Development of a Quantitative Chromatographic Fingerprint Analysis Method for Sugar Components of Xiaochaihu Capsules Based on Quality by Design Concept
Abstract
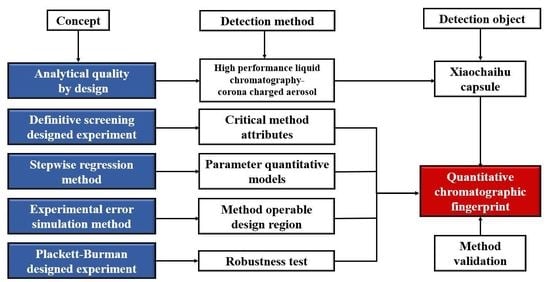
:1. Introduction
2. Materials and Reagents
3. Methods
3.1. Sample Preparation
3.1.1. Preparation of the Chemical Reference Solution
3.1.2. Preparation of the Sample Solution
3.2. HPLC Analysis
3.3. Experimental Design
3.3.1. DSD Experiment
3.3.2. Data Processing and Model Validation
3.3.3. Plackett–Burman Designed Experiment
3.4. LC-Q-TOF-Ms Analysis
3.5. Method Validation
4. Results
4.1. Identification of CMAs
4.2. Influence of Method Parameters
4.3. MODR and Validation
4.4. Plackett–Burman Designed Experiment Result
4.5. LC-Q-TOF-MS Analysis
4.6. Method Validation
4.6.1. Fingerprint Method Validation
4.6.2. Application of Fingerprinting
4.6.3. Content Determination Method Validation
4.6.4. Applications of Content Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xingchu, G.; Ying, Z.; Huali, H.; Teng, C.; Jianyang, P.; Xiaoyu, W.; Haibin, Q. Development of an Analytical Method by Defining a Design Space: A Case Study of Saponin Determination for Panax Notoginseng Extracts. Anal. Methods 2016, 8, 2282–2289. [Google Scholar] [CrossRef]
- Kyoungmin, L.; Wokchul, Y.; Jin Hyun, J. Analytical Method Development for 19 Alkyl Halides as Potential Genotoxic Impurities by Analytical Quality by Design. Molecules 2022, 27, 4437. [Google Scholar] [CrossRef]
- Dispas, A.; Avohou, H.T.; Lebrun, P.; Hubert, P.; Hubert, C. ‘Quality by Design’ Approach for the Analysis of Impurities in Pharmaceutical Drug Products and Drug Substances. TrAC, Trends Anal. Chem. 2018, 101, 24–33. [Google Scholar] [CrossRef]
- Taevernier, L.; Wynendaele, E.; D Hondt, M.; De Spiegeleer, B. Analytical Quality-by-Design Approach for Sample Treatment of BSA-containing Solutions. J. Pharm. Anal. 2015, 5, 27–32. [Google Scholar] [CrossRef]
- Jayagopal, B.; Murugesh, S. QbD-mediated RP-UPLC Method Development Invoking an FMEA-based Risk Assessment to Estimate Nintedanib Degradation Products and Their Pathways. Arabian J. Chem. 2020, 13, 7087–7103. [Google Scholar] [CrossRef]
- Shangxin, G.; Jing, L.; Bo, L.; Baixiu, Z.; Xingchu, G.; Xiaohui, F. Continuous Flow Synthesis of N-Doped Carbon Quantum Dots for Total Phenol Content Detection. Chemosensors 2022, 10, 334. [Google Scholar] [CrossRef]
- Jingyuan, S.; Wen, C.; Haibin, Q.; Jianyang, P.; Xingchu, G. A Novel Quality by Design Approach for Developing an HPLC Method to Analyze Herbal Extracts: A Case Study of Sugar Content Analysis. PLoS ONE 2018, 13, e0198515. [Google Scholar] [CrossRef] [Green Version]
- Musters, J.; Van Den Bos, L.; Kellenbach, E. Applying QbD Principles to Develop a Generic UHPLC Method Which Facilitates Continual Improvement and Innovation Throughout the Product Lifecycle for a Commercial API. Org. Process Res. Dev. 2013, 17, 87–96. [Google Scholar] [CrossRef]
- Vogt, F.G.; Kord, A.S. Development of Quality-by-Design Analytical Methods. J. Pharm. Sci. 2011, 100, 797–812. [Google Scholar] [CrossRef]
- Rozet, E.; Lebrun, P.; Debrus, B.; Boulanger, B.; Hubert, P. Design Spaces for Analytical Methods. TrAC Trends Anal. Chem. 2013, 42, 157–167. [Google Scholar] [CrossRef]
- Raman, N.; Mallu, U.R.; Bapatu, H.R. Analytical Quality by Design Approach to Test Method Development and Validation in Drug Substance Manufacturing. J. Chem. 2015, 2015, 435129. [Google Scholar] [CrossRef]
- Peraman, R.; Bhadraya, K.; Reddy, Y.P. Analytical Quality by Design: A Tool for Regulatory Flexibility and Robust Analytics. Int. J. Anal. Chem. 2015, 2015, 868727. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Deshpande, M.; Zaheer, Z.; Shinde, D.B.; Arote, R. Quality by Design Approach: Regulatory Need. Arabian J. Chem. 2017, 10, S3412–S3425. [Google Scholar] [CrossRef] [Green Version]
- Bastogne, T.; Caputo, F.; Prina-Mello, A.; Borgos, S.; Barberi-Heyob, M. A State of the Art in Analytical Quality-by-Design and Perspectives in Characterization of Nano-Enabled Medicinal Products. J. Pharm. Biomed. Anal. 2022, 219, 114911. [Google Scholar] [CrossRef]
- Volta e Sousa, L.; Goncalves, R.; Menezes, J.C.; Ramos, A. Analytical Method Lifecycle Management in Pharmaceutical Industry: A Review. Aaps Pharmscitech 2021, 22, 128. [Google Scholar] [CrossRef]
- Rathore, A.S. Roadmap for Implementation of Quality by Design (QbD) for Biotechnology Products. Trends Biotechnol. 2009, 27, 546–553. [Google Scholar] [CrossRef]
- Shukun, Z.; Naiqiang, C.; Yuzhen, Z.; Jiangong, H.; Junhong, L.; Dihua, L.; Lihua, C. Modified Xiaochaihu Decoction Promotes Collagen Degradation and Inhibits Pancreatic Fibrosis in Chronic Pancreatitis Rats. Chin. J. Integr. Med. 2020, 26, 599–603. [Google Scholar] [CrossRef]
- Kaibin, S.; Xinyu, Z.; Jing, L.; Rong, S. Network Pharmacological Analysis and Mechanism Prediction of Xiaochaihu Decoction in Treatment of COVID-19 with Syndrome of Pathogenic Heat Lingering in Lung and Obstructive Cardinalate. Zhongcaoyao 2020, 51, 1750–1760. [Google Scholar] [CrossRef]
- Shanyong, W.; Ninghua, J.; Yanbo, S.; Xibin, Z.; Zhili, G.; Jintao, L.; Yanyan, H. Effects of Xiaochaihu Decoction Combined with Irbesartan on Intestinal Flora and Lipid Metabolism in Patients with Hypertension. Zhonghua Zhongyiyao Xuekan 2022, 40, 169–172. [Google Scholar] [CrossRef]
- Commission, C.P. Pharmacopoeia of the People’s Republic of China: Part I; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Yujiao, H.; Fen, X.; Shijun, Z. Difference of Chemical Compositions in Fu Zheng Fang with Different Dosage Forms Based on HPLC-Q-Exactive Orbitrap/MS Combined with Multivariate Statistical Analysis. Curr. Pharm. Anal. 2021, 17, 710–722. [Google Scholar] [CrossRef]
- Yanfang, Y.; Guijun, Z.; Qiyu, S.; Liang, L.; Hui, P.; Jingjuan, W.; Li, X. Simultaneous Determination of 8 Compounds in Gancao-Ganjiang-Tang by HPLC-DAD and Analysis of the Relations between Compatibility, Dosage, and Contents of Medicines. J. Evid. -Based Complement. Altern. Med. 2017, 2017, 4703632. [Google Scholar] [CrossRef] [Green Version]
- Xiaohui, F.; Zhengliang, Y.; Yiyu, C. A Computational Method Based on Information Fusion for Evaluating the Similarity of Multiple Chromatographic Fingerprints of TCM. Gaodeng Xuexiao Huaxue Xuebao 2006, 27, 26–29. [Google Scholar]
- Qinglian, Y.; Tao, Q. Research of Digital Based on Network Model in the Fingerprint of Traditional Chinese Medicines (TCM). In Proceedings of the 3rd International Conference on Intelligent Computing and Cognitive Informatics (ICICCI), Mexico City, Mexico, 19–21 December 2018; p. 01005. [Google Scholar]
- Xiaoyuan, L.; Wenwen, J.; Me, S.; Yue, S.; Hongming, L.; Lei, N.; Hengchang, Z. Quality Evaluation of Traditional Chinese Medicines Based on Fingerprinting. J. Sep. Sci. 2020, 43, 6–17. [Google Scholar] [CrossRef]
- Xiangqin, W.; Fei, S.; Wei, Y.; Le, C.; Shumei, W.; Shengwang, L. Fingerprinting of Mineral Medicine Natrii Sulfas by Fourier Transform Infrared Spectroscopy. Spectroscopy 2021, 36, 38–43. [Google Scholar]
- Xue, Z.; Hongwei, W.; Lina, L.; Shihan, T.; Huihui, L.; Hongjun, Y. Establishment and Application of Quality Evaluation Method for Xiaochaihu Granules Based on Calibrator Samples. Zhongguo Zhongyao Zazhi 2022, 47, 85–94. [Google Scholar] [CrossRef]
- Aoxue, L.; Tongtong, X.; Yu, Y.; Qingyu, W.; Dandan, Z.; Yiwei, S. Study on Determination of Seven Components in Xiaochaihu Granules by QAMS. Yaowu Pingjia Yanjiu 2020, 43, 2217–2221. [Google Scholar] [CrossRef]
- Guangzheng, X.; Hui, W.; Yingqian, D.; Keyi, X.; Weibo, Z.; Xingchu, G. Research Progress on Quality Control Methods for Xiaochaihu Preparations. Separations 2021, 8, 199. [Google Scholar] [CrossRef]
- Jiaying, W.; Bingyong, M.; Jiayu, G.; Shumao, C.; Qiuxiang, Z. Isolation and Identification of the Intestinal Bacteria Capable of Utilizing Stachyose and its Utilization Characteristics. Shipin Yu Fajiao Gongye 2020, 46, 16–23. [Google Scholar] [CrossRef]
- Wei, L.; Jiawei, W.; Lingyuan, M.; Xin, W.; Xia, X.; Baowei, Y. Application and Effects of Stachyose on Enterobacteria: Research Progress. Chin. J. Microecol. 2017, 29, 1110–1113, 1117. [Google Scholar] [CrossRef]
- Jiaying, W.; Minxuan, C.; Tianci, J.; Shunhe, W.; Shumao, C.; Xin, T.; Bingyong, M. Utilization Characteristics of Stachyose by Bifidobacterium and Lactobacillus. Shipin Yu Fajiao Gongye 2021, 47, 13–20. [Google Scholar] [CrossRef]
- Zeqi, C.; Wei, G.; Fei, L.; Chengyi, Z.; Fang, Z.; Wenzhu, L.; Jianyang, P.; Haibin, Q. Simultaneous Determination of Seven Saccharides in the Intermediates of Danshen Chuanxiongqin Injection by HPLC-ELSD. Zhongguo Xiandai Yingyong Yaoxue 2021, 38, 1349–1353. [Google Scholar] [CrossRef]
- Yang, Z.; Desheng, X.; Li, L.; Furong, Q.; Jiiongliang, C.; Guanglin, X. A LC-MS/MS Method for the Determination of Stachyose in Rat Plasma and its Application to a Pharmacokinetic Study. J. Pharm. Biomed. Anal. 2016, 123, 24–30. [Google Scholar] [CrossRef]
- Ghosh, R.; Kline, P. HPLC with Charged Aerosol Detector (CAD) as a Quality Control Platform for Analysis of Carbohydrate Polymers. BMC Res. Notes 2019, 12, 268. [Google Scholar] [CrossRef]
- Linlin, W.; Shunnan, Z.; Lihong, Z.; Haoshu, X.; Xingchu, G.; Sijie, Z.; Jianyang, P.; Haibin, Q. Establishment and Validation of the Quantitative Analysis of Multi-Components by Single Marker for the Quality Control of Qishen Yiqi Dripping Pills by High-Performance Liquid Chromatography with Charged Aerosol Detection. Phytochem. Anal. 2021, 32, 942–956. [Google Scholar] [CrossRef]
- Johanne, P.; Julian, W. Hydrophilic Interaction Chromatography Coupled with Charged Aerosol Detection for Simultaneous Quantitation of Carbohydrates, Polyols and Ions in Food and Beverages. Molecules 2019, 24, 4333. [Google Scholar] [CrossRef] [Green Version]
- Aneta, S.; Ewa, J.-R.; Anna, S. High-Performance Liquid Chromatography Determination of Free Sugars and Mannitol in Mushrooms Using Corona Charged Aerosol Detection. Food Anal. Method 2021, 14, 209–216. [Google Scholar] [CrossRef]
- Ying, W.; Yuanxi, L.; Hongshui, Y.; Weiyi, X.; Jianming, C.; Hongyu, J.; Shuangcheng, M. Comparison between Charged Aerosol Detector and Evaporative Light Scattering Detector for Analysis of Sugar in Zhusheyong Yiqi Fumai and Study on Accuracy of Methods. Zhongguo Zhongyao Zazhi 2020, 45, 5511–5517. [Google Scholar] [CrossRef]
- Jingyuan, S.; Haibin, Q.; Xingchu, G. Comparison of Two Algorithms for Development of Design Space-Overlapping Method and Probability-Based Method. Zhongguo Zhongyao Zazhi 2018, 43, 2074–2080. [Google Scholar] [CrossRef]




| t/min | B% |
|---|---|
| 0 | X1 |
| X2 | X3 |
| X4 | 50 |
| X4 + 5 | 50 |
| Level | Phase B Content in Mobile Phase at 0 min X1/% | Closing Time of the First Gradient X2/min | Phase B Content in Mobile Phase at the Beginning of the Second Gradient X3/% | Closing Time of the Second Gradient X4/min | Column Temperature X5/°C | Flow Rate X6 /(mL/min) |
|---|---|---|---|---|---|---|
| −1 | 78.0 | 8.0 | 71.0 | 28.0 | 26.0 | 0.60 |
| 0 | 80.0 | 10.0 | 73.0 | 30.0 | 28.0 | 0.70 |
| 1 | 82.0 | 12.0 | 75.0 | 32.0 | 30.0 | 0.80 |
| Run | X1/% | X2/min | X3/% | X4/min | X5/°C | X6/(mL/min) | Y1 | Y2/% | Y3/min |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 78.0 | 8.0 | 71.0 | 30.0 | 30.0 | 0.60 | 15 | 88.86 | 24.43 |
| 2 | 82.0 | 10.0 | 75.0 | 28.0 | 30.0 | 0.60 | 15 | 85.26 | 27.18 |
| 3 | 78.0 | 10.0 | 71.0 | 32.0 | 26.0 | 0.80 | 12 | 89.63 | 22.92 |
| 4 | 80.0 | 10.0 | 73.0 | 30.0 | 28.0 | 0.70 | 11 | 91.21 | 25.27 |
| 5 | 82.0 | 8.0 | 71.0 | 32.0 | 28.0 | 0.80 | 12 | 90.45 | 21.88 |
| 6 | 80.0 | 12.0 | 75.0 | 32.0 | 30.0 | 0.80 | 9 | 93.88 | 26.83 |
| 7 | 80.0 | 8.0 | 71.0 | 28.0 | 26.0 | 0.60 | 12 | 92.43 | 24.03 |
| 8 | 82.0 | 12.0 | 71.0 | 28.0 | 26.0 | 0.70 | 11 | 92.16 | 24.95 |
| 9 | 82.0 | 12.0 | 75.0 | 30.0 | 26.0 | 0.80 | 11 | 91.93 | 26.29 |
| 10 | 78.0 | 8.0 | 75.0 | 28.0 | 26.0 | 0.80 | 10 | 92.51 | 22.27 |
| 11 | 82.0 | 12.0 | 71.0 | 32.0 | 30.0 | 0.60 | 14 | 88.22 | 27.41 |
| 12 | 78.0 | 12.0 | 75.0 | 28.0 | 28.0 | 0.60 | 14 | 89.58 | 27.98 |
| 13 | 78.0 | 8.0 | 75.0 | 32.0 | 30.0 | 0.70 | 12 | 90.51 | 25.73 |
| 14 | 82.0 | 8.0 | 73.0 | 28.0 | 30.0 | 0.80 | 14 | 88.44 | 22.09 |
| 15 | 78.0 | 12.0 | 71.0 | 28.0 | 30.0 | 0.80 | 12 | 90.4 | 23.19 |
| 16 | 78.0 | 12.0 | 73.0 | 32.0 | 26.0 | 0.60 | 13 | 89.47 | 28.68 |
| 17 | 82.0 | 8.0 | 75.0 | 32.0 | 26.0 | 0.60 | 17 | 86.24 | 27.78 |
| 18 | 80.0 | 10.0 | 73.0 | 30.0 | 28.0 | 0.70 | 11 | 91.59 | 25.25 |
| 19 | 80.0 | 10.0 | 73.0 | 30.0 | 28.0 | 0.70 | 11 | 91.32 | 25.25 |
| 20 | 80.0 | 10.0 | 73.0 | 30.0 | 28.0 | 0.70 | 11 | 91.63 | 25.24 |
| Run | X1/% | X2/min | X3/% | X4/min | X5/°C | X6/(mL/min) | Y1 | Y2/% | Y3/min |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 78.0 | 9.0 | 74.0 | 30.0 | 31.0 | 0.59 | 14 | 90.05 | 26.60 |
| 2 | 78.0 | 8.0 | 73.0 | 31.0 | 31.0 | 0.61 | 13 | 89.78 | 25.36 |
| 3 | 79.0 | 8.0 | 74.0 | 31.0 | 29.0 | 0.61 | 9 | 93.69 | 26.09 |
| 4 | 78.0 | 9.0 | 74.0 | 31.0 | 29.0 | 0.61 | 10 | 93.16 | 26.59 |
| 5 | 78.0 | 9.0 | 73.0 | 30.0 | 29.0 | 0.61 | 12 | 91.10 | 25.66 |
| 6 | 79.0 | 9.0 | 73.0 | 31.0 | 31.0 | 0.59 | 12 | 91.34 | 26.39 |
| 7 | 78.5 | 8.5 | 73.5 | 30.5 | 30.0 | 0.60 | 14 | 89.26 | 26.00 |
| 8 | 78.0 | 8.0 | 73.0 | 30.0 | 29.0 | 0.59 | 13 | 90.37 | 25.46 |
| 9 | 78.5 | 8.5 | 73.5 | 30.5 | 30.0 | 0.60 | 14 | 89.85 | 25.99 |
| 10 | 79.0 | 8.0 | 74.0 | 30.0 | 29.0 | 0.59 | 11 | 91.85 | 26.08 |
| 11 | 79.0 | 8.0 | 73.0 | 30.0 | 31.0 | 0.61 | 12 | 91.86 | 25.03 |
| 12 | 79.0 | 9.0 | 73.0 | 31.0 | 29.0 | 0.59 | 12 | 92.46 | 26.40 |
| 13 | 78.5.0 | 8.5 | 73.5 | 30.5 | 30.0 | 0.60 | 13 | 90.67 | 25.94 |
| 14 | 79.0 | 9.0 | 74.0 | 30.0 | 31.0 | 0.61 | 13 | 90.37 | 26.14 |
| 15 | 78.0 | 8.0 | 74.0 | 31.0 | 31.0 | 0.59 | 13 | 91.51 | 26.21 |
| Y1 | Y2/% | Y3/min | ||||
|---|---|---|---|---|---|---|
| Item | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value |
| Constants | 10.942 | 0.000 | 91.463 | 0.000 | 25.254 | 0.000 |
| X1 | 0.429 | 0.036 | −0.590 | 0.000 | 0.170 | 0.000 |
| X2 | −0.571 | 0.009 | 0.443 | 0.001 | 1.223 | 0.000 |
| X3 | - | - | −0.160 | 0.092 | 1.088 | 0.000 |
| X4 | - | - | −0.170 | 0.077 | 0.682 | 0.000 |
| X5 | 0.357 | 0.073 | −0.629 | 0.000 | - | - |
| X6 | −1.429 | 0.000 | 1.227 | 0.000 | −1.573 | 0.000 |
| X12 | 2.337 | 0.000 | −3.323 | 0.000 | −0.208 | 0.000 |
| X22 | - | - | 2.788 | 0.000 | 0.236 | 0.000 |
| X32 | - | - | - | - | 0.223 | 0.000 |
| X42 | −1.288 | 0.020 | 0.543 | 0.044 | −0.460 | 0.000 |
| X52 | 0.962 | 0.068 | −1.690 | 0.000 | 0.554 | 0.000 |
| X62 | - | - | - | - | −0.374 | 0.000 |
| X1 X2 | −1.125 | 0.001 | 1.106 | 0.000 | −0.323 | 0.000 |
| X1 X4 | - | - | −0.401 | 0.008 | −0.062 | 0.002 |
| Methods | Gradient 1 | Gradient 2 | Peak Number | Percentage of Common Peak/% | Retention Time of the Last Peak/min | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phase B Content in Mobile Phase at the Beginning/% | Closing Time/min | Phase B Content in Mobile Phase at the Beginning/% | Closing Time/min | Predicted Value | Measured Value | Predicted Value | Measured Value | Predicted Value | Measured Value | |
| A | 78.0 | 10.0 | 74.0 | 28.0 | 14 | 14 | 88.26 | 91.72 | 26.025 | 26.683 |
| B | 78.5 | 8.5 | 73.5 | 30.5 | 14 | 14 | 90.19 | 91.50 | 26.236 | 25.504 |
| C | 78.0 | 9.0 | 74.0 | 30.0 | 15 | 14 | 89.43 | 88.65 | 26.515 | 26.445 |
| Concentration Level | Ribitol | Fructose | Sucrose | Stachyose |
|---|---|---|---|---|
| Low level recovery rate (%) | 102.4 | 105.4 | 106.6 | 103.5 |
| 103.4 | 105.0 | 106.4 | 99.64 | |
| 105.3 | 103.8 | 105.4 | 99.71 | |
| Medium level recovery rate (%) | 101.1 | 99.95 | 101.5 | 95.20 |
| 102.9 | 103.3 | 103.9 | 102.1 | |
| 104.8 | 102.8 | 103.1 | 101.2 | |
| High level recovery rate (%) | 97.39 | 97.59 | 100.6 | 98.58 |
| 97.78 | 96.87 | 98.86 | 98.26 | |
| 97.14 | 94.50 | 99.28 | 98.82 | |
| Average recovery rate (%) | 101.4 | 101.0 | 102.9 | 99.66 |
| RSD (%) | 3.142 | 3.896 | 2.880 | 2.414 |
| Sample Number | Ribitol (%) | Fructose (%) | Sucrose (%) | Stachyose (%) |
|---|---|---|---|---|
| S1 | 2.004 | 2.012 | 4.666 | 0.5578 |
| S2 | 1.205 | 4.819 | 3.504 | 0.6880 |
| S3 | 1.206 | 5.021 | 3.413 | 0.8859 |
| S4 | 1.411 | 5.737 | 3.750 | 0.4003 |
| S5 | 1.854 | 2.263 | 3.877 | 0.5058 |
| S6 | 2.200 | 9.018 | 4.549 | 1.815 |
| S7 | 2.281 | 7.209 | 4.838 | 2.255 |
| S8 | 2.209 | 6.478 | 5.109 | 2.411 |
| S9 | 2.236 | 7.244 | 5.316 | 2.430 |
| S10 | 2.185 | 7.935 | 5.320 | 2.310 |
| S11 | 0.8985 | 1.815 | 2.735 | 0 * |
| S12 | 1.042 | 2.303 | 2.054 | 0.412 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, J.; Wu, G.; Wu, L.; Qu, H.; Gong, P.; Xie, Y.; Zhou, P.; Gong, X. Development of a Quantitative Chromatographic Fingerprint Analysis Method for Sugar Components of Xiaochaihu Capsules Based on Quality by Design Concept. Separations 2023, 10, 13. https://doi.org/10.3390/separations10010013
Lan J, Wu G, Wu L, Qu H, Gong P, Xie Y, Zhou P, Gong X. Development of a Quantitative Chromatographic Fingerprint Analysis Method for Sugar Components of Xiaochaihu Capsules Based on Quality by Design Concept. Separations. 2023; 10(1):13. https://doi.org/10.3390/separations10010013
Chicago/Turabian StyleLan, Jing, Gelin Wu, Linlin Wu, Haibin Qu, Ping Gong, Yongjian Xie, Peng Zhou, and Xingchu Gong. 2023. "Development of a Quantitative Chromatographic Fingerprint Analysis Method for Sugar Components of Xiaochaihu Capsules Based on Quality by Design Concept" Separations 10, no. 1: 13. https://doi.org/10.3390/separations10010013
APA StyleLan, J., Wu, G., Wu, L., Qu, H., Gong, P., Xie, Y., Zhou, P., & Gong, X. (2023). Development of a Quantitative Chromatographic Fingerprint Analysis Method for Sugar Components of Xiaochaihu Capsules Based on Quality by Design Concept. Separations, 10(1), 13. https://doi.org/10.3390/separations10010013