J. A clinical trial of orally administered alkaline reduced water. Biomed. Sci. Lett. 2007, 13, 83–89. [Google Scholar]
- Ignacio, R.M.C.; Joo, K.-B.; Lee, K.-J.J. Clinical effect and mechanism of alkaline reduced water. J. Food Drug Anal. 2012, 20, 394–397. [Google Scholar] [CrossRef]
- Jackson, K.; Dressler, N.; Ben-Shushan, R.S.; Meerson, A.; LeBaron, T.W.; Tamir, S.J. Effects of alkaline-electrolyzed and hydrogen-rich water, in a high-fat-diet nonalcoholic fatty liver disease mouse model. World J. Gastroenterol. 2018, 24, 5095. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-J.; Park, S.-K.; Kim, J.-W.; Kim, G.-Y.; Ryang, Y.-S.; Kim, G.-H.; Cho, H.-C.; Kim, S.-K.; Kim, H.-W.J. Anticancer effect of alkaline reduced water (international conference on mind body science: Physical and physiological approach joint with the eighteenth symposium on life information science). J. Int. Soc. Life Inf. Sci. 2004, 22, 302–305. [Google Scholar]
- Bertoni, M.; Oliveri, F.; Manghetti, M.; Boccolini, E.; Bellomini, M.G.; Blandizzi, C.; Bonino, F.; Del Tacca, M.J. Effects of a bicarbonate-alkaline mineral water on gastric functions and functional dyspepsia: A preclinical and clinical study. Pharmacol. Res. 2002, 46, 525–531. [Google Scholar] [CrossRef]
- Koufman, J.A.; Johnston, N.J. Potential benefits of ph 8.8 alkaline drinking water as an adjunct in the treatment of reflux disease. Ann. Otol. Rhinol. Laryngol. 2012, 121, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Yoon, H.; Kim, H.S.; Choi, Y.J.; Shin, C.M.; Park, Y.S.; Kim, N.; Lee, D.H. Effects of alkaline-reduced drinking water on irritable bowel syndrome with diarrhea: A randomized double-blind, placebo-controlled pilot study. Evid.-Based Complement. Altern. Med. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungewiß, H.; Vielmuth, F.; Suzuki, S.T.; Maiser, A.; Harz, H.; Leonhardt, H.; Kugelmann, D.; Schlegel, N.; Waschke, J. Desmoglein 2 regulates the intestinal epithelial barrier via p38 mitogen-activated protein kinase. Sci. Rep. 2017, 7, 6329. [Google Scholar] [CrossRef]
- Rias, Y.A.; Kurniawan, A.L.; Chang, C.W.; Gordon, C.J.; Tsai, H.T. Synergistic effects of regular walking and alkaline electrolyzed water on decreasing inflammation and oxidative stress, and increasing quality of life in individuals with type 2 diabetes: A community based randomized controlled trial. Antioxidants 2020, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Chang, J.-J.; Chien, C.-T.; Yang, M.-C.; Chien, H.-F.J. Antioxidant sol-gel improves cutaneous wound healing in streptozotocin-induced diabetic rats. Exp. Diabetes Res. 2012, 2012. [Google Scholar] [CrossRef]
- Vergauwen, H.; Tambuyzer, B.; Jennes, K.; Degroote, J.; Wang, W.; De Smet, S.; Michiels, J.; Van Ginneken, C. Trolox and ascorbic acid reduce direct and indirect oxidative stress in the ipec-j2 cells, an in vitro model for the porcine gastrointestinal tract. PLoS ONE 2015, 10, e0120485. [Google Scholar] [CrossRef] [Green Version]
- Hamasaki, T.; Harada, G.; Nakamichi, N.; Kabayama, S.; Teruya, K.; Fugetsu, B.; Gong, W.; Sakata, I.; Shirahata, S.J. Electrochemically reduced water exerts superior reactive oxygen species scavenging activity in ht1080 cells than the equivalent level of hydrogen-dissolved water. PLoS ONE 2017, 12, e0171192. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H. The effect of mapk inhibitors and ros modulators on cell growth and death of h2o2-treated hela cells. Mol. Med. Rep. 2013, 8, 557–564. [Google Scholar] [CrossRef]
- Rehfeldt, S.C.H.; Laufer, S.; Goettert, M.I. A highly selective in vitro jnk3 inhibitor, fmu200, restores mitochondrial membrane potential and reduces oxidative stress and apoptosis in sh-sy5y cells. Int. J. Mol. Sci. 2021, 22, 3701. [Google Scholar] [CrossRef]
- Ren, H.; Meng, Q.; Yepuri, N.; Du, X.; Sarpong, J.O.; Cooney, R.N. Protective effects of glutathione on oxidative injury induced by hydrogen peroxide in intestinal epithelial cells. J. Surg. Res. 2018, 222, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Begum, R.; Kim, C.-S.; Fadriquela, A.; Bajgai, J.; Jing, X.; Kim, D.-H.; Kim, S.-K.; Lee, K.-J. Molecular hydrogen protects against oxidative stress-induced raw 264.7 macrophage cells through the activation of nrf2 and inhibition of mapk signaling pathway. Mol. Cell Toxicol. 2020, 16, 103–118. [Google Scholar] [CrossRef]
- Konyalioglu, S.; Armagan, G.; Yalcin, A.; Atalayin, C.; Dagci, T.J. Effects of resveratrol on hydrogen peroxide-induced oxidative stress in embryonic neural stem cells. Neural Regen. Res. 2013, 8, 485. [Google Scholar]
- Mirzaei, M.; Mirdamadi, S.; Safavi, M.; Zare, D.; Hadizadeh, M.; Asadi, M.M. Synthesis, in vitro and cellular antioxidant activity evaluation of novel peptides derived from saccharomyces cerevisiae protein hydrolysate: Structure–function relationship. Amino Acids 2019, 51, 1167–1175. [Google Scholar] [CrossRef]
- Park, W.H. Anti-apoptotic effect of caspase inhibitors on h2o2-treated hela cells through early suppression of its oxidative stress. Oncol. Rep. 2014, 31, 2413–2421. [Google Scholar] [CrossRef] [Green Version]
- Kaminskyy, V.O.; Zhivotovsky, B.J. Free radicals in cross talk between autophagy and apoptosis. Antioxid. Redox Signal. 2014, 21, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Knaus, U.J. Ros in gastrointestinal inflammation: Rescue or sabotage? Br. J. Pharmacol. 2017, 174, 1704–1718. [Google Scholar] [CrossRef] [Green Version]
- Debattisti, V.; Gerencser, A.A.; Saotome, M.; Das, S.; Hajnóczky, G.J. Ros control mitochondrial motility through p38 and the motor adaptor miro/trak. Cell Rep. 2017, 21, 1667–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Hussien, R.; Brooks, G.A.J. H2o2-induced mitochondrial fragmentation in c2c12 myocytes. Free Radic. Biol. Med. 2010, 49, 1646–1654. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.C.; Chiu, H.W.; Hung, J.C.; Hong, J.R. Beta-nodavirus b2 protein induces hydrogen peroxide production, leading to drp1-recruited mitochondrial fragmentation and cell death via mitochondrial targeting. Apoptosis 2014, 19, 1457–1470. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Aung, L.H.; Prabhakar, B.S.; Li, P. The mitochondrial ubiquitin ligase plays an anti-apoptotic role in cardiomyocytes by regulating mitochondrial fission. J. Cell. Mol. Med. 2016, 20, 2278–2288. [Google Scholar] [CrossRef] [PubMed]
- Willems, P.H.; Rossignol, R.; Dieteren, C.E.; Murphy, M.P.; Koopman, W.J. Redox homeostasis and mitochondrial dynamics. Cell Metab. 2015, 22, 207–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, X.; Huang, S.; Yu, Q.; Yu, H.; Yan, S.S.J. Blockade of drp1 rescues oxidative stress-induced osteoblast dysfunction. Biochem. Biophys. Res. Commun. 2015, 468, 719–725. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Kwon, S.Y.; Woo, S.-Y.; Seo, W.D.; Kim, D.Y.J. Antioxidative effects of chrysoeriol via activation of the nrf2 signaling pathway and modulation of mitochondrial function. Molecules 2021, 26, 313. [Google Scholar] [CrossRef]
- Li, C.-J.; Sun, L.-Y.; Pang, C.-Y.J. Synergistic protection of n-acetylcysteine and ascorbic acid 2-phosphate on human mesenchymal stem cells against mitoptosis, necroptosis and apoptosis. Sci. Rep. 2015, 5, 9819. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Saotome, M.; Nobuhara, M.; Sakamoto, A.; Urushida, T.; Katoh, H.; Satoh, H.; Funaki, M.; Hayashi, H. Roles of mitochondrial fragmentation and reactive oxygen species in mitochondrial dysfunction and myocardial insulin resistance. Exp. Cell Res. 2014, 323, 314–325. [Google Scholar] [CrossRef] [Green Version]
- Quintana-Cabrera, R.; Manjarrés-Raza, I.; Vicente-Gutiérrez, C.; Corrado, M.; Bolaños, J.P.; Scorrano, L. Opa1 relies on cristae preservation and atp synthase to curtail reactive oxygen species accumulation in mitochondria. Redox Biol. 2021, 41, 101944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lee, M.D.; Wilson, C.; McCarron, J.G. Hydrogen peroxide depolarizes mitochondria and inhibits ip3-evoked Ca2+ release in the endothelium of intact arteries. Cell Calcium 2019, 84, 102108. [Google Scholar] [CrossRef]
- Cossu, A.; Posadino, A.M.; Giordo, R.; Emanueli, C.; Sanguinetti, A.M.; Piscopo, A.; Poiana, M.; Capobianco, G.; Piga, A.; Pintus, G.J. Apricot melanoidins prevent oxidative endothelial cell death by counteracting mitochondrial oxidation and membrane depolarization. PLoS ONE 2012, 7, e48817. [Google Scholar] [CrossRef] [Green Version]
- Distelmaier, F.; Visch, H.-J.; Smeitink, J.A.; Mayatepek, E.; Koopman, W.J.; Willems, P.H.J. The antioxidant trolox restores mitochondrial membrane potential and Ca2+-stimulated atp production in human complex i deficiency. J. Mol. Med. 2009, 87, 515–522. [Google Scholar] [CrossRef] [Green Version]
- Marquez, J.; Park, N.; Garcia, M.V.F.; Kim, H.K.; Han, J.J. Hs-1793 protects c2c12 cells from oxidative stress via mitochondrial function regulation. Mol. Cell Toxicol. 2020, 16, 359–365. [Google Scholar] [CrossRef]
- Park, C.; Lee, H.; Park, S.-H.; Hong, S.H.; Song, K.S.; Cha, H.-J.; Kim, G.-Y.; Chang, Y.-C.; Kim, S.; Kim, H.-S.; et al. Indole-6-carboxaldehyde prevents oxidative stress-induced mitochondrial dysfunction, DNA damage and apoptosis in c2c12 skeletal myoblasts by regulating the ros-ampk signaling pathway. Mol. Cell Toxicol. 2020, 16, 455–467. [Google Scholar] [CrossRef]
- Kalathil, S.G.; Thanavala, Y.J. High immunosuppressive burden in cancer patients: A major hurdle for cancer immunotherapy. Cancer Immunol. Immunother. 2016, 65, 813–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.-P.; Zhang, X.; Tan, Q.-L.; Xu, W.-X.; Zhou, C.-Y.; Luo, M.; Li, X.; Huang, R.-Y.; Zeng, X.J. Nf-κb pathways are involved in m1 polarization of raw 264.7 macrophage by polyporus polysaccharide in the tumor microenvironment. PLoS ONE 2017, 12, e0188317. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.-T.; Yan, W.-H.; Cao, Y.; Yan, J.-K.; Cai, W. Neutralization of il-6 and tnf-α ameliorates intestinal permeability in dss-induced colitis. Cytokine 2016, 83, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Ajuebor, M.N.; Das, A.M.; Virág, L.; Flower, R.J.; Szabó, C.; Perretti, M.J. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: Evidence for an inhibitory loop involving endogenous il-10. J. Immunol. 1999, 162, 1685–1691. [Google Scholar]
- Ajuebor, M.N.; Swain, M.G.J. Role of chemokines and chemokine receptors in the gastrointestinal tract. Immunology 2002, 105, 137–143. [Google Scholar] [CrossRef]
- Cassatella, M.A.J. The production of cytokines by polymorphonuclear neutrophils. Immunolol. Today 1995, 16, 21–26. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonio, J.M.; Fadriquela, A.; Jeong, Y.J.; Kim, C.-S.; Kim, S.-K. Alkaline Reduced Water Attenuates Oxidative Stress-Induced Mitochondrial Dysfunction and Innate Immune Response Triggered by Intestinal Epithelial Dysfunction. Processes 2021, 9, 1828. https://doi.org/10.3390/pr9101828
Antonio JM, Fadriquela A, Jeong YJ, Kim C-S, Kim S-K. Alkaline Reduced Water Attenuates Oxidative Stress-Induced Mitochondrial Dysfunction and Innate Immune Response Triggered by Intestinal Epithelial Dysfunction. Processes. 2021; 9(10):1828. https://doi.org/10.3390/pr9101828
Chicago/Turabian StyleAntonio, Jayson M., Ailyn Fadriquela, Yun Ju Jeong, Cheol-Su Kim, and Soo-Ki Kim. 2021. "Alkaline Reduced Water Attenuates Oxidative Stress-Induced Mitochondrial Dysfunction and Innate Immune Response Triggered by Intestinal Epithelial Dysfunction" Processes 9, no. 10: 1828. https://doi.org/10.3390/pr9101828
APA StyleAntonio, J. M., Fadriquela, A., Jeong, Y. J., Kim, C. -S., & Kim, S. -K. (2021). Alkaline Reduced Water Attenuates Oxidative Stress-Induced Mitochondrial Dysfunction and Innate Immune Response Triggered by Intestinal Epithelial Dysfunction. Processes, 9(10), 1828. https://doi.org/10.3390/pr9101828