Single and Binary Adsorption Behaviour and Mechanisms of Cd2+, Cu2+ and Ni2+ onto Modified Biochar in Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Modified Biochar
2.3. Batch Adsorption Experiments
3. Results and Discussion
3.1. Adsorption of Cd2+, Cu2+ and Ni2+ in the Single–Metal System
3.2. Adsorption of Cd2+, Cu2+ and Ni2+ in the Binary–Metal System
3.2.1. Cd2+–Ni2+ System
3.2.2. Cu2+–Ni2+ System
3.2.3. Cu2+–Cd2+ System
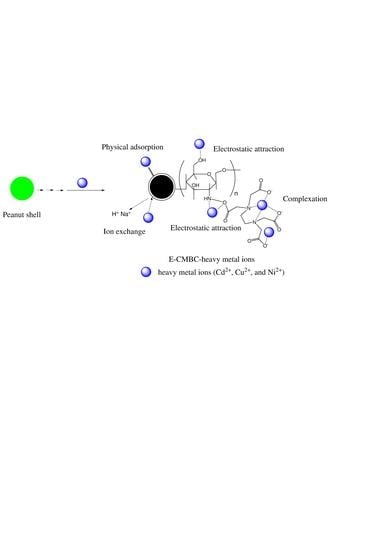
3.3. Analysis of Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, Z.; Wang, Z.; Wu, X.; Li, M.; Liu, X. Competitive adsorption of tylosin, sulfamethoxazole and Cu(II) onnano-hydroxyapatitemodified biochar in water. Chemosphere 2020, 240, 124884. [Google Scholar] [CrossRef]
- Munusamy, T.; Ya-Ting, J.; Jiunn-Fwu, L. Enhanced adsorption of inorganic and organicpollutants by amine modified sodium montmorillonite nanosheets. RSC Adv. 2015, 5, 20583. [Google Scholar] [CrossRef]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines inaquatic environments: A critical review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions fromwastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; et al. Biochar as a tool for effective management of drought and heavymetal toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef] [PubMed]
- Pohl, A. Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Pei, X.; Gan, L.; Tong, Z.; Gao, H.; Meng, S.; Zhang, W.; Wang, P.; Chen, Y. Robust cellulose-based composite adsorption membrane for heavymetal removal. J. Hazard. Mater. 2021, 406, 124746. [Google Scholar] [CrossRef]
- Yu, Y.; Zhong, Y.; Wang, M.; Guo, Z. Electrochemical behavior of aluminium anode in super-gravity field and its application in copper removal from wastewater by electrocoagulation. Chemosphere 2021, 272, 129614. [Google Scholar] [CrossRef]
- Wu, J.; Wang, T.; Wang, J.; Zhang, Y.; Pan, W. A novel modified method for the efficient removal of Pb and Cd from wastewater by biochar: Enhanced the ion exchange and precipitation capacity. Sci. Total Environ. 2021, 754, 142150. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Rafatullah, M. Recent advances in soil microbial fuel cells for soil contaminants remediation. Chemosphere 2021, 272, 129691. [Google Scholar] [CrossRef]
- Won, S.W.; Kotte, P.; Wei, W.; Lim, A.; Yun, Y.S. Biosorbents for recovery of precious metals. Bioresour. Technol. 2014, 160, 203–212. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Yin, D.; Sillanpää, M.E.T. Adsorption of Cd(II) and Pb(II) by a novel EGTA-modified chitosan material: Kinetics and isotherms. J. Colloid Interface Sci. 2013, 409, 174–182. [Google Scholar] [CrossRef]
- Wu, D.; Hu, L.; Wang, Y.; Wei, Q.; Yan, L.; Yan, T.; Li, Y.; Du, B. EDTA modified ß-cyclodextrin/chitosan for rapid removal of Pb(II) and acid red from aqueous solution. J. Colloid Interface Sci. 2018, 523, 56–64. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–222. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Wei, D.; Li, B.; Huang, H.; Lin, L.; Zhang, J.; Yang, Y.; Guo, J.; Tang, L.; Zeng, G.; Zhou, Y. Biochar-based functional materials in the purification of agricultural wastewater: Fabrication, application and future research needs. Chemosphere 2018, 197, 165–180. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Yue, Q.Y. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Chen, S.S.; Tsang DC, W.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef]
- Li, R.; Liang, W.; Huang, H.; Jiang, S.; Guo, D.; Li, M.; Zhang, Z.; Ali, A.; Wang, J.J. Removal of cadmium(II) cations from an aqueous solution with aminothiourea chitosan strengthened magnetic biochar. J. Appl. Polym. Sci. 2018, 135, 46239. [Google Scholar] [CrossRef]
- Lv, D.; Liu, Y.; Zhou, J.; Yang, K.; Lou, Z.; Baig, S.A.; Xu, X. Application of EDTA-functionalized bamboo activated carbon (BAC) for Pb(II) and Cu(II) removal from aqueous solutions. Appl. Surf. Sci. 2018, 428, 648–658. [Google Scholar] [CrossRef]
- Sajjadi, B.; Broome, J.W.; Chen, W.Y.; Mattern, D.L.; Egiebor, N.O.; Hammer, N.; Smith, C.L. Urea functionalization of ultrasound-treated biochar: A feasible strategy for enhancing heavy metal adsorption capacity. Ultrason. Sonochem. 2019, 51, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, R.; Yang, R.; Yang, F.; Chen, J.; Li, Y.; Zhou, R.; Xu, J. Synthesis of amino-functionalized biochar/spinel ferrite magnetic composites for low-cost and efficient elimination of Ni(II) from wastewater. Sci. Total Environ. 2020, 722, 137822. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Lian, F.; Yu, Z.; Zhu, L.; Xing, B.; Qiu, W. Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution. Chem. Eng. J. 2014, 242, 36–42. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, J.; Liu, Y.; Guo, J.; Ren, J.; Zhou, F. Preparation of iminodiacetic acid-modified magnetic biochar by carbonization, magnetization and functional modification for Cd(II) removal in water. Fuel 2018, 233, 469–479. [Google Scholar] [CrossRef]
- Ni, B.J.; Huang, Q.S.; Wang, C.; Ni, T.Y.; Sun, J.; Wei, W. Competitive adsorption of heavy metalsin aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere 2019, 219, 351–357. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef]
- Zheng, L.; Gao, Y.; Du, J.; Zhang, W.; Huang, Y.; Wang, L.; Zhao, Q.; Pan, X. A novel, recyclable magnetic biochar modified by chitosan-EDTA for the effective removal of Pb(II) from aqueous solution. RSC Adv. 2020, 10, 40196. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Wang, X.; Jing, F.; Chang, R.; Chen, J. Oxidative ageing of biochar and hydrochar alleviating competitive sorption of Cd(II) and Cu(II). Sci. Total Environ. 2020, 725, 138419. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.S. Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Li, Y.; Liu, Y.; Chen, Y.; Li, H.; Li, L.; Xu, F.; Jiang, H.; Chen, L. Hydroxyapatite modified sludge-based biochar for the adsorption of Cu2+and Cd2+: Adsorption behavior and mechanisms. Bioresour. Technol. 2021, 321, 124413. [Google Scholar] [CrossRef]
- Qu, J.; Liu, Y.; Cheng, L.; Jiang, Z.; Zhang, G.; Deng, F.; Wang, L.; Han, W.; Zhang, Y. Green synthesis of hydrophilic activated carbon supported sulfide nZVI for enhanced Pb(II) scavenging from water: Characterization, kinetics, isotherms and mechanisms. J. Hazard. Mater. 2021, 403, 123607. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, T.; Ren, H.; Kruse, A.; Cui, R. Polyethylene imine modified hydrochar adsorption for chromium (VI) and nickel (II) removal from aqueous solution. Bioresour. Technol. 2018, 247, 370–379. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R. H2O2 treatment enhanced the heavy metals removal by manure biocharin aqueous solutions. Sci. Total Environ. 2018, 628–629, 1139–1148. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L.; Wang, H.; Yu, H.; Gao, B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. 2013, 20, 358–368. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, H. Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater. Water Res. 2014, 48, 396–405. [Google Scholar] [CrossRef]
- Liu, R.; Lian, R. Non-competitive and competitive adsorption of Cd2+, Ni2+, and Cu2+ by biogenic vaterite. Sci. Total Environ. 2019, 659, 122–130. [Google Scholar] [CrossRef]
- Tan, P.; Hu, Y.; Bi, Q. Competitive adsorption of Cu2+, Cd2+and Ni2+from an aqueous solution on graphene oxide membranes. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 56–64. [Google Scholar] [CrossRef]
- Ahmad, Z.; Gao, B.; Mosa, A.; Yu, H.; Yin, X.; Bashir, A.; Ghoveisi, H.; Wang, S. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod. 2018, 180, 437–449. [Google Scholar] [CrossRef]
- Nightingale, E.R., Jr. Phenomenological theory of ion solvation. Effective radii of hydratedions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Park, J.H.; Ok, Y.S.; Kim, S.H.; Cho, J.S.; Heo, J.S.; Delaune, R.D.; Seo, D.C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, R.; Sun, Y.; Zhou, X.; Baig, S.A.; Xu, X. Multifunctional nanocomposites Fe3O4@SiO2-EDTA for Pb (II) and Cu (II) removal from aqueous solutions. Appl. Surf. Sci. 2016, 369, 267–276. [Google Scholar] [CrossRef]
- Shan, R.; Shi, Y.; Gu, Y.; Wang, Y.; Yuan, H. Single and competitive adsorption affinity of heavy metals toward peanut shell-derived biochar and its mechanisms in aqueous systems. Chin. J. Chem. Eng. 2020, 28, 1375–1383. [Google Scholar] [CrossRef]
- Wu, S.; Wang, F.; Yuan, H.; Zhang, L.; Mao, S.; Liu, X.; Alharbi, N.S.; Rohani, S.; Lu, J. Fabrication of xanthate-modifiedchitosan/poly (N-isopropylacrylamide) composite hydrogel for the selective adsorption of Cu (II), Pb (II)and Ni (II) metal ions. Chem. Eng. Res. Des. 2018, 139, 197–210. [Google Scholar] [CrossRef]





| Metal Ion | Pseudo–First–Order Model | Pseudo–Second–Order Model | Avrami Fractional–Order Model | |||
|---|---|---|---|---|---|---|
| Cd2+ | qe (mg g−1) | 57.0 | qe (mg g−1) | 59.0 | qe (mg g−1) | 59.75 |
| k1 (min−1) | 0.17 | k2 (g mg−1 min−1) | 0.0048 | k3 (min−1) | 0.17 | |
| R2 | 0.81 | R2 | 0.98 | R2 | 0.99 | |
| Cu2+ | qe (mg g−1) | 45.0 | qe (mg g−1) | 46.3 | qe (mg g−1) | 47.64 |
| k1 (min−1) | 0.22 | k2 (g mg−1 min−1) | 0.0089 | k3 (min−1) | 0.36 | |
| R2 | 0.62 | R2 | 0.88 | R2 | 0.95 | |
| Ni2+ | qe (mg g−1) | 38.48 | qe (mg g−1) | 39.84 | qe (mg g−1) | 40.24 |
| k1 (min−1) | 0.17 | k2 (g mg−1 min−1) | 0.0072 | k3 (min−1) | 0.18 | |
| R2 | 0.82 | R2 | 0.97 | R2 | 0.99 | |
| Metal Ion | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (L mg−1) | R2 | KF (L mg−1) | n | R2 | |
| Cd2+ | 61.95 | 3.24 | 0.98 | 36.35 | 0.11 | 0.62 |
| Cu2+ | 48.73 | 1.72 | 0.97 | 27.89 | 0.11 | 0.68 |
| Ni2+ | 40.38 | 6.61 | 0.96 | 26.15 | 0.094 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Gao, Y.; Du, J.; Zhang, W.; Huang, Y.; Zhao, Q.; Duan, L.; Liu, Y.; Naidu, R.; Pan, X. Single and Binary Adsorption Behaviour and Mechanisms of Cd2+, Cu2+ and Ni2+ onto Modified Biochar in Aqueous Solutions. Processes 2021, 9, 1829. https://doi.org/10.3390/pr9101829
Zheng L, Gao Y, Du J, Zhang W, Huang Y, Zhao Q, Duan L, Liu Y, Naidu R, Pan X. Single and Binary Adsorption Behaviour and Mechanisms of Cd2+, Cu2+ and Ni2+ onto Modified Biochar in Aqueous Solutions. Processes. 2021; 9(10):1829. https://doi.org/10.3390/pr9101829
Chicago/Turabian StyleZheng, Liwen, Yongchao Gao, Jianhua Du, Wen Zhang, Yujie Huang, Qingqing Zhao, Luchun Duan, Yanju Liu, Ravi Naidu, and Xiangliang Pan. 2021. "Single and Binary Adsorption Behaviour and Mechanisms of Cd2+, Cu2+ and Ni2+ onto Modified Biochar in Aqueous Solutions" Processes 9, no. 10: 1829. https://doi.org/10.3390/pr9101829
APA StyleZheng, L., Gao, Y., Du, J., Zhang, W., Huang, Y., Zhao, Q., Duan, L., Liu, Y., Naidu, R., & Pan, X. (2021). Single and Binary Adsorption Behaviour and Mechanisms of Cd2+, Cu2+ and Ni2+ onto Modified Biochar in Aqueous Solutions. Processes, 9(10), 1829. https://doi.org/10.3390/pr9101829