Salting-Out Assisted Liquid-Liquid Extraction for UPLC-MS/MS Determination of Thyroxine and Steroid Hormones in Human Serum and Fish Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Stock and Calibration Standards Solutions
2.3. Samples
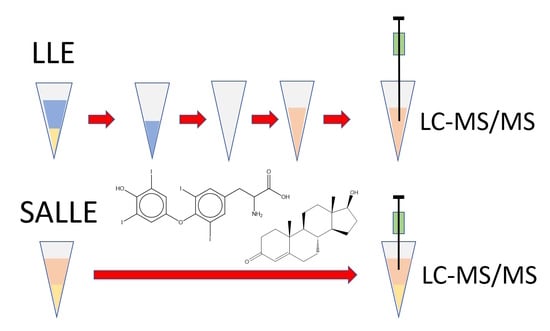
2.4. Sample Preparation
- Pipetting into a microcentrifuge tube:
- 150 µL sample of either human serum, fish plasma, calibration standard, or blank water
- 25 µL internal standard solution in methanol (ISTD)
- 100 µL saturated ammonium sulfate
- 225 µL acetonitrile (ACN).
- Capping the tube, shaking for 1 min, and centrifuging for 10 min at 2300 G.
- Pipetting 100 µL from the top phase solvent to an autosampler micro-vial, capping, and putting it into the UPLC-MS/MS instrument.
2.5. Instrumental Method Development
2.6. Method Validation
- SALLE:
- ◦
- 150 µL quality control human serum
- ◦
- 25 µL methanol (instead of ISTD)
- ◦
- 100 µL saturated ammonium sulfate
- ◦
- 225 µL ACN
- PPT:
- ◦
- 150 µL quality control human serum
- ◦
- 25 µL methanol (instead of ISTD)
- ◦
- 325 µL ACN
- LLE:
- ◦
- 150 µL quality control human serum
- ◦
- 25 µL methanol (instead of ISTD)
- ◦
- 325 µL MTBE
2.7. Data Analysis
3. Results
3.1. UPLC-MS/MS Instrumental Method
3.2. Exploring Solvents for the SALLE Method
3.3. Ammonium Sulphate vs. Sodium Chloride
3.4. Reducing Solvent Volume
3.5. Method Validation
3.6. Measurements in Fish Plasma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, G.; Lightman, S. The Human Stress Response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Liyanarachchi, K.; Ross, R.; Debono, M. Human Studies on Hypothalamo-Pituitary-Adrenal (HPA) Axis. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, A.; Reyes-López, F.E.; Tallo-Parra, O.; Lopez-Bejar, M.; Tort, L. Comparative Assessment of Cortisol in Plasma, Skin Mucus and Scales as a Measure of the Hypothalamic-Pituitary-Interrenal Axis Activity in Fish. Aquaculture 2019, 506, 410–416. [Google Scholar] [CrossRef]
- Sadoul, B.; Geffroy, B. Measuring Cortisol, the Major Stress Hormone in Fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in Teleosts: Dynamics, Mechanisms of Action, and Metabolic Regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Cabrera-Busto, J.; Mancera, J.M.; Ruiz-Jarabo, I. Cortisol and Dexamethasone Mediate Glucocorticoid Actions in the Lesser Spotted Catshark (Scyliorhinus canicula). Biology 2022, 11, 56. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in Fishes: A Diversity of Responses with Particular Reference to Changes in Circulating Corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Ciani, E.; von Krogh, K.; Nourizadeh-Lillabadi, R.; Mayer, I.; Fontaine, R.; Weltzien, F.-A. Sexual Maturation in Atlantic Salmon Male Parr May Be Triggered Both in Late Summer and Early Spring under Standard Farming Conditions. Aquaculture 2021, 544, 737086. [Google Scholar] [CrossRef]
- Blanton, M.L.; Specker, J.L. The Hypothalamic-Pituitary-Thyroid (HPT) Axis in Fish and Its Role in Fish Development and Reproduction. Crit. Rev. Toxicol. 2007, 37, 97–115. [Google Scholar] [CrossRef]
- Deal, C.K.; Volkoff, H. The Role of the Thyroid Axis in Fish. Front. Endocrinol. 2020, 11, 596585. [Google Scholar] [CrossRef]
- Ortiga-Carvalho, T.M.; Chiamolera, M.I.; Pazos-Moura, C.C.; Wondisford, F.E. Hypothalamus-Pituitary-Thyroid Axis. In Comprehensive Physiology; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 1387–1428. ISBN 978-0-470-65071-4. [Google Scholar]
- Chen, X.; Walter, K.M.; Miller, G.W.; Lein, P.J.; Puschner, B. Simultaneous Quantification of T4, T3, RT3, 3,5-T2 and 3,3′-T2 in Larval Zebrafish (Danio rerio) as a Model to Study Exposure to Polychlorinated Biphenyls. Biomed. Chromatogr. 2018, 32, e4185. [Google Scholar] [CrossRef] [Green Version]
- Peter, M.C.S. The Role of Thyroid Hormones in Stress Response of Fish. Gen. Comp. Endocrinol. 2011, 172, 198–210. [Google Scholar] [CrossRef]
- Deal, C.K.; Volkoff, H. Response of the Thyroid Axis and Appetite-Regulating Peptides to Fasting and Overfeeding in Goldfish (Carassius auratus). Mol. Cell. Endocrinol. 2021, 528, 111229. [Google Scholar] [CrossRef]
- Cao, Y.; Tveten, A.-K.; Stene, A. Establishment of a Non-Invasive Method for Stress Evaluation in Farmed Salmon Based on Direct Fecal Corticoid Metabolites Measurement. Fish Shellfish Immunol. 2017, 66, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.E.; Keevil, B.; Huhtaniemi, I.T. Mass Spectrometry and Immunoassay: How to Measure Steroid Hormones Today and Tomorrow. Eur. J. Endocrinol. 2015, 173, D1–D12. [Google Scholar] [CrossRef] [Green Version]
- Karashima, S.; Osaka, I. Rapidity and Precision of Steroid Hormone Measurement. J. Clin. Med. 2022, 11, 956. [Google Scholar] [CrossRef]
- Hillebrand, J.J.; Wickenhagen, W.V.; Heijboer, A.C. Improving Science by Overcoming Laboratory Pitfalls With Hormone Measurements. J. Clin. Endocrinol. Metab. 2021, 106, e1504–e1512. [Google Scholar] [CrossRef]
- Noyes, P.D.; Lema, S.C.; Roberts, S.C.; Cooper, E.M.; Stapleton, H.M. Rapid Method for the Measurement of Circulating Thyroid Hormones in Low Volumes of Teleost Fish Plasma by LC-ESI/MS/MS. Anal. Bioanal. Chem. 2014, 406, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Ghazal, K.; Brabant, S.; Prie, D.; Piketty, M.-L. Hormone Immunoassay Interference: A 2021 Update. Ann. Lab. Med. 2022, 42, 3–23. [Google Scholar] [CrossRef]
- Hoofnagle, A.N.; Wener, M.H. The Fundamental Flaws of Immunoassays and Potential Solutions Using Tandem Mass Spectrometry. J. Immunol. Methods 2009, 347, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Bird, R.; Schmeling, M.W.; Hoofnagle, A.N. Using Mass Spectrometry to Overcome the Longstanding Inaccuracy of a Commercially-Available Clinical Testosterone Immunoassay. J. Chromatogr. B 2021, 1183, 122969. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, G.D. Spurious Serum Hormone Immunoassay Results: Causes, Recognition, Management. touchREV. Endocrinol. 2022, 18, 141–147. [Google Scholar] [CrossRef]
- Blackwell, B.R.; Ankley, G.T. Simultaneous Determination of a Suite of Endogenous Steroids by LC-APPI-MS: Application to the Identification of Endocrine Disruptors in Aquatic Toxicology. J. Chromatogr. B 2021, 1163, 122513. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Luong, X.; Sedlak, D.L.; Helbing, C.C.; Hayes, T. Quantification of 11 Thyroid Hormones and Associated Metabolites in Blood Using Isotope-Dilution Liquid Chromatography Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2016, 408, 5429–5442. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.-Z.; Kroll, K.J.; Webb, M.; Denslow, N.D. Quantification of Steroid Hormones in Low Volume Plasma and Tissue Homogenates of Fish Using LC-MS/MS. Gen. Comp. Endocrinol. 2020, 296, 113543. [Google Scholar] [CrossRef]
- Moein, M.M.; El Beqqali, A.; Abdel-Rehim, M. Bioanalytical Method Development and Validation: Critical Concepts and Strategies. J. Chromatogr. B 2017, 1043, 3–11. [Google Scholar] [CrossRef]
- Budzinski, H.; Devier, M.H.; Labadie, P.; Togola, A. Analysis of Hormonal Steroids in Fish Plasma and Bile by Coupling Solid-Phase Extraction to GC/MS. Anal. Bioanal. Chem. 2006, 386, 1429–1439. [Google Scholar] [CrossRef]
- Domenech-Coca, C.; Mariné-Casadó, R.; Caimari, A.; Arola, L.; del Bas, J.M.; Bladé, C.; Rodriguez-Naranjo, M.I. Dual Liquid-Liquid Extraction Followed by LC-MS/MS Method for the Simultaneous Quantification of Melatonin, Cortisol, Triiodothyronine, Thyroxine and Testosterone Levels in Serum: Applications to a Photoperiod Study in Rats. J. Chromatogr. B 2019, 1108, 11–16. [Google Scholar] [CrossRef]
- Woźniak, B.; Matraszek-Żuchowska, I.; Witek, S.; Posyniak, A. Development of LC-MS/MS Confirmatory Method for the Determination of Testosterone in Bovine Serum. J. Vet. Res. 2017, 61, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wang, H.; Huan, X.; Cao, N.; Guan, H.; Zhang, H.; Cheng, X.; Wang, C. Simultaneous LC-MS/MS Bioanalysis of Alkaloids, Terpenoids, and Flavonoids in Rat Plasma through Salting-out-Assisted Liquid-Liquid Extraction after Oral Administration of Extract from Tetradium ruticarpum and Glycyrrhiza uralensis: A Sample Preparation Strategy to Broaden Analyte Coverage of Herbal Medicines. Anal. Bioanal. Chem. 2021, 413, 5871–5884. [Google Scholar] [CrossRef]
- Li, M.; Zhuang, B.; Lu, Y.; An, L.; Wang, Z.-G. Salt-Induced Liquid–Liquid Phase Separation: Combined Experimental and Theoretical Investigation of Water–Acetonitrile–Salt Mixtures. J. Am. Chem. Soc. 2021, 143, 773–784. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Weng, N. Salting-out Assisted Liquid-Liquid Extraction for Bioanalysis. Bioanalysis 2013, 5, 1583–1598. [Google Scholar] [CrossRef]
- Valente, I.M.; Gonçalves, L.M.; Rodrigues, J.A. Another Glimpse over the Salting-out Assisted Liquid–Liquid Extraction in Acetonitrile/Water Mixtures. J. Chromatogr. A 2013, 1308, 58–62. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H.; Kim, E.; El-Shourbagy, T.A. Salting-out Assisted Liquid/Liquid Extraction with Acetonitrile: A New High Throughput Sample Preparation Technique for Good Laboratory Practice Bioanalysis Using Liquid Chromatography–Mass Spectrometry. Biomed. Chromatogr. 2009, 23, 419–425. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, X.; Zhang, Y.; Chen, H.; Li, G.; Xu, Y.; Zhao, Q.; Song, W.; Jin, H.; Ding, L. Dynamic Microwave-Assisted Extraction Coupled with Salting-out Liquid-Liquid Extraction for Determination of Steroid Hormones in Fish Tissues. J. Agric. Food Chem. 2012, 60, 10343–10351. [Google Scholar] [CrossRef]
- Buhrman, D.L.; Price, P.I.; Rudewicz, P.J. Quantitation of SR 27417 in Human Plasma Using Electrospray Liquid Chromatography-Tandem Mass Spectrometry: A Study of Ion Suppression. J. Am. Soc. Mass Spectrom. 1996, 7, 1099–1105. [Google Scholar] [CrossRef] [Green Version]
- Kvamsøe, M.M.; Hansen, K.R.; Skadberg, Ø.; Vethe, N.T.; Brede, C. Salting Out-Assisted Liquid–Liquid Extraction for Liquid Chromatography–Tandem Mass Spectrometry Measurement of Tacrolimus, Sirolimus, Everolimus, and Cyclosporine a in Whole Blood. Ther. Drug Monit. 2020, 42, 695–701. [Google Scholar] [CrossRef]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem: Gembloux, Belgium, 2014; ISBN 978-91-87461-59-0. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters—A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-429-12752-6. [Google Scholar]
- Nadarajah, N.; Skadberg, Ø.; Adaway, J.; Brede, C. Multiplexed Analysis of Steroid Hormones in Saliva by LC-MS/MS with 2-Hydrazinopyridine Derivatization. Clin. Mass Spectrom. 2017, 4–5, 1–10. [Google Scholar] [CrossRef]
- Boggs, A.S.P.; Bowden, J.A.; Galligan, T.M.; Guillette, L.J.; Kucklick, J.R. Development of a Multi-Class Steroid Hormone Screening Method Using Liquid Chromatography/Tandem Mass Spectrometry (LC-MS/MS). Anal. Bioanal. Chem. 2016, 408, 4179–4190. [Google Scholar] [CrossRef] [Green Version]
- Ke, Y.; Bertin, J.; Gonthier, R.; Simard, J.-N.; Labrie, F. A Sensitive, Simple and Robust LC–MS/MS Method for the Simultaneous Quantification of Seven Androgen- and Estrogen-Related Steroids in Postmenopausal Serum. J. Steroid Biochem. Mol. Biol. 2014, 144, 523–534. [Google Scholar] [CrossRef]
- Yong, S.; Chen, Y.; Lee, T.K.; Lee, H.K. Determination of Total Thyroxine in Human Serum by Hollow Fiber Liquid-Phase Microextraction and Liquid Chromatography–Tandem Mass Spectrometry. Talanta 2014, 126, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Alzweiri, M.; Watson, D.G.; Robertson, C.; Sills, G.J.; Parkinson, J.A. Comparison of Different Water-Miscible Solvents for the Preparation of Plasma and Urine Samples in Metabolic Profiling Studies. Talanta 2008, 74, 1060–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, S.A.; Entezari, M.H. Salting out in ACN/Water Systems: Hofmeister Effects and Partition of Quercetin. J. Mol. Liq. 2020, 312, 113331. [Google Scholar] [CrossRef]
- Jutfelt, F.; Olsen, R.E.; Björnsson, B.T.; Sundell, K. Parr–Smolt Transformation and Dietary Vegetable Lipids Affect Intestinal Nutrient Uptake, Barrier Function and Plasma Cortisol Levels in Atlantic Salmon. Aquaculture 2007, 273, 298–311. [Google Scholar] [CrossRef]
- Barton, B.A.; Iwama, G.K. Physiological Changes in Fish from Stress in Aquaculture with Emphasis on the Response and Effects of Corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Meling, V.A.; Berge, K.; Knudsen, D.L.; Rønning, P.O.; Brede, C. Monitoring Farmed Fish Welfare by Measurement of Cortisol as a Stress Marker in Fish Feces by Liquid Chromatography Coupled with Tandem Mass Spectrometry. Molecules 2022, 27, 2481. [Google Scholar] [CrossRef]




| Compound Name | Molecule Mass (Da) | Parent ion (m/z) | Daughter Ion (m/z) | Dwell Time (s) | Cone Voltage (V) | CE (eV) | Retention Time (min) |
|---|---|---|---|---|---|---|---|
| Cortisol | 362.21 | 363.2 | 121.1 | 0.015 | 33 | 23 | 2.87 |
| D4 Cortisol | 367.2 | 121.1 | 0.015 | 33 | 23 | 2.87 | |
| Cortisone | 360.19 | 361.2 | 163.1 | 0.015 | 38 | 25 | 2.7 |
| D8 Cortisone | 369.2 | 169 | 0.015 | 38 | 25 | 2.7 | |
| Testosterone | 288.21 | 289.3 | 97.1 | 0.015 | 33 | 20 | 3.63 |
| D3Testosterone | 292.2 | 97.1 | 0.015 | 33 | 20 | 3.63 | |
| Thyroxine | 776.87 | 777.69 | 731.7 | 0.015 | 45 | 25 | 3.2 |
| 777.69 | 604.9 | 0.015 | 45 | 40 | 3.2 | ||
| 777.69 | 351 | 0.015 | 45 | 45 | 3.2 | ||
| 13C6 thyroxine | 783.69 | 737.7 | 0.015 | 45 | 25 | 3.2 |
| Parameters | Thyroxine | Cortisol | Cortisone | Testosterone | |
|---|---|---|---|---|---|
| Precision (RSD %) | Repeatability | 13 | 14 | 14 | 13 |
| Intermediate | 13 | 17 | 14 | 13 | |
| Relative bias (%) | low | 76 | 127 | 96 | 96 |
| medium | 92 | 55 | 81 | 90 | |
| high | 95 | 75 | 87 | 102 | |
| LOD (ng/mL) | 0.04 | 0.02 | 0.07 | 0.01 | |
| LOQ (ng/mL) | 0.18 | 0.15 | 0.12 | 0.08 | |
| Linearity (R2) | 0.997–0.999 | 0.998–0.999 | 0.998–0.999 | 0.999 | |
| Liner range (ng/mL) | 0.18–200 | 0.15–100 | 0.12–100 | 0.08–100 | |
| Mean + SE (ng/mL) | ||||||
|---|---|---|---|---|---|---|
| N | n | Thyroxine | Cortisol | Cortisone | Testosterone | |
| Net 1 | 9 | 3 | 2.28 + 0.38 a | 14.1 + 1.8 b | 13.8 + 1.1 a | 0.32 + 0.04 a |
| Net 2 | 9 | 3 | 1.79 + 0.25 ab | 89.2 + 7.9 a | 32.9 + 0.7 b | 0.28 + 0.04 a |
| Net 3 | 9 | 3 | 1.23 + 0.19 b | 21.1 + 2.2 b | 18.5 + 1.4 c | 0.32 + 0.04 a |
| Hansen Solubility Parameters | |||
|---|---|---|---|
| Substance | δD (Dispersion) | δP (Polarity) | δH (Hydrogen Bonding) |
| Thyroxine Testosterone | 23.4 18.6 | 5.2 5.4 | 13.5 6.2 |
| Cortisol Cortisone | 19.2 19.7 | 10.1 11.8 | 9.2 7.8 |
| Acetonitrile | 15.3 | 18 | 6.1 |
| Methanol | 14.7 | 12.3 | 22.3 |
| Acetone | 15.5 | 10.4 | 7 |
| 1-propanol | 16 | 6.8 | 17.4 |
| 2-propanol | 15.8 | 6.1 | 16.4 |
| MTBE | 14.8 | 4.3 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urge, A.Y.; Pampanin, D.M.; Martino, M.E.; Knudsen, D.L.; Brede, C. Salting-Out Assisted Liquid-Liquid Extraction for UPLC-MS/MS Determination of Thyroxine and Steroid Hormones in Human Serum and Fish Plasma. Separations 2023, 10, 240. https://doi.org/10.3390/separations10040240
Urge AY, Pampanin DM, Martino ME, Knudsen DL, Brede C. Salting-Out Assisted Liquid-Liquid Extraction for UPLC-MS/MS Determination of Thyroxine and Steroid Hormones in Human Serum and Fish Plasma. Separations. 2023; 10(4):240. https://doi.org/10.3390/separations10040240
Chicago/Turabian StyleUrge, Alemnesh Yirda, Daniela Maria Pampanin, Maria Elena Martino, David Lausten Knudsen, and Cato Brede. 2023. "Salting-Out Assisted Liquid-Liquid Extraction for UPLC-MS/MS Determination of Thyroxine and Steroid Hormones in Human Serum and Fish Plasma" Separations 10, no. 4: 240. https://doi.org/10.3390/separations10040240
APA StyleUrge, A. Y., Pampanin, D. M., Martino, M. E., Knudsen, D. L., & Brede, C. (2023). Salting-Out Assisted Liquid-Liquid Extraction for UPLC-MS/MS Determination of Thyroxine and Steroid Hormones in Human Serum and Fish Plasma. Separations, 10(4), 240. https://doi.org/10.3390/separations10040240