The Potential of Fibroblast Transdifferentiation to Neuron Using Hydrogels
Abstract
:1. Introduction
2. Why Fibroblasts
3. How Does Epigenetics Modulate Cellular Reprogramming and Lineage Choice?
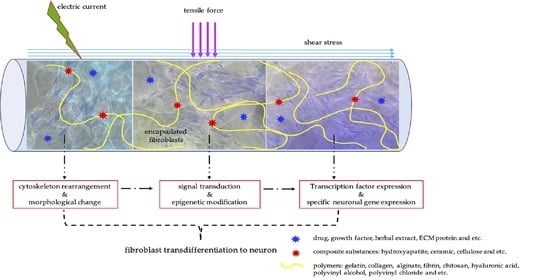
4. Biomimetic Biomaterial for Fibroblast Reprogramming
5. Mechanotransduction and Epigenetics
6. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAF complex | BRM-associated factors |
| Ca2+ | Calcium ion |
| DNA | Deoxyribonucleic acid |
| HA | Hydroxyapatite |
| hiNSCs | Human induced neural stem cells |
| hMSCs | Human mesenchymal stem cells |
| iPSCs | Induced pluripotent stem cells |
| NPCs | Neural progenitor cells |
| NSC | Neural stem cells |
| NSE | Neuron-specific enolase |
| Ptf1a | Pancreas Associated Transcription Factor 1a |
| RET | Rearranged during transfection |
| RNA | Ribonucleic acid |
| TUBB3 | Tubulin Beta 3 Class III |
References
- Singer, N.G.; Caplan, A.I. Mesenchymal stem cells: Mechanisms of inflammation. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 457–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Miguel, M.P.; Fuentes-Julián, S.; Blázquez-Martínez, A.; Pascual, C.Y.; Aller, M.A.; Arias, J.; Arnalich-Montiel, F. Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Curr. Mol. Med. 2012, 12, 574–591. [Google Scholar] [CrossRef]
- Roelofs, R.F.; Fischer, D.F.; Houtman, S.H.; Sluijs, J.A.; Van Haren, W.; Van Leeuwen, F.W.; Hol, E.M. Adult human subventricular, subgranular, and subpial zones contain astrocytes with a specialized intermediate filament cytoskeleton. Glia 2005, 52, 289–300. [Google Scholar] [CrossRef]
- Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Neural stem cell niches and homing: Recruitment and integration into functional tissues. ILAR J. 2009, 51, 3–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, X.; Xu, L.; Li, B.; Sun, Q.; Ji, Q.; Huang, D.; Zhao, L.; Xiao, Y. Chemical conversion of human lung fibroblasts into neuronal cells. Int. J. Mol. Med. 2018, 41, 1463–1468. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Chen, R.; Wu, X.; Zhao, Y.; Fan, Y.; Xiao, Z.; Han, J.; Sun, L.; Wang, X.; Dai, J. Rapid and efficient conversion of human fibroblasts into functional neurons by small molecules. Stem Cell Rep. 2019, 13, 862–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, D.; Liu, X.; Zhang, M.; Zou, M.; Deng, Q.; Sun, D.; Bian, X.; Cai, Y.; Guo, Y.; Liu, S.; et al. Direct reprogramming of fibroblasts into neural stem cells by single non-neural progenitor transcription factor Ptf1a. Nat. Commun. 2018, 9, 2865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Südhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.P.; Yang, N.; Vierbuchen, T.; Ostermeier, A.; Fuentes, D.R.; Yang, T.Q.; Citri, A.; Sebastiano, V.; Marro, S.; Südhof, T.C.; et al. Induction of human neuronal cells by defined transcription factors. Nature 2011, 476, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Wapinski, O.L.; Vierbuchen, T.; Qu, K.; Lee, Q.Y.; Chanda, S.; Fuentes, D.R.; Giresi, P.G.; Ng, Y.H.; Marro, S.; Neff, N.F.; et al. Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell 2013, 155, 621–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.N.; Freitas, B.C.; Qian, H.; Lux, J.; Acab, A.; Trujillo, C.A.; Herai, R.H.; Nguyen Huu, V.A.; Wen, J.H.; Joshi-Barr, S.; et al. Layered hydrogels accelerate iPSC-derived neuronal maturation and reveal migration defects caused by MeCP2 dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, 3185–3190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Rizzi, R.; Di Pasquale, E.; Portararo, P.; Papait, R.; Cattaneo, P.; Latronico, M.V.; Altomare, C.; Sala, L.; Zaza, A.; Hirsch, E.; et al. Post-natal cardiomyocytes can generate iPS cells with an enhanced capacity toward cardiomyogenic re-differentation. Cell Death Differ. 2012, 19, 1162–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewitzky, M.; Yamanaka, S. Reprogramming somatic cells towards pluripotency by defined factors. Curr. Opin. Biotechnol. 2007, 18, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Palmer, T.D.; Rosman, G.J.; Osborne, W.R.; Miller, A.D. Genetically modified skin fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc. Natl. Acad. Sci. USA 1991, 88, 1330–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collas, P.; Taranger, C.K. Epigenetic reprogramming of nuclei using cell extracts. Stem Cell Rev. 2006, 2, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Collas, P.; Taranger, C.K.; Boquest, A.C.; Noer, A.; Dahl, J.A. On the way to reprogramming cells to pluripotency using cell-free extracts. Reprod. Biomed. Online 2006, 12, 762–770. [Google Scholar] [CrossRef]
- Taranger, C.K.; Noer, A.; Sørensen, A.L.; Håkelien, A.M.; Boquest, A.C.; Collas, P. Induction of dedifferentiation, genomewide transcriptional programming, and epigenetic reprogramming by extracts of carcinoma and embryonic stem cells. Mol. Biol. Cell 2005, 16, 5719–5735. [Google Scholar] [CrossRef] [Green Version]
- Neri, T.; Monti, M.; Rebuzzini, P.; Merico, V.; Garagna, S.; Redi, C.A.; Zuccotti, M. Mouse fibroblasts are reprogrammed to Oct-4 and Rex-1 gene expression and alkaline phosphatase activity by embryonic stem cell extracts. Cloning Stem Cells 2007, 9, 394–406. [Google Scholar] [CrossRef]
- Xu, Y.N.; Guan, N.; Wang, Z.D.; Shan, Z.Y.; Shen, J.L.; Zhang, Q.H.; Jin, L.H.; Lei, L. ES cell extract-induced expression of pluripotent factors in somatic cells. Anat. Rec. 2009, 292, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, N.; Li, D.; Li, X.; Jin, L.; Lei, L. Induction of epigenetic reprogramming in fibroblast by extracts of carcinoma. Afr. J. Biotechnol. 2012, 11, 2855–2861. [Google Scholar]
- Lindvall, O.; Kokaia, Z.; Martinez-Serrano, A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat. Med. 2004, 10, S42–S50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sugano, E.; Isago, H.; Hiroi, T.; Tamai, M.; Tomita, H. Differentiation of neuronal cells from NIH/3T3 fibroblasts under defined conditions. Dev. Growth Differ. 2011, 53, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Xu, Z.; Luo, W.; Yan, Y.; Sun, H.; Chen, S. Direct induction of functional neurons from adult human retina derived fibroblast-like cells. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1360. [Google Scholar]
- Hao, L.; Xu, Z.; Sun, H.; Luo, W.; Yan, Y.; Wang, J.; Guo, J.; Liu, Y.; Chen, S. Direct induction of functional neuronal cells from fibroblast-like cells derived from adult human retina. Stem Cell Res. 2017, 23, 61–72. [Google Scholar] [CrossRef]
- Zhang, X.M.; Li, Q.M.; Su, D.J.; Wang, N.; Shan, Z.Y.; Jin, L.H.; Lei, L. RA induces the neural-like cells generated from epigenetic modified NIH/3T3 cells. Mol. Biol. Rep. 2010, 37, 1197–1202. [Google Scholar] [CrossRef]
- Glaser, T.; Brüstle, O. Retinoic acid induction of ES-cell-derived neurons: The radial glia connection. Trends Neurosci. 2005, 28, 397–400. [Google Scholar] [CrossRef]
- Park, S.W.; Huq, M.D.; Loh, H.H.; Wei, L.N. Retinoic acid-induced chromatin remodeling of mouse kappa opioid receptor gene. J. Neurosci. 2005, 25, 3350–3357. [Google Scholar] [CrossRef] [Green Version]
- Angrisano, T.; Sacchetti, S.; Natale, F.; Cerrato, A.; Pero, R.; Keller, S.; Peluso, S.; Perillo, B.; Avvedimento, V.E.; Fusco, A.; et al. Chromatin and DNA methylation dynamics during retinoic acid-induced RET gene transcriptional activation in neuroblastoma cells. Nucleic Acids Res. 2011, 39, 1993–2006. [Google Scholar] [CrossRef] [Green Version]
- Avaliani, N.; Pfisterer, U.; Heuer, A.; Parmar, M.; Kokaia, M.; Andersson, M. Directly converted human fibroblasts mature to neurons and show long-term survival in adult rodent hippocampus. Stem Cells Int. 2017. [Google Scholar] [CrossRef]
- Kantawong, F.; Saisuwan, C.; Soeratanapant, P.; Wanachantararak, P.; Nan, J.; Wu, J.; Chang, Y. Gynura divaricata water extract presented the possibility to enhance neuronal regeneration. Evid. Based Complement. Altern. Med. 2021, 2021. [Google Scholar] [CrossRef]
- Imhof, A. Epigenetic regulators and histone modification. Brief Funct. Genom. Proteomic 2006, 5, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sandoval, J.; Doh, S.T.; Cai, L.; López-Rodas, G.; Casaccia, P. Epigenetic modifiers are necessary but not sufficient for reprogramming non-myelinating cells into myelin gene-expressing cells. PLoS ONE 2010, 5, e13023. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Graumann, J.; Wu, G.; Araúzo-Bravo, M.J.; Han, D.W.; Greber, B.; Gentile, L.; Mann, M.; Schöler, H.R. Chromatin-remodeling components of the BAF complex facilitate reprogramming. Cell 2010, 141, 943–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koche, R.P.; Smith, Z.D.; Adli, M.; Gu, H.; Ku, M.; Gnirke, A.; Bernstein, B.E.; Meissner, A. Reprogramming factor expression initiates widespread targeted chromatin remodeling. Cell Stem Cell 2011, 8, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Moriguchi, H. Chromatin remodeling system, cancer stem-like attractors, and cellular reprogramming. Cell Mol. Life Sci. 2011, 68, 3557–3571. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, T.; Paro, R. Epigenetic reprogramming during tissue regeneration. FEBS Lett. 2011, 585, 1617–1624. [Google Scholar] [CrossRef]
- Jopling, C.; Boue, S.; Izpisua Belmonte, J.C. Dedifferentiation, transdifferentiation and reprogramming: Three routes to regeneration. Nat. Rev. Mol. Cell Biol. 2011, 12, 79–89. [Google Scholar] [CrossRef]
- Silva, J.; Nichols, J.; Theunissen, T.W.; Guo, G.; van Oosten, A.L.; Barrandon, O.; Wray, J.; Yamanaka, S.; Chambers, I.; Smith, A. Nanog is the gateway to the pluripotent ground state. Cell 2009, 138, 722–737. [Google Scholar] [CrossRef] [Green Version]
- McMurray, R.J.; Gadegaard, N.; Tsimbouri, P.M.; Burgess, K.V.; McNamara, L.E.; Tare, R.; Murawski, K.; Kingham, E.; Oreffo, R.O.; Dalby, M.J. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat. Mater. 2011, 10, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Filipp, F. A network of epigenomic and transcriptional cooperation encompassing an epigenomic master regulator in cancer. NPJ Syst. Biol. Appl. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.J.; Zahir, T.; Phillips, S.R.; Shah, D.S.; Athey, D.; Lakey, J.H.; Shoichet, M.S.; Przyborski, S.A. Neural differentiation regulated by biomimetic surfaces presenting motifs of extracellular matrix proteins. J. Biomed. Mater. Res. A 2010, 93, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.; Khoneisser, J.; Huang, P.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeki, K.; Hiramatsu, H.; Hori, A.; Hirai, Y.; Yamada, M.; Utoh, R.; Seki, M. Sacrificial alginate-assisted microfluidic engineering of cell-supportive protein microfibers for hydrogel-based cell encapsulation. ACS Omega 2020, 5, 21641–21650. [Google Scholar] [CrossRef]
- Pelham, R.J.; Wang, Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665. [Google Scholar] [CrossRef] [Green Version]
- Ueki, A.; Kidoaki, S. Manipulation of cell mechanotaxis by designing curvature of the elasticity boundary on hydrogel matrix. Biomaterials 2015, 41, 45–52. [Google Scholar] [CrossRef]
- Kantawong, F.; Kuboki, T.; Kidoaki, S. Redox gene expression of adipose-derived stem cells in response to soft hydrogel. Turk. J. Biol. 2015, 39, 682–691. [Google Scholar] [CrossRef]
- Fisher, O.Z.; Khademhosseini, A.; Langer, R.; Peppas, N.A. Bioinspired materials for controlling stem cell fate. Acc. Chem. Res. 2010, 43, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Wylie, R.G.; Ahsan, S.; Aizawa, Y.; Maxwell, K.L.; Morshead, C.M.; Shoichet, M.S. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat. Mater. 2011, 10, 799–806. [Google Scholar] [CrossRef]
- Distantina, S.; Rochmadi, R.; Fahrurrozi, M.; Wiratni, W. Preparation and Characterization of Glutaraldehyde—Crosslinked Kappa Carrageenan Hydrogel. Eng. J. 2013, 17, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Udomsom, S.; Kantawong, F. Fabrication of blended gelatin–polyvinyl alcohol–chitosan scaffold for wound regeneration. CMU J. Nat. Sci. 2020, 19, 920. [Google Scholar] [CrossRef]
- Kantawong, F.; Tanum, J.; Wattanutchariya, W.; Sooksaen, P. Variation of hydroxyapatite content in soft gelatin affects mesenchymal stem cell differentiation. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, T.; Li, Y. 3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering. Polymers 2020, 12, 1637. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heino, J.; Kapyla, J. Cellular receptors of extracellular matrix molecules. Curr. Pharm. Des. 2009, 15, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Arha, M.; Choudhary, S.; Ashton, R.S.; Bhatia, S.R.; Schaffer, D.V.; Kane, R.S. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 2009, 30, 4695–4699. [Google Scholar] [CrossRef] [Green Version]
- Kaklamani, G.; Kazaryan, D.; Bowen, J.; Iacovella, F.; Anastasiadis, S.; Deligeorgis, G. On the electrical conductivity of alginate hydrogels. Regen. Biomater. 2018, 5, 293–301. [Google Scholar] [CrossRef]
- Aadil, K.; Nathani, A.; Sharma, C.; Lenka, N.; Gupta, P. Fabrication of biocompatible alginate-poly(vinyl alcohol) nanofibers scaffolds for tissue engineering applications. Mater. Technol. 2018, 33, 507–512. [Google Scholar] [CrossRef]
- Patel, B.; McNamara, M.; Pesquera-Colom, L.; Kozik, E.; Okuzonu, J.; Hashemi, N.; Sakaguchi, D. Recovery of encapsulated adult neural progenitor cells from microfluidic-spun hydrogel fibers enhances proliferation and neuronal differentiation. ACS Omega 2020, 5, 7910–7918. [Google Scholar] [CrossRef]
- Matos, M.; Cicerone, M. Alternating Current Electric Field Effects on Neural Stem Cell Viability and Differentiation. Biotechnol. Prog. 2010, 26, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, K.; Ho, C.; Kao, C.; Ng, H.; Shie, M. Cyclic tensile stimulation enrichment of Schwann cell-laden auxetic hydrogel scaffolds towards peripheral nerve tissue engineering. Mater. Des. 2020, 195. [Google Scholar] [CrossRef]
- Kamoun, E.; Chen, X.; Eldin, M.; Kenawy, E. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Muduli, S.; Chen, L.; Li, M.; Heish, Z.; Liu, C.; Kumar, S.; Alarfaj, A.; Munusamy, M.; Benelli, G.; Murugan, K.; et al. Stem cell culture on polyvinyl alcohol hydrogels having different elasticity and immobilized with ECM-derived oligopeptides. J. Polym. Eng. 2017, 37, 647–660. [Google Scholar] [CrossRef]
- Leipzig, N.D.; Shoichet, M.S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 2009, 30, 6867–6878. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Huang, L. Feasibility of chitosan-alginate (Chi-Alg) hydrogel used as scaffold for neural tissue engineering: A pilot study in vitro. Biotechnol. Biotechnol. Equip. 2017, 31, 766–773. [Google Scholar] [CrossRef] [Green Version]
- Kippert, A.; Fitzner, D.; Helenius, J.; Simons, M. Actomyosin contractility controls cell surface area of oligodendrocytes. BMC Cell Biol. 2009, 10, 71. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Huang, A.; Zhong, Y.; Huang, L.; Yang, J.; Zhou, C.; Zhou, L.; Zhang, Y.; Fu, G. Laminin-modified gellan gum hydrogels loaded with the nerve growth factor to enhance the proliferation and differentiation of neuronal stem cells. RSC Adv. 2020, 10, 17114–17122. [Google Scholar] [CrossRef]
- Shafrir, Y.; Forgacs, G. Mechanotransduction through the cytoskeleton. Am. J. Physiol. Cell Physiol. 2002, 282, C479–C486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantawong, F.; Burgess, K.E.; Jayawardena, K.; Hart, A.; Burchmore, R.J.; Gadegaard, N.; Oreffo, R.O.; Dalby, M.J. Whole proteome analysis of osteoprogenitor differentiation induced by disordered nanotopography and mediated by ERK signalling. Biomaterials 2009, 30, 4723–4731. [Google Scholar] [CrossRef] [PubMed]
- Biggs, M.J.; Richards, R.G.; Gadegaard, N.; Wilkinson, C.D.; Oreffo, R.O.; Dalby, M.J. The use of nanoscale topography to modulate the dynamics of adhesion formation in primary osteoblasts and ERK/MAPK signalling in STRO-1+ enriched skeletal stem cells. Biomaterials 2009, 30, 5094–5103. [Google Scholar] [CrossRef] [PubMed]
- McNamara, L.E.; McMurray, R.J.; Biggs, M.J.; Kantawong, F.; Oreffo, R.O.; Dalby, M.J. Nanotopographical control of stem cell differentiation. J. Tissue Eng. 2010, 2010, 120623. [Google Scholar] [CrossRef]
- Ingber, D.E.; Tensegrity, I. Cell structure and hierarchical systems biology. J. Cell Sci. 2003, 116, 1157–1173. [Google Scholar] [CrossRef] [Green Version]
- Ingber, D.E. Tensegrity II. How structural networks influence cellular information processing networks. J. Cell Sci. 2003, 116, 1397–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingber, D.E. Tensegrity and mechanotransduction. J. Bodyw. Mov. Ther. 2008, 12, 198–200. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Herzyk, P.; Sutherland, D.; Agheli, H.; Wilkinson, C.D.; Curtis, A.S. Nanomechanotransduction and interphase nuclear organization influence on genomic control. J. Cell Biochem. 2007, 102, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Biggs, M.J.; Gadegaard, N.; Kalna, G.; Wilkinson, C.D.; Curtis, A.S. Nanotopographical stimulation of mechanotransduction and changes in interphase centromere positioning. J. Cell. Biochem. 2007, 100, 326–338. [Google Scholar] [CrossRef]
- Evans, N.D.; Minelli, C.; Gentleman, E.; LaPointe, V.; Patankar, S.N.; Kallivretaki, M.; Chen, X.; Roberts, C.J.; Stevens, M.M. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur. Cell Mater. 2009, 18, 1–13. [Google Scholar] [CrossRef]
- Jeon, K.J.; Park, S.H.; Shin, J.W.; Kang, Y.G.; Hyun, J.S.; Oh, M.J.; Kim, S.Y.; Shin, J.-W. Combined effects of flow-induced shear stress and micropatterned surface morphology on neuronal differentiation of human mesenchymal stem cells. J. Biosci. Bioeng. 2014, 117, 242–247. [Google Scholar] [CrossRef]
- Kaneko, N.; Sawamoto, K. Go with the Flow: Cerebrospinal fluid flow regulates neural stem cell proliferation. Cell Stem Cell 2018, 22, 783–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, C.L. Chromatin remodeling enzymes: Taming the machines. Third in review series on chromatin dynamics. EMBO Rep. 2002, 3, 319–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.P.; Chase, K.A. Increasing neuronal ’stemness’: Chromatin relaxation and the expression of reprogramming genes in post-mitotic neurons. Med. Hypotheses 2012, 78, 553–554. [Google Scholar] [CrossRef]
- Brosig, M. Mechanotransduction in Fibroblasts. Ph.D. Thesis, University of Basel, Basel, Switzerland, 2011. [Google Scholar]
- Guo, J.; Li, Z.C.; Feng, Y.H. Expression and activation of the reprogramming transcription factors. Biochem. Biophys. Res. Commun. 2009, 390, 1081–1086. [Google Scholar] [CrossRef]
- Kantawong, F.; Saksiriwisitkul, C.; Riyapa, C.; Limpakdee, S.; Wanachantararak, P.; Kuboki, T. Reprogramming of mouse fibroblasts into neural lineage cells using biomaterials. Bioimpacts 2018, 8, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Tsonis, P.A.; Madhavan, M.; Tancous, E.E.; Del Rio-Tsonis, K. A newt’s eye view of lens regeneration. Int. J. Dev. Biol. 2004, 48, 975–980. [Google Scholar] [CrossRef]
- Yim, E.K.; Pang, S.W.; Leong, K.W. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp. Cell Res. 2007, 313, 1820–1829. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kantawong, F. The Potential of Fibroblast Transdifferentiation to Neuron Using Hydrogels. Processes 2021, 9, 632. https://doi.org/10.3390/pr9040632
Kantawong F. The Potential of Fibroblast Transdifferentiation to Neuron Using Hydrogels. Processes. 2021; 9(4):632. https://doi.org/10.3390/pr9040632
Chicago/Turabian StyleKantawong, Fahsai. 2021. "The Potential of Fibroblast Transdifferentiation to Neuron Using Hydrogels" Processes 9, no. 4: 632. https://doi.org/10.3390/pr9040632
APA StyleKantawong, F. (2021). The Potential of Fibroblast Transdifferentiation to Neuron Using Hydrogels. Processes, 9(4), 632. https://doi.org/10.3390/pr9040632